1. Introduction
The western margin of the Indian subcontinent is globally recognized for its near-continuous Cenozoic marine sedimentary successions complemented with a unique tectonic history linked to significant geological changes during the Cenozoic (Biswas, Reference Biswas1987). The Gaj Formation in the Dwarka Basin, a key tectono-sedimentary province of western India, represents a significant geological archive of marine sedimentary sequences along with rich and diverse faunal assemblages ranging from the Miocene to Holocene. Only a few studies on the faunal composition have been attempted here by researchers like Bhatia & Mohan (Reference Bhatia and Mohan1959), Khosla (Reference Khosla1978), Khosla et al. (Reference Khosla, Mehra and Nagori1986), and Jain (Reference Jain2002) (Fig. 1). Preliminary documentation of the Tertiary marine fauna from the Kathiawar was done by Sowerby (Reference Sowerby1840), D’Archiac (Reference D’Archiac1851), d’Archiac & Haime (Reference d’Archiac and Haime1853, Reference d’Archiac and Haime1854), and Carter (Reference Carter1857). Subsequently, diverse invertebrates were reported from the Miocene of the basin which comprised of echinoids (Duncan & Sladen, Reference Duncan and Sladen1882; Duncan et al. Reference Duncan, Sladen and Blanford1883; Jain, Reference Jain2002), corals (Duncan, Reference Duncan1880; Duncan & Sladen, Reference Duncan and Sladen1882; Jain, Reference Jain2014), crabs (Stoliczka, Reference Stoliczka1871) and molluscs, that is, gastropods, bivalves and nautiloids (Duncan, Reference Duncan1880; Fedden, Reference Fedden1884; Vredenburg, Reference Vredenburg1925; Nath, Reference Nath1962; Pascoe, Reference Pascoe1964; Pandey et al. Reference Pandey, Kondo, Jain, Bahadur and Pradhan2008; Jain, Reference Jain2014; Bose et al. Reference Bose, Das and Mondal2021, Reference Bose, Das and Saha2023). Studies on microfossils were mainly done on foraminifera (Bhatia & Mohan, Reference Bhatia and Mohan1959; Chatterji, Reference Chatterji1961) and on ostracods (Khosla, Reference Khosla1978; Khosla & Pant, Reference Khosla and Pant1978; Khosla et al. Reference Khosla, Mehra and Nagori1986). Additionally, diverse calcareous algal assemblages were also studied from the Miocene-Quaternary succession of this basin (Kundal et al. Reference Kundal, Kundal and Mude2014 and the references therein). Jain (Reference Jain2014) also reported the presence of some broken and fragmented bones and ribs of the Miocene mammals and crocodilian fauna from both the Gaj and Dwarka formations.

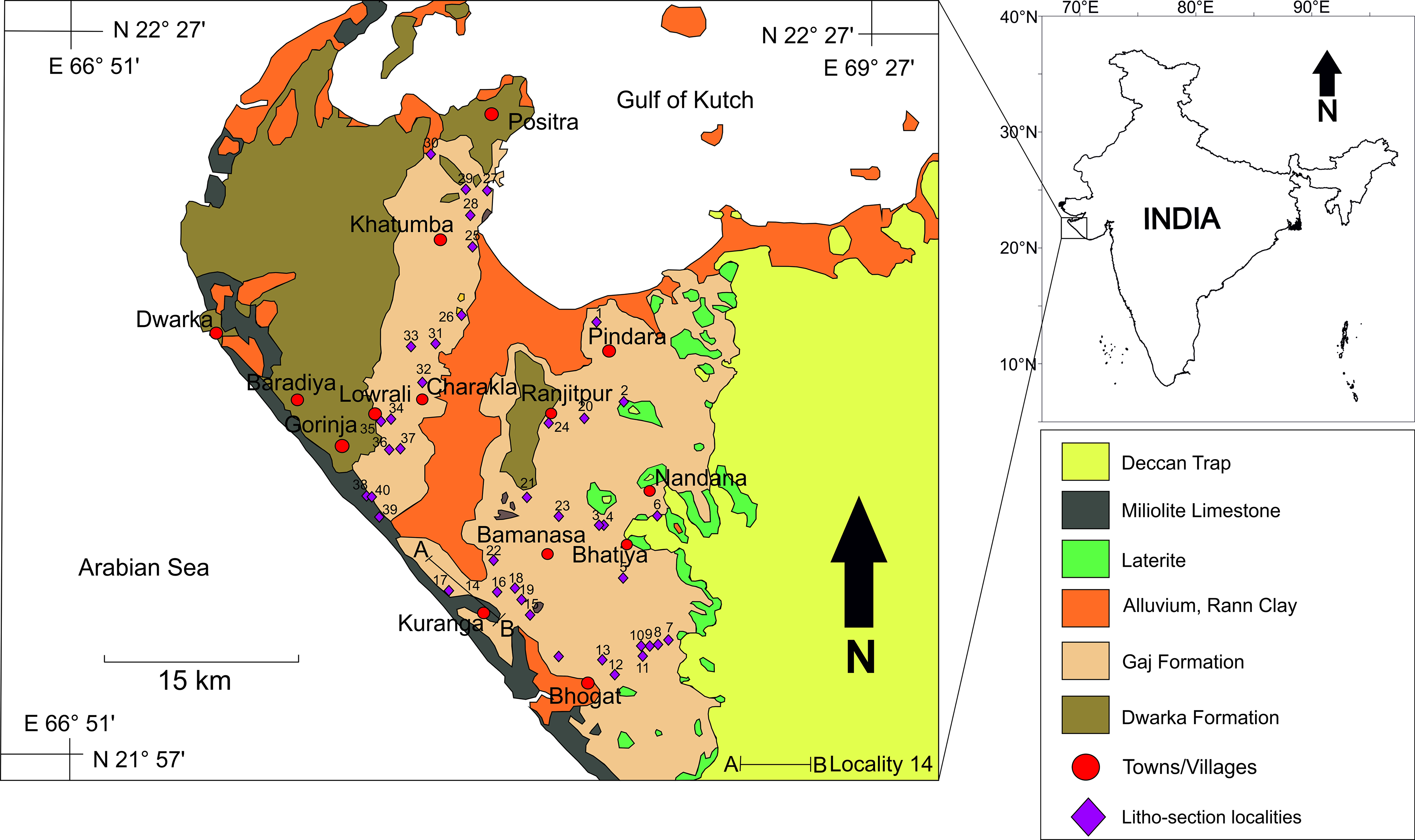
Figure 1. Geological map of the Dwarka Basin, Gujarat, western India, modified and redrawn from Jain (Reference Jain2014), showing 40 litho-section localities.
However, despite these studies, the vast availability of numerous microfossils needs to be documented, and high-resolution biostratigraphy for this region is currently absent. Additionally, a detailed and comprehensive study on paleoenvironments is still lacking. Such studies would be crucial for reconstructing the paleoclimate and paleodepositional history, as well as understanding the global climate of that time period. This study aims to address these knowledge gaps by employing a multi-proxy approach, incorporating mega-invertebrate, microfossils, mineralogical analyses, sedimentological and geochemical data. By integrating these multiple proxies, we hope to better constrain the paleoclimatic and paleodepositional scenarios and address the existing knowledge gaps within the basin during that time. This multifaceted approach will not only fill the current research gaps but also enhance our broader understanding of Miocene climate changes and their impact on marine environments.
2. Geological background
The Indian subcontinent is depicted as ‘Noah’s Ark’ due to the biotic implications of the northward drift of the Indian plate after its separation from Gondwanaland during the Cretaceous (Verma et al. Reference Verma, Khosla, Goin and Kaur2016). While passing over the Reunion hotspot, the Indian plate faced tremendous volcanic eruptions, which led to the evolution of Deccan Traps during the end of the Cretaceous–Paleogene transition (Tiwari et al. Reference Tiwari, Grevemeyer, Singh and Morgan2007). The drift culminated in the collision with the Tibetan plate, forming the Himalayan orogen during the early Miocene and eventually initiating monsoon in the Indian subcontinent (Clift & Webb, Reference Clift and Webb2019). The Kathiawar (Saurashtra) Peninsula, at the western extremity of India, was surrounded by complex tectonism, which was initiated with the splitting of the Indian subcontinent from Gondwanaland and Madagascar and further development of the Kachchh and Cambay grabens during the Mesozoic (Pandey et al. Reference Pandey, Kondo, Jain, Bahadur and Pradhan2008). The arching up of the Kathiawar Peninsula was the result of pre-Eocene thermal expansion of the crust as the Indian plate was moving north-eastward over the Reunion hotspot (Courtillot et al. Reference Courtillot, Besse, Vandamme, Montigny, Jaeger and Cappetta1986; Pandey et al. Reference Pandey, Kondo, Jain, Bahadur and Pradhan2008). This arching further resulted in upliftment as well as the development of the radial drainage pattern in the Kathiawar (Pandey et al. Reference Pandey, Kondo, Jain, Bahadur and Pradhan2008). The global sea levels were higher during the Paleogene compared to that in the Neogene (Miller et al. Reference Miller, Kominz, Browning, Wright, Mountain, Katz, Sugarman, Cramer, Christie-Blick and Pekar2005, Reference Miller, Schmelz, Browning, Rosenthal, Hess, Kopp and Wright2024). However, the tectonic uplift of the Kathiawar Peninsula raised above the sea level prevented marine sedimentation to a considerable extent in comparison to the adjacent Cambay, Kachchh and Jaisalmer basins during the Paleogene. The scenario changed during the early Miocene with the northern part of the Indian subcontinent landlocked due to the collision with the Eurasian Plate. Eventually, the major global sea-level rise during the Burdigalian submerged the northwestern coast of the Kathiawar Peninsula, thus forming a peri-cratonic sedimentary basin, that is, the Dwarka Basin (Pandey et al. Reference Pandey, Kondo, Jain, Bahadur and Pradhan2008; Jain, Reference Jain2014).
A handful of studies have been conducted on the stratigraphy and geology of the Dwarka Basin during the past few decades (Mohan & Chatterji, Reference Mohan and Chatterji1956; Bhatia & Mohan, Reference Bhatia and Mohan1959; Bhatt, Reference Bhatt2003; Jain, Reference Jain2014). However, there still remain considerable disparities and differences in opinions among researchers regarding the stratigraphic classification of the Miocene succession of the basin. Fedden (Reference Fedden1884) pioneered in systematically documenting the geology of Kathiawar and divided the Cenozoic (Tertiary) rocks of Kalyanpur and Okha Mandal Talukas into Gaj Beds (Miocene), Dwarka Beds (Pliocene) and Miliolite Formation (Plio-Pleistocene). A few subsequent workers devised a stratigraphic scheme of the basin and further recognized three distinct biohorizons based on microfossils, that is, foraminifera and ostracods (Mohan & Chatterji, Reference Mohan and Chatterji1956; Mohan, Reference Mohan1958; Bhatia & Mohan, Reference Bhatia and Mohan1959). The stratigraphy comprised of the older Bhatia Limestone (lower Burdigalian) lying unconformably on the Laterites, followed by the Visawara Limestone (upper Burdigalian), ‘Orbiculina’ Limestones (Vindobonian in (Chatterji et al. Reference Chatterji, Sah, Mohan and Rao1953)), and lastly, the Dwarka Beds of Post Miocene (Fedden, Reference Fedden1884). They concluded that the Gaj Beds were strictly Burdigalian in age, with the absence of any middle Miocene sediments in the Dwarka Basin. Further, the Gaj Beds were correlated to the upper Gaj and Kyankkoh of Burma (f1–f2) (Mohan & Chatterji, Reference Mohan and Chatterji1956). However, their study area was mainly confined to the eastern, southern and south-western parts of the Kathiawar Peninsula, which only included the Bhatia-Bhogat areas of the Dwarka Basin.
Bhatt (Reference Bhatt2003) proposed a lithostratigraphic classification of the Dwarka Basin, in which he primarily divided the Neogene-Quaternary carbonate deposits into four formations, that is, Gaj Formation (lower to middle Miocene), Dwarka Formation (middle Miocene to Pliocene), Miliolite Formation (early to middle Pleistocene), and lastly, Chaya Formation (middle to late Pleistocene) in stratigraphic order. The entire succession unconformably rests on the Deccan Traps, laterites, and bauxite. Bhatt (Reference Bhatt2003) further subdivided the Gaj Formation into two members, that is, the lower Ashapura Clay Member and the upper Ranjitpur Limestone Member. Jain (Reference Jain2014) developed a new biostratigraphic scheme for the entire sedimentary succession of the Dwarka Basin (Supplementary Table 1). He delineated five biozones based on the molluscan fauna, which had restricted occurrence in the Indo-Pacific and was strictly Miocene in age (Supplementary Table 1). He assigned the Gaj Formation to the early-middle Miocene and the Dwarka Formation to the late Miocene (Supplementary Table 1). He further subdivided the Gaj Formation into seven members based on lithology and classified them within three biozones based on bivalve molluscs biostratigraphy, that is, Zone-1 (early Miocene): Ostrea protoimbricata, Diplodonta incerta naricum and Aturia aturi Acme Zone; Zone-2 (middle Miocene): Tenagodus granti and Lyria (Harpeola) jugosa Zone; and Zone-3 (middle Miocene): Clementia lowraliensis Zone (Supplementary Table 1). Although Jain (Reference Jain2014) studied several litho-sections throughout the basin to show the seven members of the Gaj Formation, he did not provide any composite stratigraphic litholog to study the temporal facies variation.
3. Material and method
3.a. Sample collection and fieldwork
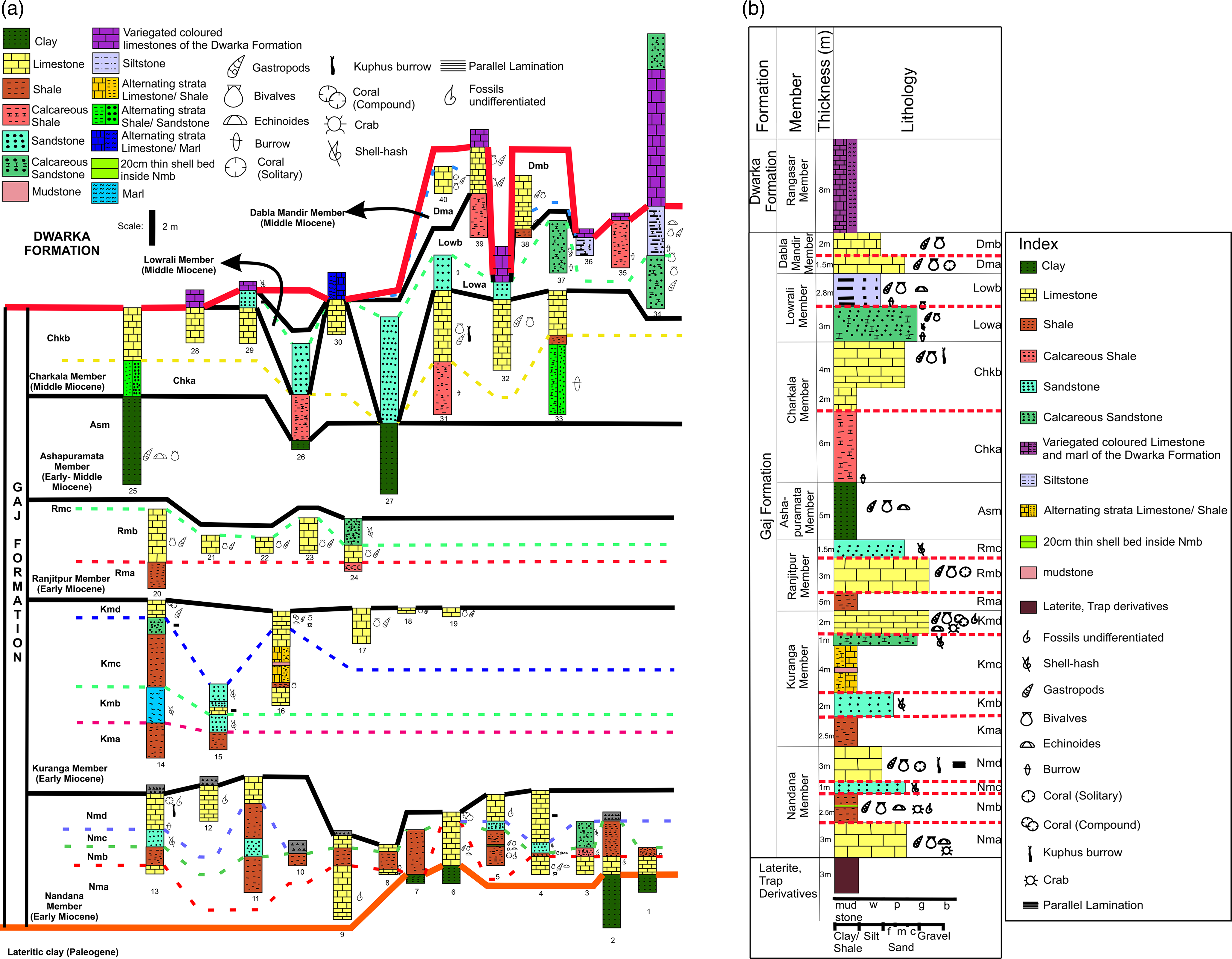
Extensive fieldwork was done in and around the Devbhoomi Dwarka District of Gujarat, and rock samples were collected from 40 different localities (Fig. 1; Supplementary Table 2). The rock samples were collected from each layer of several vertical litho-sections throughout the district. In addition, a lateral traverse (A–B) was taken along NH51 near the Kuranga Village (Fig. 1). The 40 litho-sections from the localities were correlated to construct a composite litholog of the Gaj Formation of the Dwarka Basin (Fig. 2a). The true thickness was measured from each litho-unit using measuring tape and a topographic Abney level.

Figure 2. (a) Lithostratigraphy of Miocene succession of the Gaj Formation, Dwarka Basin, showing lithologs of 40 sections. (b) Composite stratigraphic litholog of the Gaj Formation, Dwarka Basin, Western India, demarcating different litho-units within the seven litho-members. Abbreviations: w = wackestone; p = packstone; g = grainstone; b = boundstone. Grain size details: f = fine; m = medium; c = coarse.
Nineteen rock samples, predominantly carbonate lithologies with subordinate sandstone and shale components, were collected for further studies (Fig. 1). Preliminary field identification of carbonate rocks was conducted through hydrochloric acid testing, while shale, mudstone, and sandstone were differentiated based on textural, colourimetric, and mineralogical attributes. Detailed field observations encompassing sedimentary structures, stratigraphic architecture, surface characteristics, macro- and trace fossils, and bedding plane features were conducted to establish a foundation for paleoenvironmental interpretation.
3.b. Petrographic method
All collected samples underwent detailed thin-section studies, with particular emphasis on biotic constituents, including larger benthic foraminifera (LBF), smaller planktic and benthic foraminifera, coralline algae and corals. Thin sections were analysed under transmitted light microscopy (Olympus BX51; Zeiss Axio Imager M2m microscope and Leica DM 2700P with fitted camera Flexacam C3) for detailed observation. The detailed petrographic study, microfacies analysis and carbonate rock classification adhered to the frameworks established by Folk (Reference Folk1962), Dunham (Reference Dunham1962) and Embry & Klovan (Reference Embry and Klovan1971). Depositional environments were interpreted through facies analysis with the integration of published literature. Eocene LBFs were classified according to the taxonomic criteria established by Hottinger (Reference Hottinger1960, Reference Hottinger1974), Drobne (Reference Drobne1977), Schaub (Reference Schaub1981), Less (Reference Less1987), Tappan & Loeblich (Reference Tappan and Loeblich1988), Sirel & Acar (Reference Sirel and Acar2008) and Özcan et al. (Reference Özcan, Less, Okay, Baldi-Beke, Kollanyi and Yilmaz2010). Shallow Benthic Zones (SBZs) were delineated based on the biostratigraphic zonation proposed by Cahuzac & Poignant (Reference Cahuzac and Poignant1997). For biozonation nomenclature based on LBF, we have followed the concept provided by Saraswati (Reference Saraswati2024).
3.c. X-ray diffractogram analysis
Powder X-ray diffraction (XRD) was carried out for bulk mineralogical analysis of each of the rock samples from each member. Approximately 5–8 grams of each rock sample were finely grinded prior to analysis. XRD patterns were generated by irradiating powdered samples with monochromatic X-rays and recording the diffraction of these rays according to Bragg’s law (Bish & Post, Reference Bish and Post2018). Resultant diffraction peaks were used to identify and quantify mineral phases present within the samples. This technique facilitated the evaluation of carbonate compositional variability. XRD peaks were identified with the JCPDS cards. XRD analysis was completed in the Rigaku smartLab machine with a scanning range from 3° to 60° at a rate of 2°/min with a step size of 0.02° at Central Research Facility, IIT (ISM) Dhanbad, India.
3.d. Image analysis software
To check warming impact and biotic response, foraminiferal chamber size and grain size in thin sections were measured using the image analysis software, ImageJ 1.54g. This software is based on the Java programming language, designed and developed by Wayne Rasband at the National Institutes of Health and the Laboratory for Optical and Computational Instrumentation (LOCI), University of Wisconsin (Schneider et al. Reference Schneider, Rasband and Eliceiri2012).
4. Results and discussion
This study presents a comprehensive analysis of the Gaj Formation in the Dwarka Basin. Seven members of the formation were examined and further divided into distinct units based on lithological differences (rock type, grain size, colour and preserved fossils) observed in the field. Petrographic, microfossil and bulk mineralogical analyses were performed on the samples from all of these units to complete a high-resolution study (Fig. 2b).
4.a. Biostratigraphy
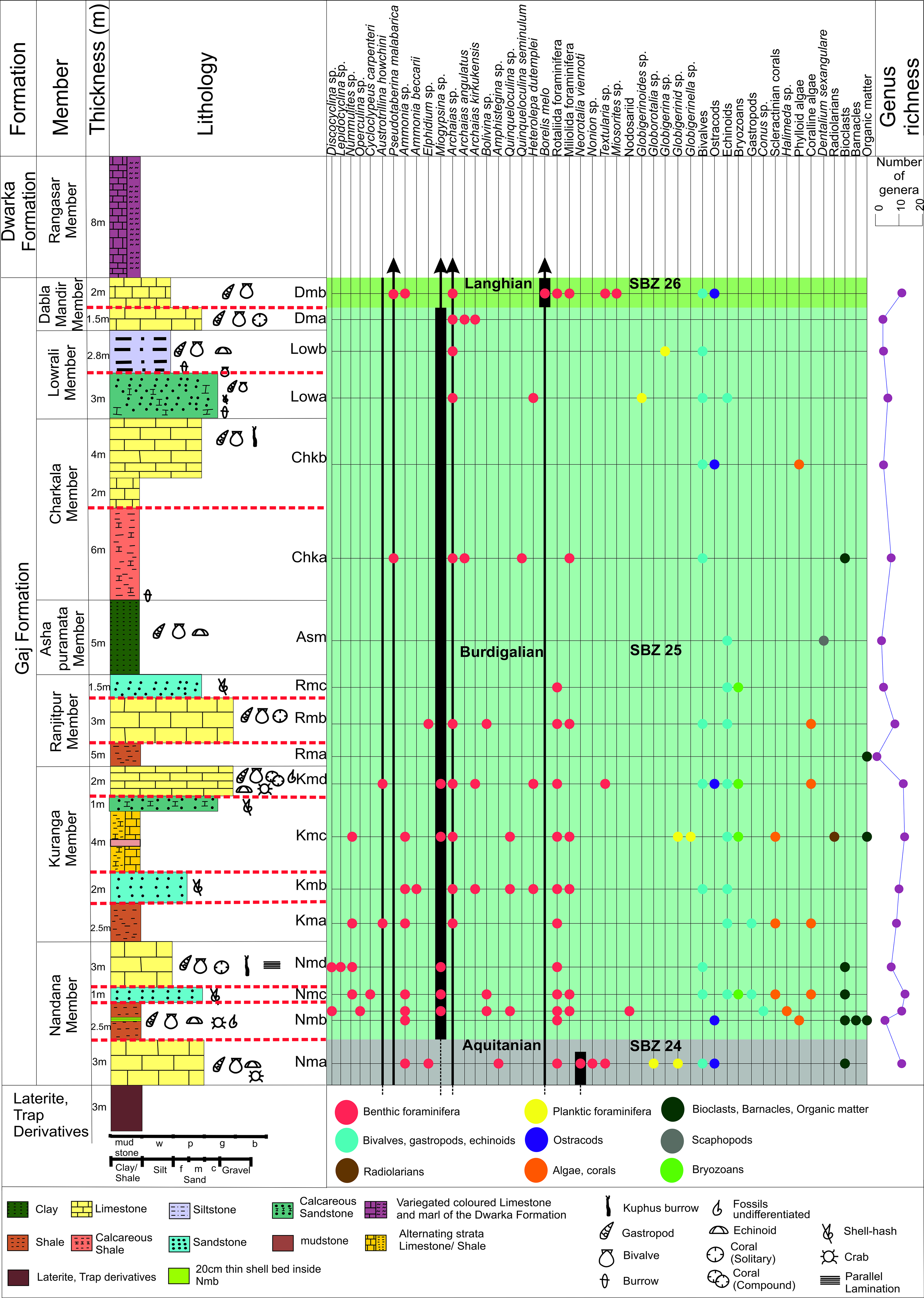
This study primarily focuses on analysing the microfacies and reconstructing the paleoenvironment of the Gaj Formation within the designated study area (Fig. 1); additionally, since the age of this formation requires clarification, the biostratigraphy of the Gaj Formation is discussed here in detail. In the study area, LBFs represent the primary biotic component of the Gaj Formation and hold significant biostratigraphic value as index fossils (Fig. 3). Petrographic analyses reveal that all the members of the Gaj Formation are characterized by a diverse and well-preserved assemblage of marine organisms, including foraminifera, ostracods, bivalves, gastropods, algae and bryozoans. Each of these groups contributes to the formation’s rich paleontological assemblage, highlighting its paleoecological perspectives and age determination of the sediments. The lowermost members of the Gaj Formation (Nandana and Kuranga members) are characterized by a diverse and abundant assemblage of taxa. Notable constituents include the LBFs (Amphistegina spp., Archaias kirkukensis, Archaias spp., Austrotrillina howchini, Cycloclypeus carpenteri, Discocyclina spp., Heterolepa dutemplei, Lepidocyclina spp., Miogypsina spp., Nummulites spp., Operculina spp., Textularia spp., and other nodosariids, rotaliidas, miliolida foraminifera), smaller benthic foraminifera (Ammonia spp., Ammonia beccarii, Bolivina spp., Elphidium spp., Neorotalia viennoti, Nonion spp., Quinqueloculina spp.), planktic foraminifera (Globigerinid spp., Globigerinella spp., Globorotalia spp.), different algae like Halimeda spp., phylloid algae, coralline red algae, bryozoans, scleractinian corals, radiolarians, other invertebrates (bivalve, gastropods (e.g., Conus spp.), barnacles, ostracod and echinoids) and some bioclasts with organic matter (Fig. 3). The overlying units contain a diverse assemblage of taxa, including the LBFs (Archaias angulatus, Archaias kirkukensis, Archaias spp., Borelis melo, Heterolepa dutemplei, Miosorites spp., Pseudotaberina malabarica, Textularia spp., and some other miliolidas and rotaliidas), smaller benthic foraminifera (Ammonia spp., Bolivina spp., Elphidium spp., Quinqueloculina seminulum), planktic foraminifera (Globigerina spp., Globigerinoides spp.), phylloid and coralline red algae, bryozoans, other invertebrates (bivalve shells, Scaphopod (Dentalium sexangulare), ostracods, echinoids) and organic matter (Fig. 3).

Figure 3. Vertical distribution of larger benthic foraminifera-associated fossil assemblages and biostratigraphic framework along with the distribution of SBZ biozones 24 to 26 defined by Cahuzac & Poignant (Reference Cahuzac and Poignant1997) within the different units of the Gaj Formation of the Dwarka Basin, India.
The SBZ is a well-established biostratigraphic framework primarily based on LBF, which is widely used for dating Neotethyan shallow marine sedimentary rocks (Serra-Kiel et al. Reference Serra-Kiel, Hottinger, Caus, Drobne, Ferrandez, Jauhri, Less, Pavlovec, Pignatti and Samso1998; Papazzoni et al. Reference Papazzoni, Fornaciari, Giusberti, Simonato and Fornaciari2023). In this study, we adopt the SBZ convention by Cahuzac & Poignant (Reference Cahuzac and Poignant1997) for defining early to middle Miocene SBZs.
There are conflicting interpretations on the age of the Gaj Formation. Previous studies by Chatterji et al. (Reference Chatterji, Sah, Mohan and Rao1953), Mohan & Chatterji (Reference Mohan and Chatterji1956) and Bhatia & Mohan (Reference Bhatia and Mohan1959) have identified age-diagnostic LBFs, such as Pseudotaberina malabarica, Austrotrillina howchini and several species of Miogypsina, within this formation. Based on these findings, these researchers assigned a Burdigalian age to the Gaj Formation. However, subsequent studies by Pandey et al. (Reference Pandey, Kondo, Jain, Bahadur and Pradhan2008) have proposed a slightly younger age for the Gaj Formation, suggesting a late Burdigalian depositional age.
In this study, we have found the lowermost member of the Gaj Formation, that is, the Nandana Member consists of dominant benthic foraminiferal fauna including Amphistegina sp., Elphidium sp., Neorotalia viennoti, Ammonia sp., Textularia sp. and Nonion sp., along with planktic foraminifera like Globigerinid sp. and Globorotalia sp. The biostratigraphic marker of the N. viennoti is given by Cahuzac & Poignant (Reference Cahuzac and Poignant1997) as SBZ 23–24. However, the assemblage of occurrence of Amphistegina sp., Elphidium sp. and Neorotalia viennoti within the lowermost part of the Nandana Member suggests a late Aquitanian age (SBZ 24) (Cahuzac & Poignant, Reference Cahuzac and Poignant1997; Yazdi-Moghadam et al. Reference Yazdi-Moghadam, Sarfi, Ghasemi-Nejad, Sadeghi and Sharifi2021) (Fig. 3). This implies that the boundary between Nma and Nmb units of the Nandana Member marks the transition from the Aquitanian to the Burdigalian age (Fig. 3).
The interval encompassing the Nmb to Lowb units (middle part of the Nandana Member – uppermost part of the Lowrali Member) constitutes the most substantial portion of this biostratigraphic zonation. This zone is characterized by the presence of diagnostic LBFs, including Austrotrillina howchini, Miogypsina sp. and Pseudotaberina malabarica, which collectively suggest a Rupelian–Burdigalian age (SBZ 21–25) (Cahuzac & Poignant, Reference Cahuzac and Poignant1997) (Fig. 3). Here, the occurrence of Miogypsina sp. supports an early Miocene (Burdigalian) age assignment in accordance with global biostratigraphic frameworks (Wildenborg, Reference Wildenborg1991; Cahuzac & Poignant, Reference Cahuzac and Poignant1997; Özcan et al. Reference Özcan, Less, Báldi-Beke, Kollányi and Acar2009). The lower and upper boundaries of this unit are defined by the first occurrence (FO) and the last occurrence (LO) of all Miogypsina sp. (Ogg et al. Reference Ogg, Ogg and Gradstein2016), and the unit corresponds to SBZ 25 or a Burdigalian age (Fig. 3).
The uppermost units of the Dabla Mandir Member are characterized by an abundance of Borelis melo, Archaias angulatus, Archaias kirkukensis, Archaias spp., Pseudotaberina malabarica and some other rotaliidas. While Archaias spp. and Pseudotaberina malabarica are long-ranging taxa, Borelis melo is particularly dominant and can be considered a marker taxon for this unit, potentially defined as ‘Borelis melo, Taxa Zone’. The abundance of Borelis melo within this unit suggests a Langhian–Serravallian age, that is, SBZ 26 (Fig. 3), aligning with the Borelis melo biozone defined by Cahuzac & Poignant (Reference Cahuzac and Poignant1997). The biostratigraphic significance of this taxon, particularly as a middle Miocene index fossil, has been widely recognized. The first appearance of Borelis melo and the disappearance of Miogypsina sp. mark the boundaries of this biozone (Ogg et al. Reference Ogg, Ogg and Gradstein2016) (Fig. 3). However, a precise biochronology is not possible because the majority of our samples fall within SBZ 25 only; therefore, estimates of sedimentation-rate variation through most of the record could be obtained only where distinct upper and lower boundary intervals were differentiated. On this basis, the estimated average sedimentation rate for the SBZ 25-assigned units of the Gaj Formation is approximately ∼1.1 cm/ka.
4.b. Depositional environments
Based on the combined results of petrographic and mineralogical analyses alongside microfossil content, a high-resolution microfacies model has been proposed to understand paleoenvironmental conditions. This model illustrates the various depositional environments represented in the Gaj Formation during the Aquitanian to Langhian time interval. By correlating local observations with global sea-level fluctuation and sea surface temperature profile records, this research contributes to our knowledge of Miocene climate and environmental conditions (Supplementary Fig. S1.1).
4.b.1. Nandana Member
4.b.1.a. Nma Unit
Basal Nandana Member (Nma), a ∼10 m thick succession rich in bivalves, gastropods and crustaceans, unconformably overlies the Deccan Traps and associated lateritic deposits (Fig. 4a, b). Petrographic analysis indicates a fine-grained, micritic limestone with abundant bioclasts, predominantly derived from bivalve and foraminiferal fragments (Fig. 5a). The presence of intraclasts is minimal, occurrences of prominent calcite crystals were observed, distinguished by their characteristic high-order variegated interference colours (Fig. 5a), and the rock is classified as a biomicrite (Folk, Reference Folk1962). The microfossil assemblage of the unit is dominated by rotaliidas, including Amphistegina sp., Globorotalia sp., Elphidium sp., Ammonia sp., Textularia sp., Nonion sp. and Neorotalia viennoti (Fig. 6a–c; Supplementary Fig. S1.2). Additionally, planktic foraminifera, like Globigerinids sp., are also present within this unit (Fig. 6c). Some bivalve and ostracod fragments are also identified from the petrographic study. XRD data reveal the dominance of calcium carbonate (CaCO₃) and silicon dioxide (SiO₂) within the sediments (Fig. 7).

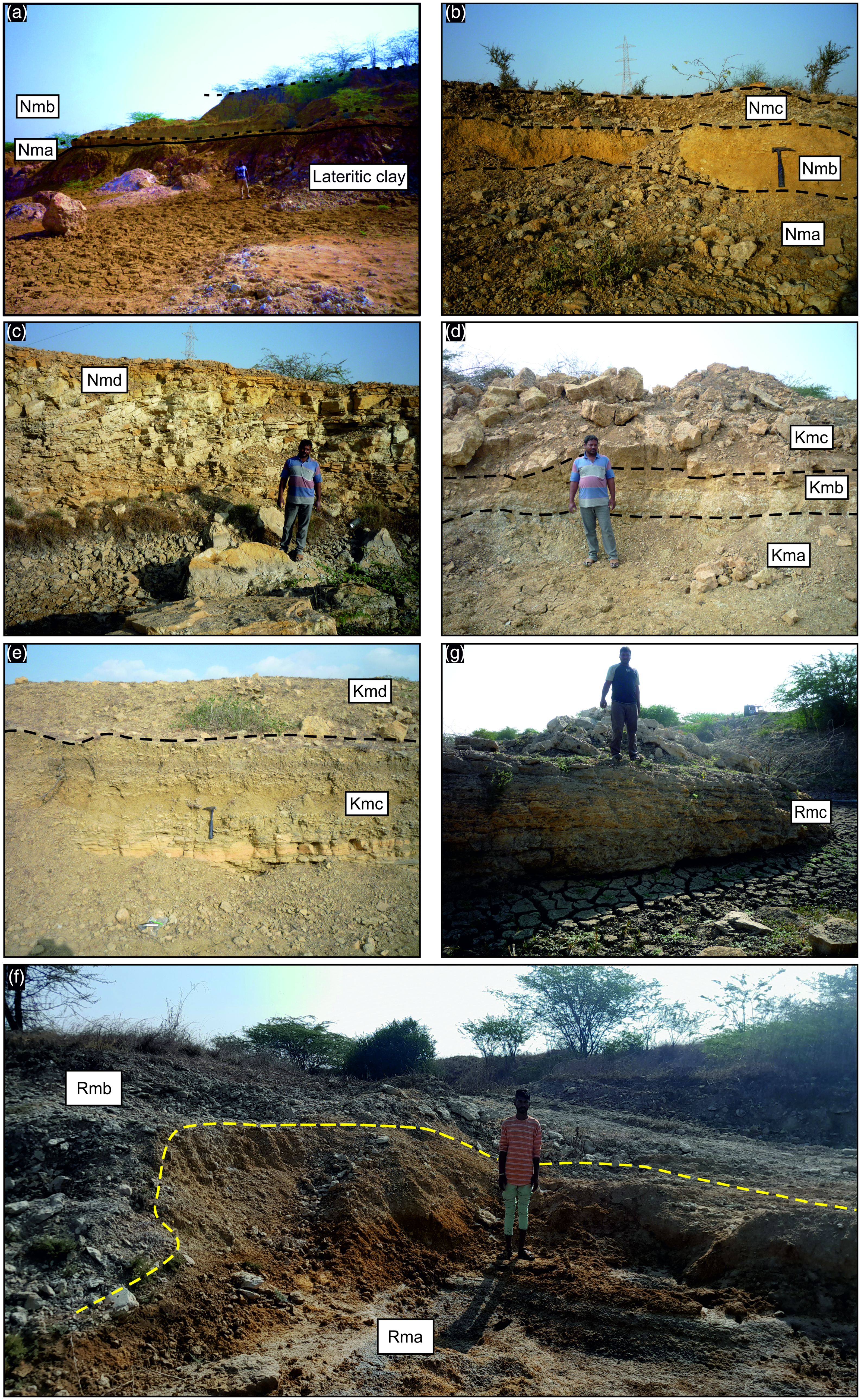
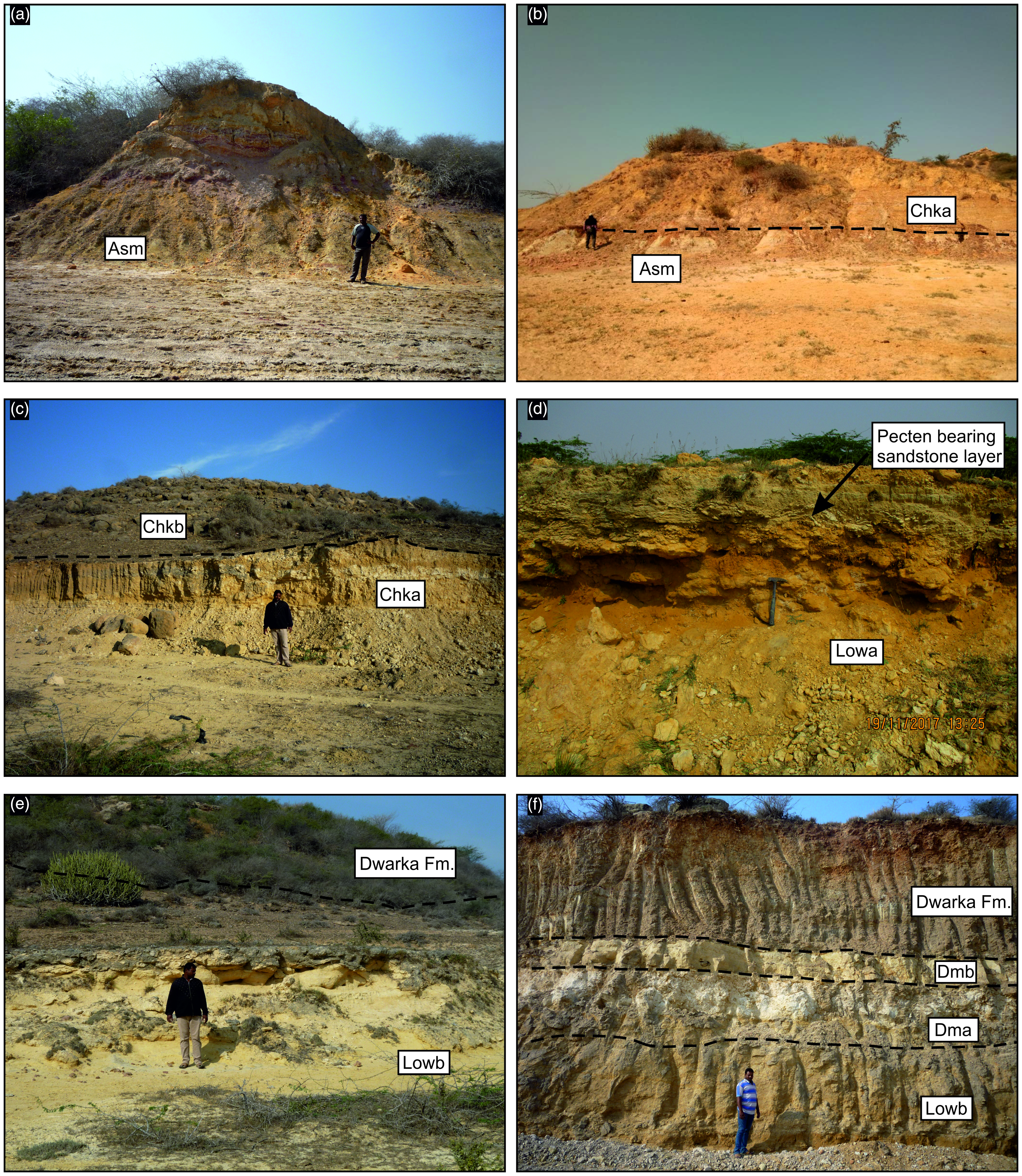
Figure 4. Field photographs (a) showing the Nma Unit, comprising basement lateritic clay, overlain by the Nmb Unit; (b) depicts the Nmb Unit, positioned between the underlying Nma Unit and the overlying Nmc Unit; (c) showcases the Nmd Unit; (d) illustrates the Kma Unit, succeeded by the Kmb and Kmc units; (e) highlights the Kmc Unit, which underlies the Kmd Unit; (f) shows the Rma Unit, capped by the Rmb Unit and (g) presents the Rmc Unit.

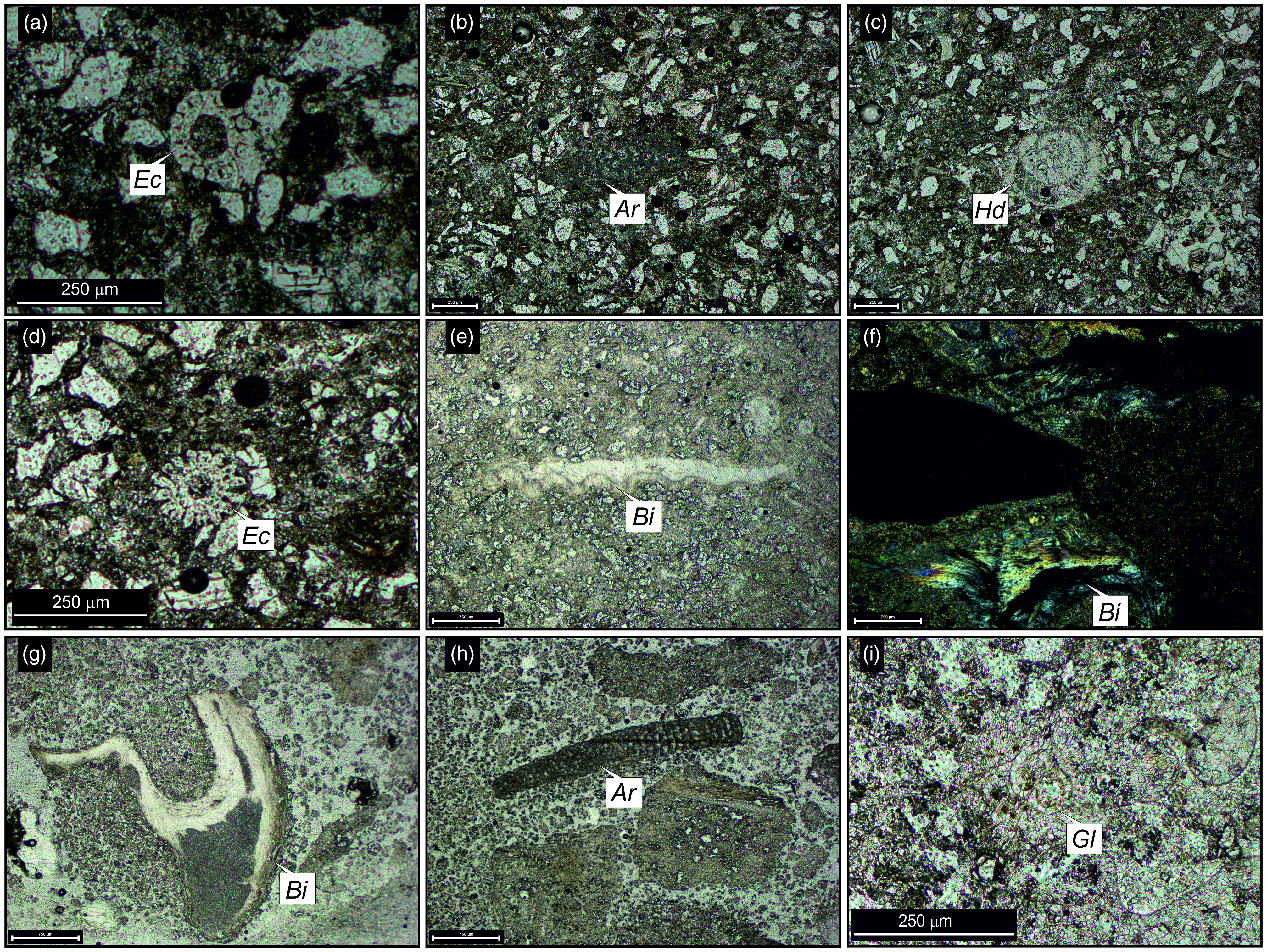
Figure 5. Representative petrographic thin-section images from different members of the Gaj Formation. (a) Thin-section photomicrograph of biomicrite from the Nandana Member (Nma), (b, c) Thin-section photomicrographs of bioclastic arenite from the Kuranga Member (Kma), (d) Thin-section photomicrographs of siltstone from the Ranjitpur Member (Rma), (e) Thin-section photomicrographs of claystone from the Ashapuramata Member (Asm), (f) Thin-section photomicrograph of intramicrite from the Charkala Member (Chkb), (g) Photomicrograph of coarse-grained sandstone from the Lowrali Member (Lowa) and (h) Photomicrograph of biomicrite from the Dabla Mandir Member (Dmb). Fig. 5b has been taken under plane polars. Abbreviations: Scleractinian corals (Sc).

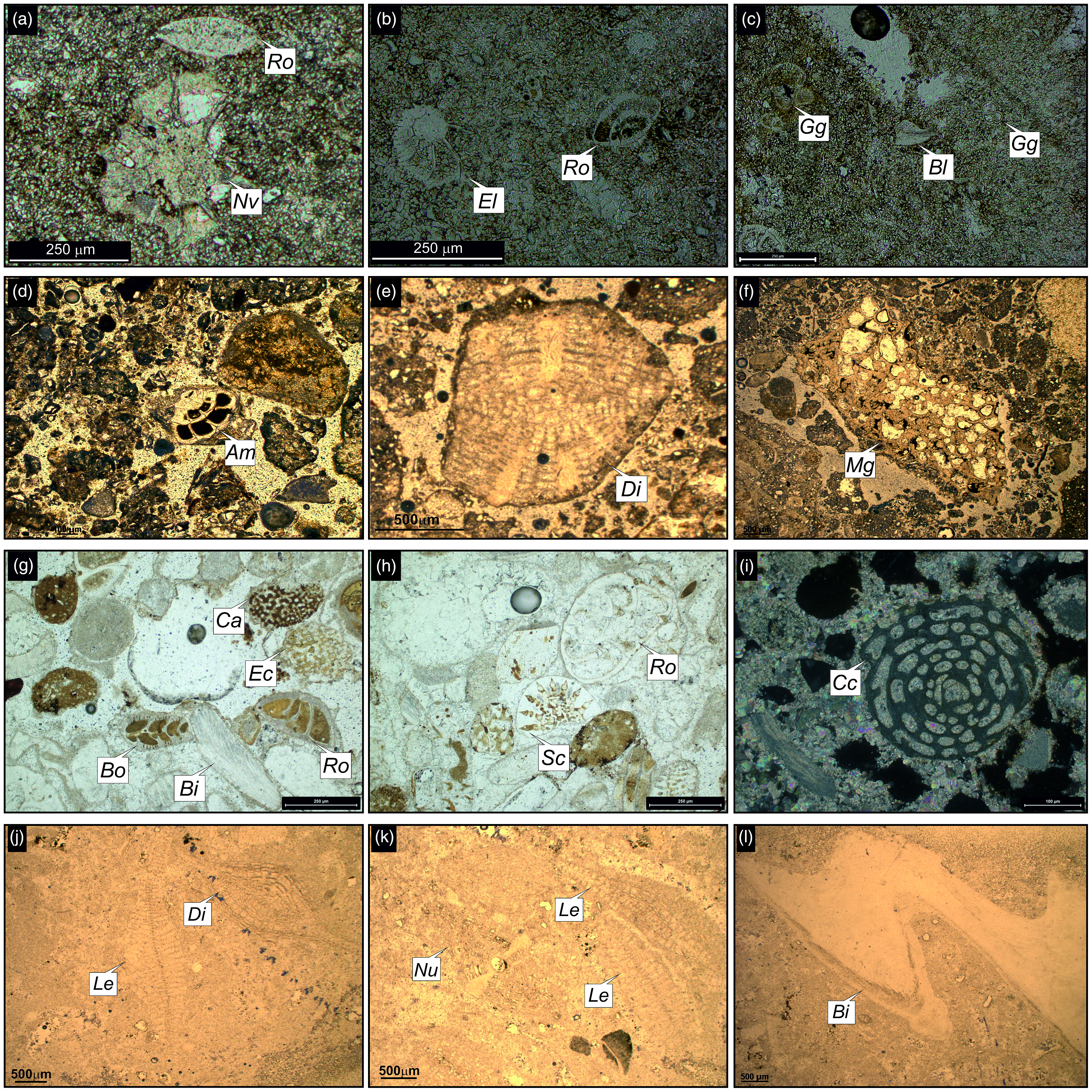
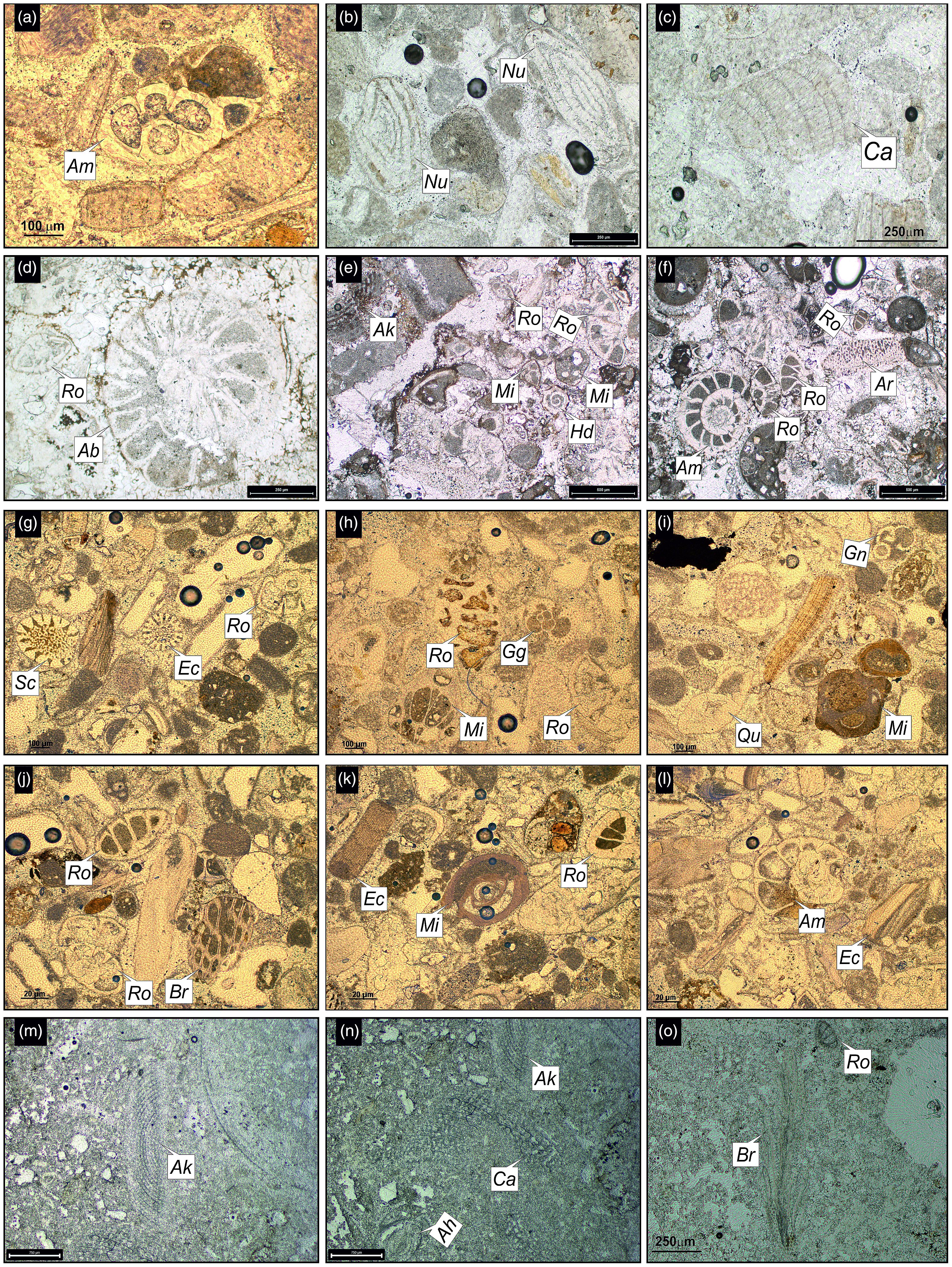
Figure 6. Thin-section photomicrographs of the identified larger benthic foraminifera and associated fossils from the Nandana Member (a–c) Nma, (d–f) Nmb, (g–i) Nmc, (j–l) Nmd of the Gaj Formation. Abbreviations: Ammonia sp. (Am), Bioclasts (Bl), Bivalves (Bi), Bolivina sp. (Bo), Coralline algae (Ca), Cycloclypeus carpenteri (Cc), Discocyclina sp. (Di), Echinoids (Ec), Elphidium sp. (El), Globigerinid foraminifera (Gg), Lepidocyclina sp. (Le), Miogypsina sp. (Mg), Neorotalia viennoti (Nv), Nummulites sp. (Nu), Rotaliida (Ro), Scleractinian corals (Sc). Fig. 6i has been taken under crossed nicols.
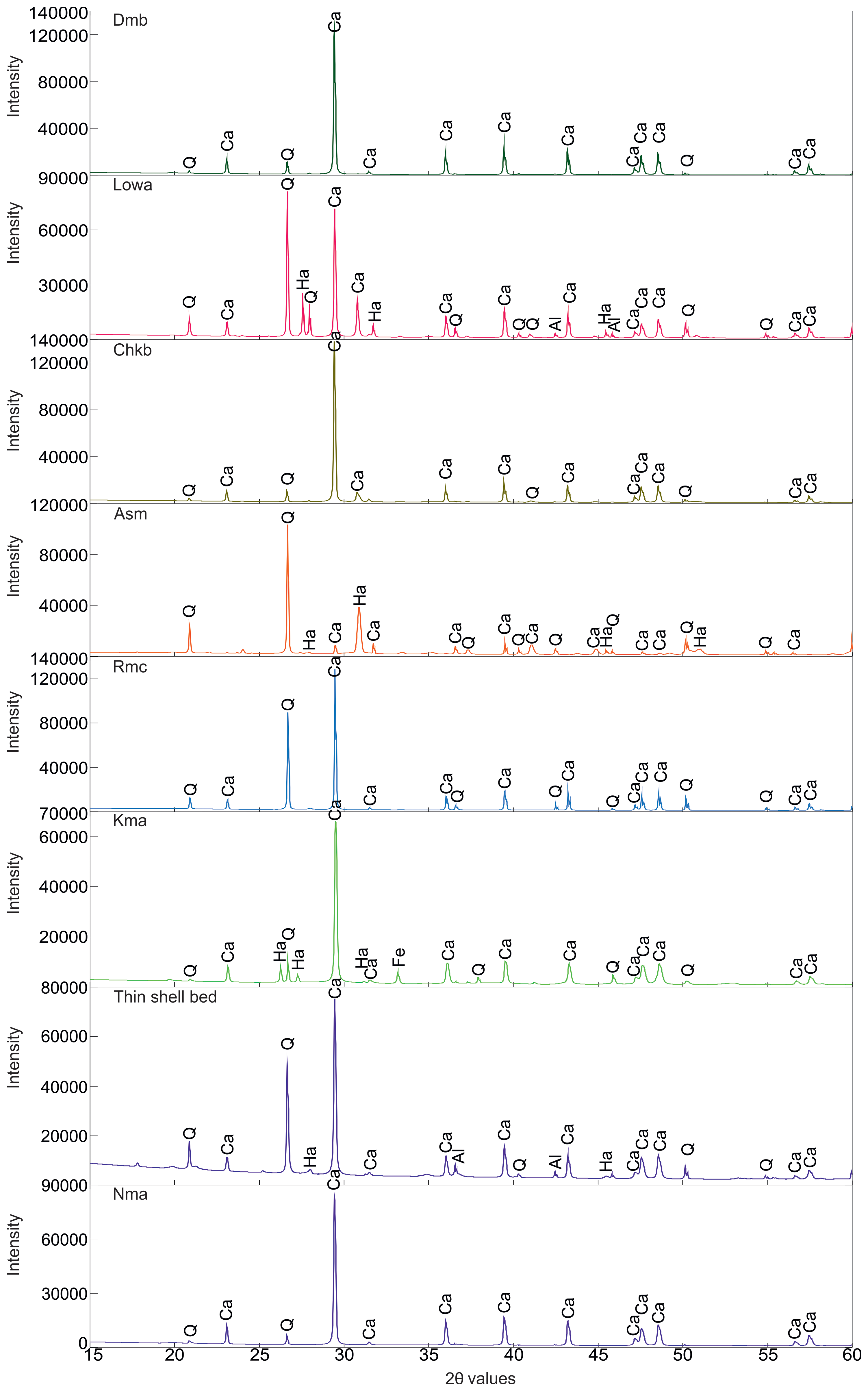
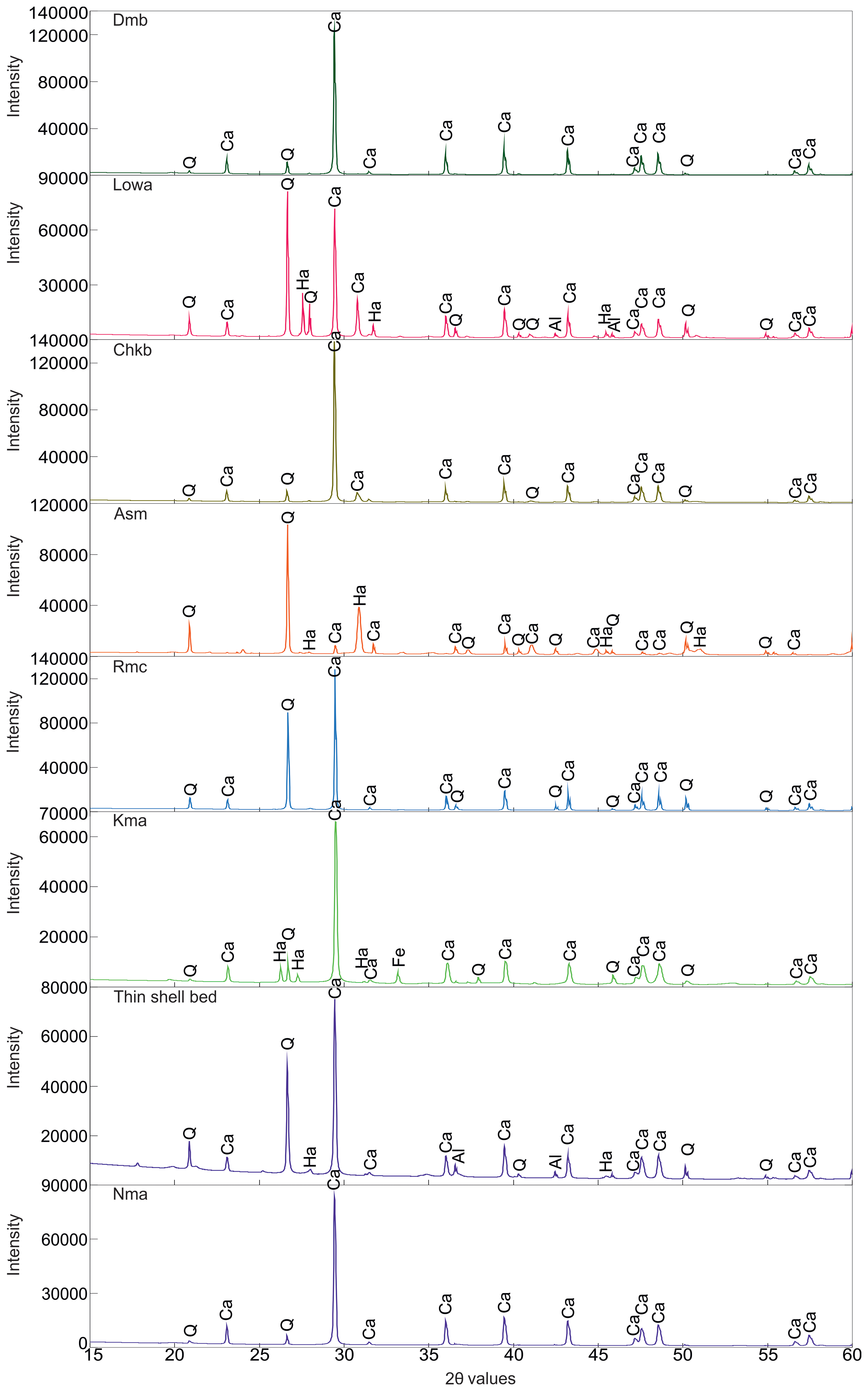
Figure 7. X-ray diffraction (XRD) patterns of lithologies from the different members of the Gaj Formation (marked by specific colour code). Nandana Member (Nma and Thin shell bed), Kuranga (Kma), Ranjitpur (Rmc), Ashapuramata (Asm), Charkala (Chkb), Lowrali (Lowa) and Dabla Mandir Member (Dmb). Quartz (Q) and calcite (Ca) are identified as the primary mineral constituents across all analysed members, whereas halite (Ha), aluminium oxide (Al), iron oxide (Fe), and copper iron sulphide (Cu) are the minor mineral constituents.
The abundance of micrite suggests deposition in a low-energy environment. The presence of benthic foraminifera such as Amphistegina sp., Elphidium sp., Ammonia sp., Textularia sp. and Nonion sp. indicates a shallow marine, low-energy, normal marine salinity shelf environment associated with warm, well-oxygenated and nutrient-rich conditions (Murray, Reference Murray2006; Boudaugher-Fadel, Reference Boudaugher-Fadel2018) (Fig. 8a). The presence of planktonic foraminifera suggests a relatively higher depositional depth, that is, the middle to outer neritic zone region (Kennett & Stott, Reference Kennett and Stott1991). Overall, it represents a low-energy environment, below the fair-weather wave base within the shallow marine regime (Fig. 8a). The depositional environment identified from microfossil assemblage is complemented by a diverse macrofaunal assemblage, including gastropods such as Persististrombus cf. depertitus, Persististrombus sp., Tibia cf. indica, Lyncina cf. prunum, Conus (Lithoconus) cf. literatus and Conus (Lithoconus) cf. ineditus, and bivalves such as Tellina cf. inflexuosa, Tellina cf. protocandida, Periglypta cf. mekranika and Clementia sp. reported by Jain (Reference Jain2014) and Bose et al. (Reference Bose, Das and Saha2023). This macrofaunal assemblage is a well-documented indicator of shallow marine environments during the Neogene (Abott, Reference Abbott1960; McCarthy, Reference McCarthy2007; Harzhauser & Kronenberg, Reference Harzhauser and Kronenberg2013; Avila et al. Reference Ávila, Melo, Berning, Cordeiro, Landau and da Silva2016).
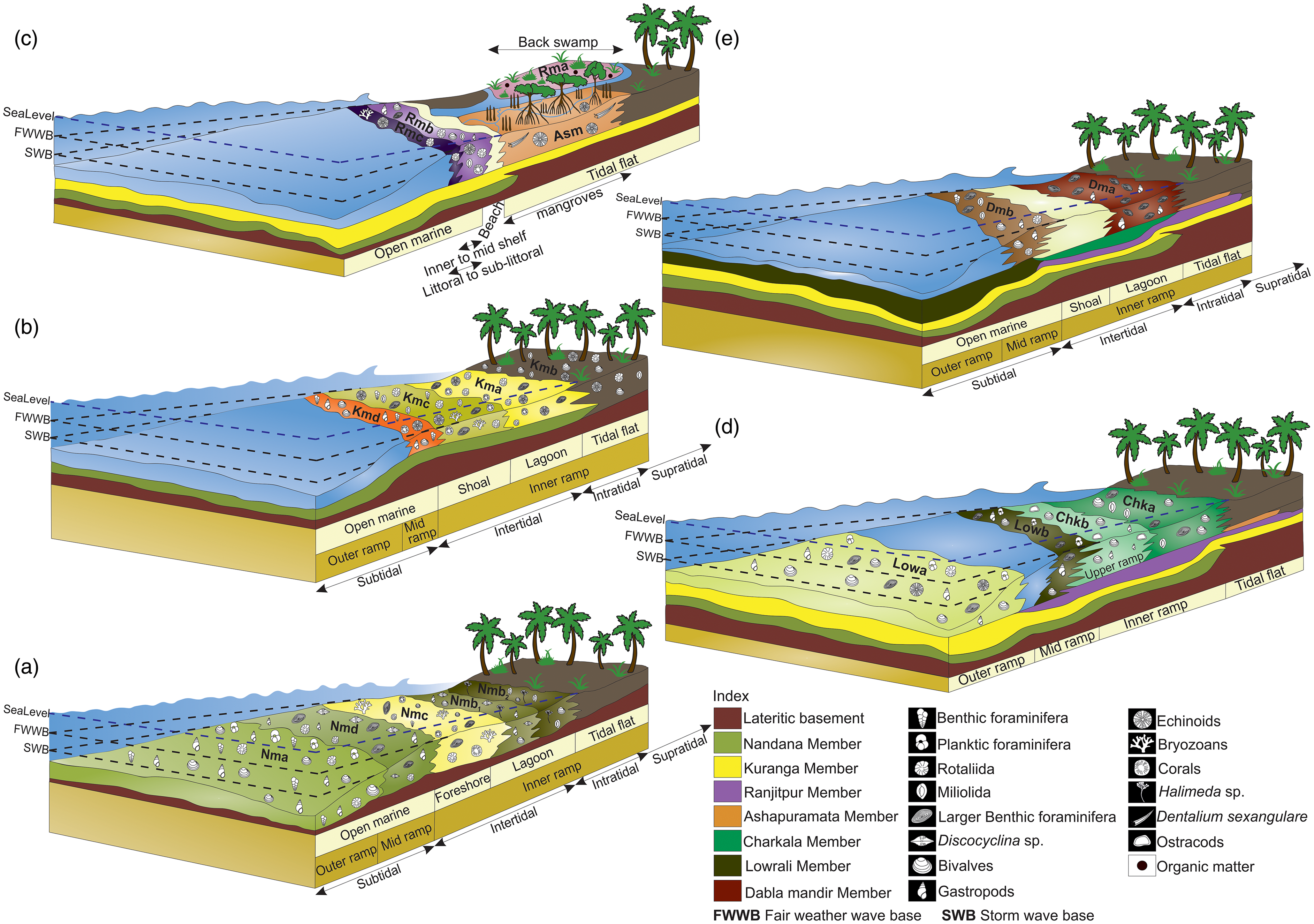
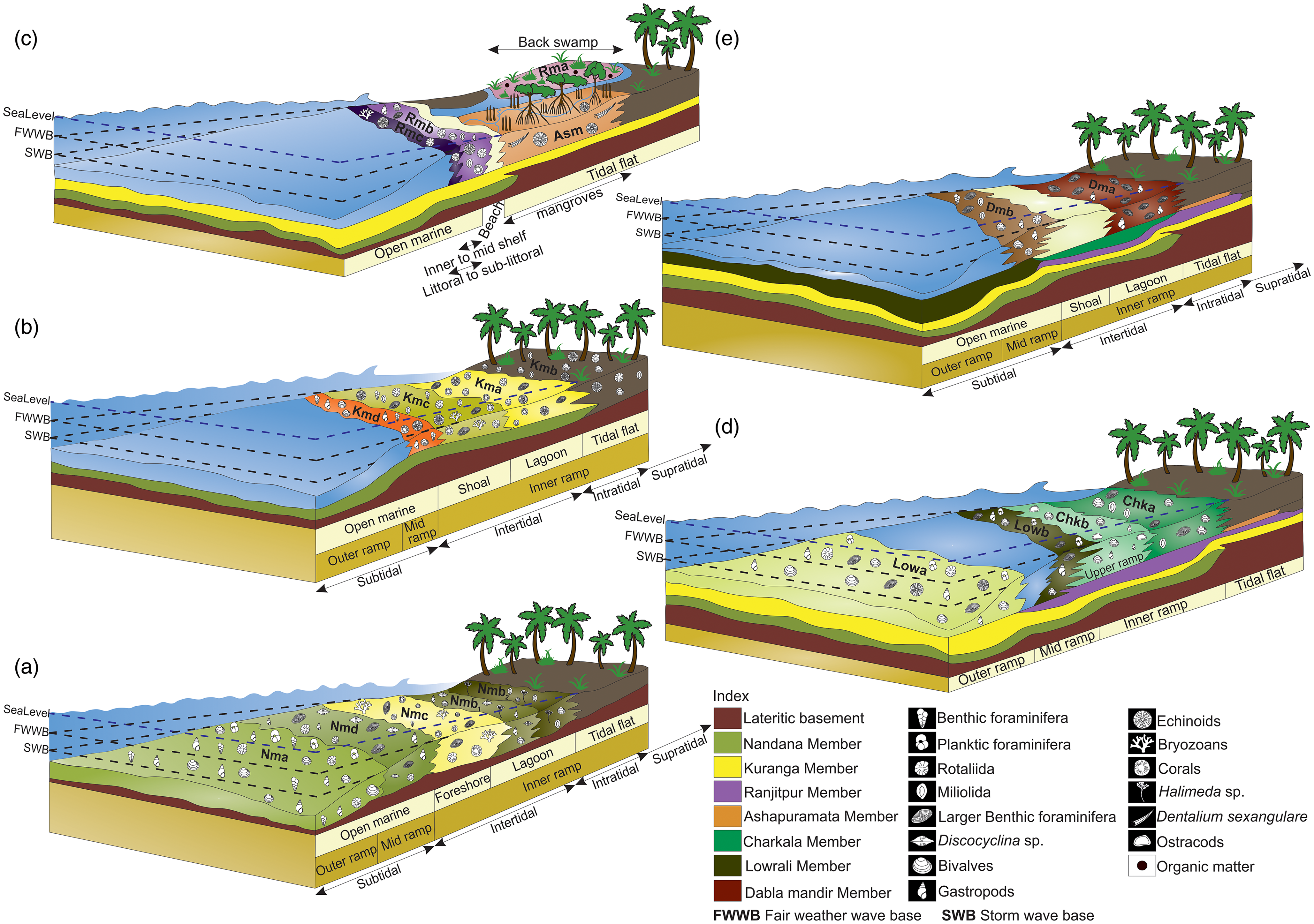
Figure 8. A schematic representation of the depositional model and the inferred paleoclimatic regime of the (a) Nandana, (b) Kuranga, (c) Ranjitpur and Ashapuramata, (d) Charkala and Lowrali, and (e) Dabla Mandir Member that influenced sediment deposition within the Dwarka Basin, western India, during the Aquitanian to Langhian time interval.
4.b.1.b. Nmb Unit
The overlying turritelline gastropod-dominated assemblages (TDAs) Shale Unit (Nmb) is characterized by a dark-coloured, fossiliferous shale dominated by an assemblage of highly diverse turritelline gastropods associated with bivalves, echinoids, crustaceans, and fish teeth (Fig. 4a, b).
The silty shale facies are comprised of microcrystalline silt-sized particles placed in a clay-rich matrix (Supplementary Fig. S1.3). This rock is mostly comprised of quartz and calcite particles smaller than ∼60 µm (coarse silt and finer) (Supplementary Fig. S1.3). Bioclast grains contain common benthic foraminifera like Miogypsina sp., Operculina sp., Quinqueloculina sp., Ammonia sp., Discocyclina sp. and Bolivina sp. (Fig. 6d–f; Supplementary Fig. S1.3). Other foraminifera groups like Nodosariid and Miliolida are present in this section. This section is rich in Halimeda sp., with flat thallus segments (Supplementary Fig. S1.3). Some broken gastropod shells are also very well preserved within the clay matrix. A petrographic study confirms the evidence of a distinct small unit with varying lithologies within this shale unit (Supplementary Fig. S1.3). XRD peaks are dominated by SiO₂ and CaCO₃, with minor amounts of aluminium oxide (Al₂O₃) (Supplementary Fig. S1.4). It indicates that the underlying unit is dominated by CaCO₃; with the onset of the TDA bed, siliciclastic peaks become prominent. These facies comprised poorly to moderately sorted, angular quartz grains scattered in the micritic matrix made up of mostly calcite. In some parts, the matrix is extremely fine-grained (Supplementary Fig. S1.3). The monocrystalline quartz grains, muscovite and plagioclase feldspar are also present in this section as other subordinate minerals. The benthic foraminifera of the Rotaliida group, Ammonia sp., and ostracods are predominant skeletal grains. Several barnacles and fragmented barnacle debris are other major biogenic components within these samples. Some fragmented algal remains and organic matter are well preserved (Supplementary Fig. S1.3). The orientation of bioclasts does not exhibit any noticeable preference; random disorders are often seen within these siltstone facies. Mineralogical data reveal that this small unit consists primarily of SiO₂ and CaCO₃, with minor sodium chloride (NaCl) and Al₂O₃ (Fig. 7).
Shallow marine shelf conditions support large populations of Rotaliida and Miliolida groupings and commonly thrive in well-oxygenated, nutrient-rich waters (BouDagher-Fadel, Reference BouDagher-Fadel2008; Chaudhuri et al. Reference Chaudhuri, De, Srivastava, Chattopadhyay and Bhaumik2022). Ammonia usually thrives in shallow shelf environments (Murray, Reference Murray2006; Dupuy et al. Reference Dupuy, Rossignol, Geslin and Pascal2010) and can survive fluctuations of temperatures, water salinity and nutrient input (Schnitker, Reference Schnitker1974; Debenay et al. Reference Debenay, Bénéteau, Zhang, Stouff, Geslin, Redois and Fernandez-Gonzalez1998). Quinqueloculina flourishes in shallow, tropical, well-oxygenated, warm-temperate seas, preferring to live in near-shore environments (Bandy & Arnal, Reference Bandy and Arnal1957; Chaudhuri et al. Reference Chaudhuri, De, Srivastava, Chattopadhyay and Bhaumik2022). The coexistence of Ammonia and Quinqueloculina implies deposition in the intertidal to subtidal zone of the continental shelf, favoured by oxygen-rich conditions (Murray, Reference Murray2006; Gupta & Platon, Reference Gupta and Platon2006; Chaudhuri et al. Reference Chaudhuri, De, Srivastava, Chattopadhyay and Bhaumik2022). The occurrence of LBFs such as Miogypsina sp. indicates shallow marine environmental conditions with ambient sunlight (BouDagher-Fadel, Reference BouDagher-Fadel2008). Besides, Halimeda, green algae is commonly inhabited in tropical, subtidal, to lower intertidal zones in wave-affected habitats (Dragastan & Herbig, Reference Dragastan and Herbig2007). The large-sized barnacles from the middle siltstone units indicate the deposition was in the subtidal to intertidal environment with enough nutrient supply for higher growth rates (Menge, Reference Menge2000) (Fig. 8a). The biotic association of LBFs such as Operculina and Miogypsina, along with algae and other mollusc fragments, signifies that a highly nutrient-rich environment prevailed during that time.
Based on analyses of the petrography, grain size and fossil assemblage, fluctuations are evident between subtidal and intertidal environments, suggesting significant environmental fluctuations. These observations likely reflect dynamic shifts in sedimentary conditions leading to the coexistence of characteristics typical of both subtidal and intertidal zones within these units. The median grain size of sediment demonstrates a seaward coarsening trend across the tidal flats, coupled with a transition from coarse sand in the subtidal zone to mud in the supratidal zone, reflecting typical accumulation patterns influenced by the selective sorting effects of wave dynamics and tidal currents within the subtidal and shallow intertidal flat regions (Davis, Reference Davis and Davis1978; Pettijohn et al. Reference Pettijohn, Potter and Siever1987). The microfossil association and petrographic study suggest the deposition of sediments of these units under tropical, warm, nutrient-rich, well-oxygenated conditions in subtidal–intertidal conditions.
4.b.1.c. Nmc Unit
The Nandana Sandstone Unit (Nmc) overlies the TDA Shale Unit (Nmb) and is characterized by medium-grained sandstones containing a significant amount of shell hash (Fig. 4b). Petrographic analysis of the fine-grained sandstone reveals pale yellow colour sections. The rock is rich in bioclasts, including bryozoans, foraminifera (Ammonia sp., Miogypsina sp., Cycloclypeus carpenteri, Nummulites sp., Rotaliida, Miliolida), bivalve fragments, echinoid spines, scleractinian coral fragments and algal remains (Fig. 6g–i; Supplementary Fig. S1.5). XRD data indicate that this rock unit is composed of CaCO₃ and SiO₂ (Supplementary Fig. S1.4). The fine-grained nature of the matrix often obscures grain boundaries. The microfossil assemblage, dominated by benthic foraminifera along with coralline red algae, is indicative of a shallow marine environment. The high abundance of abraded and fragmented gastropod and bivalve shells, typically of shell hash, along with their bimodal orientation, suggests deposition in a high-energy, shallow marine environment (Fig. 8a). This can be considered a type 1 fair-weather wave concentration, and the depositional environment is considered a foreshore environment (Fürsich & Oschmann, Reference Fürsich and Oschmann1993). The transition from a subtidal to a foreshore environment is often marked by a decrease in water depth and increased wave energy. The resulting sedimentary facies typically reflect these changes, with coarser-grained sediments, such as sands, becoming more dominant in the foreshore environment from the underlying Nmb Unit (Fig. 8a). The poor sorting of both the matrix and skeletal components further supports this interpretation.
4.b.1.d. Nmd Unit
The upper Nandana Limestone Unit (Nmd) is characterized by fossiliferous wackestone, exhibiting extensive bioturbation and karst features (Fig. 4c). From field observation, the fossil assemblage includes corals, molluscs and occasional Kuphus burrows. Petrographic analysis revealed a pale yellow to yellow limestone dominated by a micritic matrix with abundant bioclasts, including bivalve shells and LBFs (Fig. 6j–l; Supplementary Fig. S1.5). The presence of calcite crystals with high-order interference colour suggests diagenetic alteration. In the mud-supported nature with embedded grains, the rock is classified as a biomicrite due to the dominance of bioclasts within a micritic matrix and wackestone (Dunham, Reference Dunham1962). Petrographic analysis revealed a diverse assemblage of fossils, including LBFs such as Lepidocyclina sp., Discocyclina sp., Miogypsina sp. and Nummulites sp., along with the presence of macrofossil shells, algal remains and other bioclasts (Fig. 6j–l; Supplementary Fig. S1.5). The gastropod assemblage includes species such as Xenophora gajensis, Persististrombus deperditus and Tibia indica. The assemblage of these micro taxa is commonly associated with warm, shallow marine environments, in carbonate platforms and, more specifically, in mid-ramp settings (Hottinger, Reference Hottinger1982; BouDagher-Fadel, Reference BouDagher-Fadel2008). Miogypsina-Miogypsinoides inhabit in shallow marine limestones with calcareous algae and need curved surfaces such as macroalgae or seagrass to grow, as they cannot thrive on flat surfaces (BouDagher-Fadel, Reference BouDagher-Fadel2008; Boudagher-Fadel & David Price, Reference Boudagher-Fadel and David Price2013). Nummulites, along with Lepidocyclina, prefer shallow marine environments within the photic zone, often in wave-influenced settings from inner to mid-ramp areas (Gilham & Bristow, Reference Gilham and Bristow1998; Racey et al. Reference Racey, Bailey, Beckett, Gallagher, Hampton and McQuilken2001). The predominance of micritic sediments and well-preserved foraminifera suggests a low-energy depositional environment, further supported by the abundance of large, flat Lepidocyclina and Discocyclina specimens (Fig. 8a) (Sinclair et al. Reference Sinclair, Sayer and Tucker1998; Racey et al. Reference Racey, Bailey, Beckett, Gallagher, Hampton and McQuilken2001; Beavington-Penney et al. Reference Beavington-Penney, Wright and Racey2006; Banerjee et al. Reference Banerjee, Khanolkar and Saraswati2018). Sedimentation likely occurred below the fair-weather wave base and deposited in a mid-ramp setting with 50–60m of water depth. The observed transition from a foreshore (Nmc) to a mid-ramp environment (Nmd) is relatively abrupt, bypassing intermediate environments. This abrupt transition from a foreshore to a mid-ramp environment can be attributed to significant tectonic changes, such as the closure of the Tethys Seaway due to the development of the Gomphotherium-Landbridge during the early Burdigalian (∼19 Ma), which led to rapid changes in sea level and sedimentation patterns (Chattopadhyay et al. Reference Chattopadhyay, Venu Gopal and Dahakey2025).
4.b.2. Kuranga Member
The Kuranga Member, ∼12 m thick, conformably overlies the Nandana Member and is well exposed across the basin. Based on lithological variations, fossil content and field observations, this member has been divided into four distinct units (Fig. 2b).
4.b.2.a. Kma Unit
This Kma Unit is characterized by the presence of grey and red shale (Fig. 4d). Petrographic study indicates that the rock unit is highly fossiliferous, with skeletal grains making up over 85% of its volume, classifying it as shale (Fig. 5b, c). This suggests a depositional environment with high biological activity and favourable preservation conditions. The remaining 15% consists of a detrital matrix, likely composed of quartz or feldspar (Fig. 5c). XRD data shows the peaks are dominated by CaCO₃ and SiO₂, with minor amounts of NaCl and iron oxide (Fe₂O₃) (Fig. 7). Petrographic analysis reveals a diverse fossil assemblage, LBFs (Nummulites sp., Austrotrillina howchini), smaller benthic foraminifera (Ammonia sp., other Rotaliida), echinoids, scleractinian corals, fragments of gastropods and algal remains (Fig. 9a-c; Supplementary Fig. S1.6). The alternation of red and grey shale indicates lagoonal deposition with intermittent siliciclastic input: red from marginal land input and grey with foraminifera from deeper zones (Fig. 8b) (Banerjee et al. Reference Banerjee, Chattoraj, Saraswati, Dasgupta, Sarkar and Bumby2012). The fossil assemblages, along with the dominance of skeletal grains and the absence of significant detrital input, as evidenced by petrographic analysis, suggest a lagoonal depositional environment.

Figure 9. Thin-section photomicrographs of larger benthic foraminifera and associated fossils from the Kuranga Member (a–c) Kma, (d–f) Kmb, (g–l) Kmc, (m–o) Kmd of the Gaj Formation. Abbreviations: Ammonia beccarii (Ab), Ammonia sp. (Am), Archaias kirkukensis (Ak), Archaias sp. (Ar), Austrotrillina howchini (Ah), Bryozoans (Br), Coralline algae (Ca), Echinoids (Ec), Globigerinella sp. (Gn), Globigerinid foraminifera (Gg), Heterolepa dutemplei (Hd), Miliolida (Mi), Nummulites sp. (Nu), Quinqueloculina sp. (Qu), Rotaliida (Ro), Scleractinian corals (Sc).
4.b.2.b. Kmb Unit
overlying Kmb Unit is composed of fine-grained sandstones (Fig. 4d). The low matrix content (<15%) supports its classification as arenite. Isolated monocrystalline quartz grains were observed along with micas as accessory minerals (Supplementary Fig. S1.6). The unit exhibits an excellent preservation of various fossils, including Rotaliida foraminifera, Ammonia beccarii, Ammonia sp., some miliolida foraminifera, Quinqueloculina sp., Archaias kirkukensis, Heterolepa dutemplei, Archaias sp., echinoid and bivalve shell fragments (Fig. 9d–f; Supplementary Fig. S1.6). Archaias kirkukensis and other Archaias sp. are typically associated with carbonate-rich environments and are known to thrive in the photic zone, often in shallow, tropical and subtropical tidal flats (Bassi et al. Reference Bassi, Braga, Pignatti, Fujita, Nebelsick, Renema and Iryu2024). The presence of Heterolepa dutemplei indicates well-lit conditions and low sedimentation rates typical of tidal flats and the inner shelf (Saidova, Reference Saidova and Bezrukov1969). The assemblage of these foraminiferal species suggests deposition in tidal flat to littoral environments, extending into the inner shelf, where conditions are typically shallow, calm and influenced by tidal cycles and water depth fluctuations (Fig. 8b) (Murray, Reference Murray2006; Bassi et al. Reference Bassi, Braga, Pignatti, Fujita, Nebelsick, Renema and Iryu2024). The petrographic study, along with the nature of sandstone, corroborates the same and deposition in a transgressive sea.
4.b.2.c. Kmc Unit
The overlying Kmc Unit is marked by alternating layers of shale and calcareous mudstone, with occasional thin Pecten-bearing intervals and fine-grained calcareous sandstone layers at the top in certain areas (Fig. 4d, e). The microfossil assemblage is dominated by benthic foraminifera, including miliolida, Ammonia sp., Quinqueloculina sp., Archaias sp., rotaliidas and LBFs such as Miogypsina sp. (Fig. 9g–l; Supplementary Fig. S1.7). Planktonic foraminifera like Globigerinid sp. and Globigerinella sp. are also present (Fig. 9h, i). Additionally, echinoid fragments, bryozoans and scleractinian corals are also observed in the thin section (Fig. 9g, j–l; Supplementary Fig. S1.7). The studied petrographic sections display textural and compositional features typical of a lithic arenite. Well-rounded monocrystalline quartz grains and lath-shaped mica grains (accessory) are prominent in this section (Supplementary Fig. S1.7). SiO₂, CaCO₃, with minor amounts of Fe₂O₃, and copper iron sulphide (Cu₅FeS₄) are present, as shown in XRD data (Supplementary Fig. S1.4). The assemblage of LBFs suggests deposition in an intertidal to subtidal environment (Hottinger, Reference Hottinger1982; BouDagher-Fadel, Reference BouDagher-Fadel2008; Chaudhuri et al. Reference Chaudhuri, De, Srivastava, Chattopadhyay and Bhaumik2022). The presence of planktic foraminifera further indicates a tendency towards greater water depths, supporting a predominantly subtidal setting (Fig. 8b). The alternation of mudstone and shale within this sedimentary sequence reflects variations in water flow energy during deposition. Mudstone typically forms in calmer, low-energy environments, whereas shale deposition occurs under slightly higher-energy conditions, such as slow-moving waters (Boggs, Reference Boggs2006). The presence of shell hash in the upper part of the succession indicates a reduction in water depth and a transition to a higher-energy environment (Fig. 8b). This suggests that significant hydrodynamic changes were present throughout the succession.
4.b.2.d. Kmd Unit
The overlying Kmd Unit is distinguished by a highly fossiliferous limestone unit (Fig. 4e). Petrographic analysis reveals a light grey colouration, predominantly composed of allochemical grains, with bioclasts (foraminifera, bivalve shell fragments, ostracods and echinoid elements) as the primary component in the micritic matrix (Fig. 9m–o; Supplementary Fig. S1.8). Microscopic examination in thin-section analysis revealed a diverse assemblage of microfossils, including smaller benthic foraminifera and LBFs such as Heterolepa dutemplei, Miogypsina sp., Archaias kirkukensis and Austrotrillina howchini (Fig. 9m, n; Supplementary Fig. S1.8). Other microfossils identified include ostracods, bivalve fragments, bryozoans and coralline algae (Fig. 9n, o; Supplementary Fig. S1.8). The fossil assemblage, more specifically with Heterolepa dutemplei and Austrotrillina howchini, has been identified as an inner-shelf benthic foraminiferal species occurring at water depths of approximately 50–60 m in tropical to subtropical environments (Szarek et al. Reference Szarek, Kuhnt, Kawamura and Kitazato2006; Martin et al. Reference Martin, Culver, Leorri, Mallinson, Buzas, Hayek and Shazili2018). The presence of micritic sediments also confirms the low-energy depositional environment (Fig. 8b). Towards the top of the sedimentary sequence, the presence of shell hash signifies a reduction in water depth, indicative of a high-energy depositional environment, leading to the accumulation of coarse-grained sediments, including shell debris.
4.b.3. Ranjitpur Member
The Ranjitpur Member, approximately 10 m thick, conformably overlies the Kuranga Member (Kmd Unit) and is prominently exposed in regions near Bamanasa, Gaga and Ranjitpur Villages in Dwarka, Gujarat (Figs. 1 and 2b). This member is further categorized into three distinct stratigraphic units, that is, Rma, Rmb and Rmc units (Fig. 2b).
4.b.3.a. Rma Unit
Field observations reveal that the Ranjitpur Brown Shale Unit (Rma) is primarily composed of brown shale, with some intermittent siltstones, which serves as its defining lithological characteristic (Fig. 4f). The petrographic thin section exhibits an extremely fine-grained texture dominated by sub-angular to sub-rounded quartz grains, alkali feldspar and plagioclase feldspar (minor) (Fig. 5d; Supplementary Fig. S1.9). No microfossils were observed in this section from petrographic as well as field observations; however, traces of organic matter were identified (Fig. 10a). Based on its textural and mineralogical characteristics, the rock is classified as siltstone. XRD data also substantiate the petrographic observation that this rock is primarily composed of SiO₂ and CaCO₃ as secondary minerals (Supplementary Fig. S1.4). The presence of siltstone with no fossils is often interpreted as indicative of a relative sea-level fall (Banerjee et al. Reference Banerjee, Khanolkar and Saraswati2018). Such deposits, which lack fossil content, are commonly associated with a restricted depositional environment (Fig. 8c), such as coastal marshes or back swamp conditions, where organic material accumulates under low-energy conditions (Biswas, Reference Biswas1992).

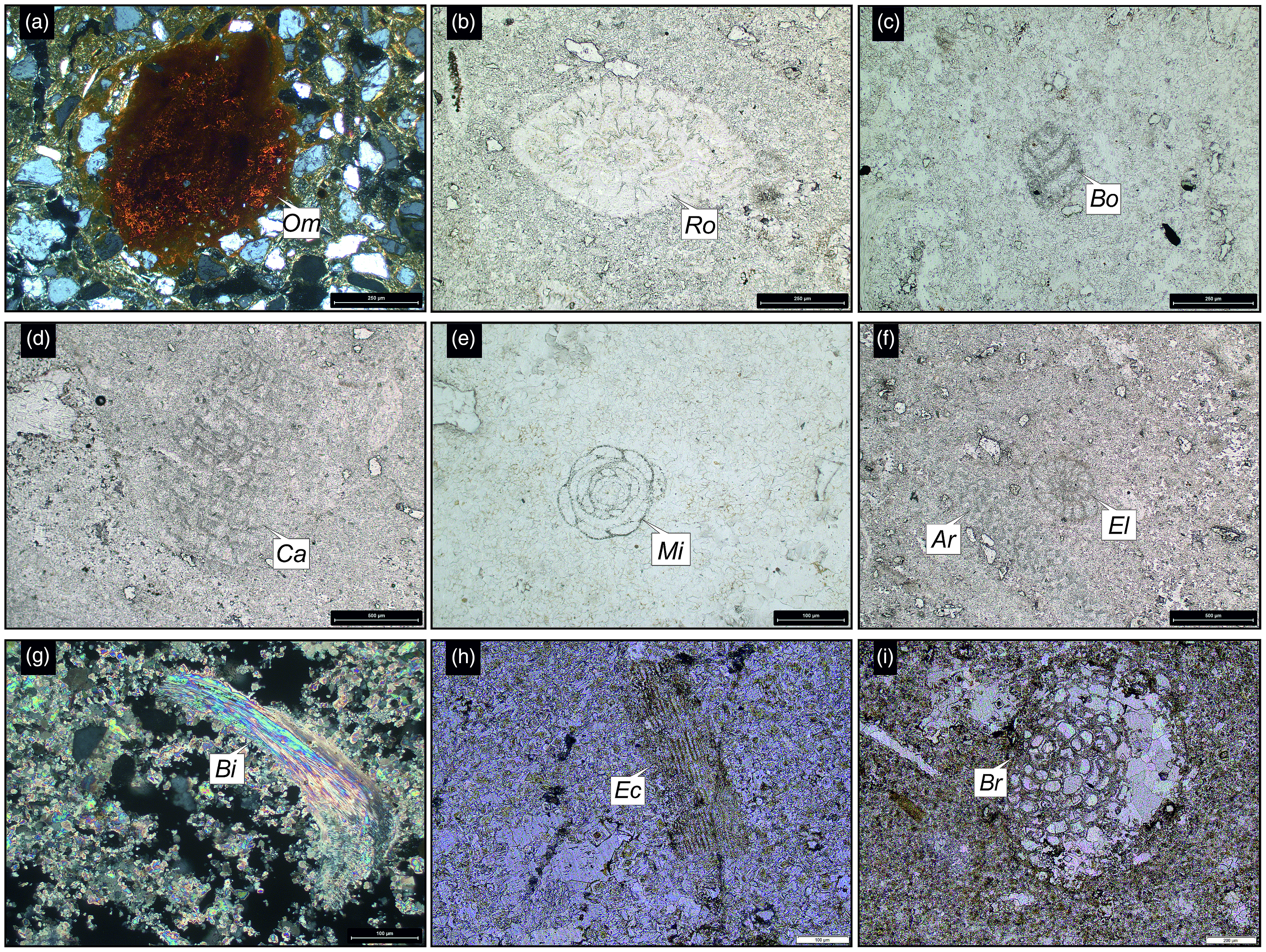
Figure 10. Thin-section photomicrographs of the (a) Rma, (b–g) Rmb and (h, i) Rmc units and their associated fossils from the Ranjitpur Member of the Gaj Formation. Abbreviations: Archaias sp. (Ar), Bivalves (Bi), Bolivina sp. (Bo), Bryozoans (Br), Coralline algae (Ca), Echinoids (Ec), Elphidium sp. (El), Miliolida (Mi), Organic matter (Om), Rotaliida (Ro). Fig. 10a, g has been taken under crossed nicols.
4.b.3.b. Rmb Unit
The overlying Ranjitpur Limestone Unit (Rmb) is characterized by a highly fossiliferous grainstone, abundant with molluscan fossils (bivalves and gastropods) and both compound and solitary corals (Fig. 4f). Petrographic study reveals that the rock is mainly composed of limestone, with its allochemical fraction predominantly made up of bioclasts of foraminifera, bivalve and echinoid shells (Fig. 10b–g; Supplementary Fig. S1.9). Thin-section study reveals that microcrystalline calcite fills the foraminiferal moulds (Supplementary Fig. S1.9). The petrographic analysis identifies various organisms, including LBFs, Archaias sp., smaller benthic foraminifera like Elphidium sp., Bolivina sp., miliolida and rotaliida foraminifera, and some coralline algae, echinoids and fragments of bivalve shells (Fig. 10b–g). Field observations reveal the presence of several shallow marine gastropods, such as Gibbula sp., Persististrombus cf. depertitus, Lyncina cf. prunum, Phos sp., Xenophora cf. gajensis, Lyria (Harpeola) cf. jugosa, Natica obscura, Sinum sp. and Cantharus erythrostomus. These assemblages suggest a rich and diverse shallow marine ecosystem. These facies, characterized by abundant preservation of LBFs, bivalves, gastropods and corals, indicate a mid- to inner-shelf depositional environment (Fig. 8c). This suggests that the deposition occurred under low-energy conditions, with occasional wave action that allowed for the accumulation of sediments. (Flügel, Reference Flügel2004).
4.b.3.c. Rmc Unit
The overlying Rmc Unit is characterized by medium-grained sandstone that contains abundant shell hash (Fig. 4g). Petrographic analysis indicates a sandstone composition with over 15% fine-grained matrix. The grains, ranging from sub-angular to angular, are poorly sorted and exhibit low maturity (Fig. 10h, i; Supplementary Fig. S1.9). Both monocrystalline and polycrystalline quartz grains, and some accessory minerals, including micas and iron oxides, are identified (Supplementary Fig. S1.9). The sample contains some bryozoans, echinoids and some rotaliida foraminiferal fossils (Fig. 10h, i). Mineralogical evidence points towards sandstone rock type with SiO₂ as the predominant mineral and CaCO₃ as the secondary mineral (Fig. 7). The presence of shell hash suggests elevated depositional energy, while poorly sorted grains point to rapid sediment deposition, likely occurring at the base of a slope. Evidence of a significant hydraulic jump from the underlying unit supports this interpretation. The absence of smaller foraminifera, washed out by wave action, further reflects high-energy conditions (Fig. 8c). Petrographic analysis, preservation style and grain orientation collectively indicate deposition in a high-energy sandy shore environment, transitioning from littoral to sub-littoral zones (Fig. 8c).
4.b.4. Ashapuramata Member
The overlying Ashapuramata Member (Asm), a ∼5 m thick marine sequence near Khatumba Village, Gujarat, is well exposed along ridges and mounds on the western Okha Rann (Fig. 1, 2b, and 11a, b). It consists of red claystone with macrofossils interbedded with thin calcareous mudstone and siltstone layers, lacking distinct lithologic variation (Fig. 11a, b). Petrography shows claystone with a fine clay matrix and floating anhedral feldspar with minor quartz, indicating poor sorting, minimal transport and textural immaturity (Figs. 5e and 12a). XRD peaks reveal SiO₂, NaCl and CaCO₃ as major mineral components (Fig. 7). A few larger fossils, including Echinoids and Scaphopod (Dentalium sexangulare), are present, but no other microfossils are preserved in this member (Fig. 12a, b). Various gastropod samples observed during fieldwork from this member have been used to establish the depositional environment. The Asm Unit is very rich in certain potamidid gastropods (mudwhelks) – Vicarya verneuili (Family Potamididae), which are epifaunal, actively moving herbivore grazers and are best known to thrive on mud flats and in mangrove and swamp environments (Sälgeback & Savazzi, Reference Sälgeback and Savazzi2006; Kase et al. Reference Kase, Kitao, Aguilar, Kurihara and Pandita2008; Harzhauser et al. Reference Harzhauser, Reuter, Mohtat and Piller2017). The association of abundant strombid (Family Strombidae), naticid (Family Naticidae) and conid (Family Conidae) gastropods, along with these potamidid gastropods, reveals a shallow marine environment present in between the seam areas of the intertidal and mudflat/mangroves (Harzhauser et al. Reference Harzhauser, Reuter, Mohtat and Piller2017). The depositional environment of the Asm Unit is interpreted as intertidal, mudflat or mangrove swamp settings (Fig. 8c). This interpretation is supported by the absence of microfossils, as mangrove swamps are unfavourable for microfossil preservation due to dynamic environmental conditions (Debenay et al. Reference Debenay, Guiral and Parra2004).

Figure 11. Field photographs (a) showing the Asm Unit of Ashapuramata Member; (b) depicts the Charkala Member (Chka Unit) and (c) its overlying Chkb Unit; (d) illustrates the Lowa Unit, including the uppermost Pecten-bearing sandstone layer; (e) highlights the Lowb Unit; (f) showcases the Dma Unit, situated between the underlying Lowb Unit and the overlying Dmb Unit, which is capped by the Dwarka Formation.

Figure 12. Thin-section photomicrographs of the identified fossils from the (a, b) Ashapuramata Member (Asm), (c–j) Charkala Member (Chka) and (k, l) Chkb of the Gaj Formation. Abbreviations: Archaias angulatus (Aa), Archaias sp. (Ar), Bioclasts (Bl), Bivalves (Bi), Dentalium sexangulare (Ds), Echinoids (Ec), Miliolida (Mi), Phylloid algae (Pa), Pseudotaberina malabarica (Pm), Quinqueloculina seminulum (Qs).
4.b.5. Charkala Member
The Charkala Member, overlying the Ashapuramata Member, is a ∼12 m thick succession consisting of two fossil-rich units. The lowermost unit, Chka, is a shale unit, while the upper unit, Chkb, is a limestone unit (Figs. 2b and 11b, c).
4.b.5.a. Chka unit
The thin section shows a fine-grained, calcareous shale with a clay-rich matrix, where dispersed grains suggest poor sorting and transport (Supplementary Fig. S1.9). Alkali feldspar, calcite grains and some opaque grains of organic matter are identified. The anhedral nature of most grains reflects textural immaturity and minimal sorting during deposition. The fossil assemblage includes a diverse range of organisms, such as the foraminifera Pseudotaberina malabarica, Archaias angulatus, Quinqueloculina seminulum and some other miliolida (Fig. 12c–j). Additionally, the presence of bivalve shells and bioclasts is noted during the study. XRD data supports the evidence from field and petrographic observations (Supplementary Fig. S1.4). Calcareous shale represents deposition in marine environments, typically moderately far from the shore in waters of normal salinity (Hattin, Reference Hattin1956). The fossil assemblage, including Pseudotaberina malabarica, stromboid gastropods and associated seagrass vegetation, further supports the interpretation of deposition within an inner ramp to littoral zone setting (BouDagher-Fadel, Reference BouDagher-Fadel2008). These features indicate a relatively shallow, nearshore marine environment influenced by normal salinity conditions and seagrass ecosystems (Fig. 8d).
4.b.5.b. Chkb Unit
The Chkb Unit is characterized by a calcareous limestone interval overlain by a fossiliferous packstone bed (Fig. 11c). Petrographic analysis identified large calcite crystals, anhedral, poorly sorted quartz grains set in a micritic matrix (Figs. 5f and 12k), classifying the rock as an intramicrite (Folk, Reference Folk1962). The sample lacks LBFs but contains phylloid algae, bivalve shells and ostracod shells (Fig. 12k, l). In addition, field observations also revealed the presence of gastropod species, including Globularia cf. carlei, Perisististrombus cf. deperditus, Perisististrombus sp., Natica obscura, Pachycrommium cf. harissi and Semicassis cf. vredenburgi. The presence of phylloid algae suggests deposition in shallow marine conditions, typically at depths of 5–15 m on a carbonate platform (Wray, Reference Wray1977). The associated fossil assemblage indicates a highly oxygenated environment within the photic zone, conducive to active photosynthetic activity. The occurrence of ostracods implies a rise in sea level or an increase in water depth relative to the underlying unit (Boomer et al. Reference Boomer, Horne and Slipper2003). Additionally, the presence of bioclasts supports the interpretation of a depositional setting within the upper ramp, where moderate energy and increased accommodation space prevailed (Fig. 8d) (Burchette & Wright, Reference Burchette and Wright1992). This interpretation has also been supported by XRD data (Fig. 7).
4.b.6. Lowrali Member
The Lowrali Member, approximately 6 m thick and composed of two distinct lithological units – Lowa and Lowb – conformably overlies the Charkala Member (Figs. 2b and 11d–f).
4.b.6.a. Lowa Unit
The Lowa Unit is a coarse-grained sandstone containing abundant bivalve and gastropod fossils. The lower part of the unit is bioturbated, whereas the top of this unit, Pecten, is preserved (Fig. 11d). Petrographic analysis reveals the rock as a sandstone, predominantly composed of over 90% polycrystalline quartz grains, floating in a fine-grained matrix (Fig. 5g; Supplementary Fig. S1.9). Orthoclase is the second most abundant mineral, here, with accessory mica grains (Fig. 5g). The low matrix content classifies the rock as a quartz arenite, though poor sorting and subhedral to anhedral grains reflect textural immaturity. XRD analysis shows the dominant minerals are SiO₂, CaCO₃, NaCl and minor amounts of Al₂O₃ within the peak (Fig. 7). Field observations identified gastropod species such as Perisististrombus cf. deperditus, Perisististrombus sp., Natica obscura, Pachycrommium cf. harissi, Semicassis cf. vredenburgi, Globularia cf. carlei, Conus (Lithoconus) cf. literatus and Tibia cf. indica. Thin-section analysis revealed the presence of various microfossils, including bivalve fragments, benthic foraminifera such as Heterolepa dutemplei and Archaias sp., and planktonic foraminifera like Globigerinoides sp. (Fig. 13a–e). The presence of Heterolepa dutemplei suggests deposition in an outer ramp setting (Flügel, Reference Flügel2004). The reappearance of planktic foraminifera further indicates a deepening of water compared to the underlying units (Fig. 8d). The topmost Pecten layer represents a transgressive lag concentration, signifying a deepening-upward sequence and a rise in sea level.

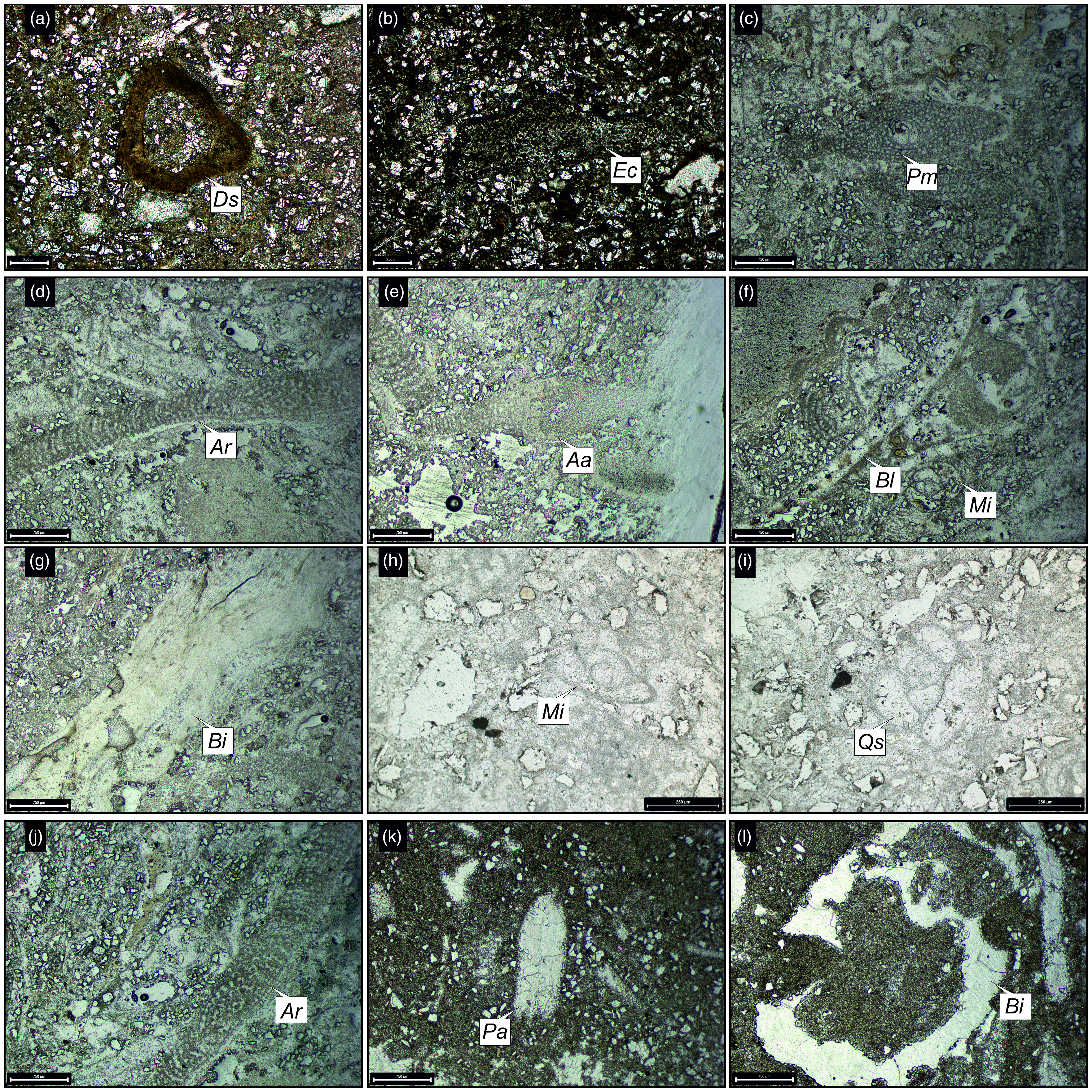
Figure 13. Thin-section photomicrographs of the (a–e) Lowa Unit and (f–i) Lowb Unit and its associated fossils from the Lowrali Member of the Gaj Formation. Abbreviations: Archaias sp. (Ar), Bivalves (Bi), Echinoids (Ec), Globigerina sp. (Gl), Heterolepa dutemplei (Hd). Fig. 13f has been taken under crossed nicols.
4.b.6.b. Lowb Unit
The Lowb Unit is a highly fossiliferous calcareous siltstone, rich in echinoids and molluscs, and exhibits significant bioturbation (Fig. 11e, f). The petrographic study suggests that the rock sample is composed of a fine-grained, clay-rich siltstone matrix, within which bivalve shells and foraminiferal tests are embedded (Fig. 13f–i; Supplementary Fig. S1.9). The microfaunal assemblage of this unit includes benthic foraminifera such as Archaias sp. and planktonic foraminifera like Globigerina sp. (Fig. 13h, i). The presence of Archaias sp. suggests deposition in a low-energy, well-lit environment within the euphotic to mesophotic zone, characterized by normal salinity and typical of inner ramp settings (Fig. 8d) (Flügel, Reference Flügel2004). Additionally, the occurrence of planktic foraminifera indicates a relative increase in water depth. The presence of minor quartz and feldspar grains suggests a slight terrigenous contribution. XRD data reveals mineralogy similar to the underlying Lowa Unit (Supplementary Fig. S1.4).
4.b.7. Dabla Mandir Member
The Dabla Mandir Member represents the youngest unit of the Gaj Formation and is conformably overlain by the Rangasar Member of the Dwarka Formation. This member, approximately 4 m thick, is prominently exposed near Dabla Mandir, and the member comprises two distinct limestone units, Dma Unit and Dmb Unit (Figs. 1, 2b, and 11f).
4.b.7.a. Dma Unit
This rock unit is primarily limestone, with sparse fossil fragments as allochems embedded in a fine micritic matrix of microcrystalline calcite (Supplementary Fig. S1.9). This rock is calcite-dominated with traces of muscovite, classified as biomicrite (Folk, Reference Folk1962), while Dunham’s scheme identifies it as a packstone due to its grain-supported texture and minor mud content. The microfossils Archaias sp., A. angulatus and A. kirkukensis were found in extraordinary abundance (Fig. 14a–d). Fieldwork also yielded a diverse and prolific gastropod assemblage, including Perisististrombus cf. deperditus, Phos sp., Natica obscura and numerous other species. Archaias angulatus and other Archaias sp. are known to inhabit shallow back-reef environments, typically within water depths of up to 30 m (Brasier, Reference Brasier1975; Hallock & Glenn, Reference Hallock and Glenn1986). Similarly, Archaias kirkukensis is adapted to thrive in shallow lagoonal settings on carbonate ramps, indicative of low-energy environments with restricted circulation (Fig. 8e) (BouDagher-Fadel, Reference BouDagher-Fadel2008). These ecological preferences suggest a depositional environment characterized by shallow, warm and well-lit marine conditions typical of shallow back-reef settings.

Figure 14. Thin-section photomicrographs of the identified larger benthic foraminifera and associated fossils from the Dabla Mandir Member (a–d) Dma Unit and (e–l) Dmb Unit of the Gaj Formation. Abbreviations: Ammonia sp. (Am), Archaias angulatus (Aa), Archaias kirkukensis (Ak), Archaias sp. (Ar), Bivalves (Bi), Borelis melo (Bm), Miliolida (Mi), Miosorites sp. (Ms), Pseudotaberina malabarica (Pm), Rotaliida (Ro).
4.b.7.b. Dmb Unit
The upper Yellow Limestone Dmb Unit is a hard, compact limestone (Fig. 11f). The petrographic analysis identifies the rock sample as a microcrystalline limestone, with a fine-grained micritic matrix exhibiting a greyish to brown colour and indistinct grain boundaries, indicative of a precipitated origin (Fig. 5h; Supplementary Fig. S1.9). The matrix contains abundant bioclasts, including fragments of bivalves, foraminifera and coralline algae, pointing to a marine depositional environment with moderate to high biological productivity. Prominent calcite veins, displaying high-order variegated interference colours under polarized light, are also present. According to Folk’s classification, the rock is classified as a biomicrite due to bioclast content exceeding 10% (Folk, Reference Folk1962). The microfossil assemblage includes LBFs such as Pseudotaberina malabarica and Archaias sp., along with smaller benthic foraminifera like miliolida, Ammonia sp., rotaliidas, Textularia sp., and Miosorites sp., are also present (Fig. 14e–l; Supplementary Fig. S1.9). Additionally, the macrofossil assemblage includes bivalve shells and diverse gastropod species like Perisististrombus cf. deperditus, Phos sp. and Natica obscura. The fossil assemblage provides key insights into the depositional environment of this unit. The consistent presence of Pseudotaberina malabarica indicates a preference for very shallow, epiphytic habitats, suggesting deposition in environments with ample light and moderate energy, typical of tidal to shallow subtidal zones (Fig. 8e) (Reuter et al. Reference Reuter, Piller, Harzhauser, Kroh, Rögl and Ćorić2011). This aligns with the ecological niche of epiphytic foraminifera, which thrives in close association with seagrass or other substrates in shallow marine settings. XRD data indicates that the rock of this unit is composed of CaCO₃ and SiO₂ mostly (Fig. 7).
The sedimentary record of the Gaj Formation provides a valuable framework for interpreting its relationship with global mean sea-level fluctuations during the late Aquitanian to Burdigalian and early Langhian. This interval corresponds to the third-order sea-level cycles identified in global eustatic sea-level curves (Haq et al. Reference Haq, Hardenbol and Vail1987; Miller et al. Reference Miller, Kominz, Browning, Wright, Mountain, Katz, Sugarman, Cramer, Christie-Blick and Pekar2005, Reference Miller, Browning, Schmelz, Kopp, Mountain and Wright2020) (Supplementary Fig. S1.1). The presence of transgressive facies, such as bioclastic limestones with shallow marine assemblages and transgressive lag deposits, suggests a close correspondence with eustatic sea-level rises during the Burdigalian and early Langhian (Supplementary Fig. S1.1). These transgressions align with the Miocene Climatic Optimum, marked by higher global temperatures and glacio-eustatic adjustments (Supplementary Fig. S1.1). The regressive phases within the Gaj Formation may reflect relative sea-level falls due to regional tectonic influences or glacial episodes (Supplementary Fig. S1.1).
4.c. Paleoenvironment and tectonic history of the Dwarka Basin and biotic response to MMCO climate warming
The complex tectonic history of the study area, by the breakup of Gondwanaland during the Cretaceous, has resulted in the formation of various structural features within the Dwarka Basin (Biswas, Reference Biswas1982). The region experienced significant tectonic and sedimentary processes during three distinct geological time periods: the late Cretaceous, the Eocene and the early Miocene (Biswas, Reference Biswas1982).
Faulting during the late Cretaceous to Paleocene, associated with the rifting of the western Indian margin, was succeeded by the emplacement of Deccan Trap volcanism (Sriram et al. Reference Sriram, Gupte, Kothari, Bisen, Waraich and Mehta2006; Bisen et al. Reference Bisen, Sriram and Gupte2010). This sequence of events triggered the formation of the Girnar fracture zone, the Dwarka fault, as well as the upliftment of the Saurashtra Arch, followed by faulting along its central axis (Sriram et al. Reference Sriram, Gupte, Kothari, Bisen, Waraich and Mehta2006; Bisen et al. Reference Bisen, Sriram and Gupte2010) (Supplementary Fig. S1.10). During the early Paleocene, the Saurashtra Arch, characterized by an already thinned crust, underwent crustal collapse driven by thermal cooling coincided with north-south extensional forces from India’s northward drift, producing a horst–graben complex (Biswas, Reference Biswas1982; Sriram et al. Reference Sriram, Gupte, Kothari, Bisen, Waraich and Mehta2006) (Supplementary Fig. S1.10). During the middle to late Miocene, the southern limb of the Saurashtra Arch experienced significant tilting, likely associated with regional tectonic events such as the Murray Ridge uplift or the intense Himalayan collision (Biswas, Reference Biswas1982; Sriram et al. Reference Sriram, Gupte, Kothari, Bisen, Waraich and Mehta2006). The paleogeographic evolution of the Saurashtra Basin provides crucial insights into the complex interplay of tectonic, climatic and sedimentary processes that shaped the region during the Paleogene and Neogene. The significant hiatus between the Laterite Formation and the Miocene sediments, as well as the absence of Eocene–Oligocene deposits, can be attributed to a combination of several factors. The arching of the Saurashtra Peninsula resulted in differential tectonic uplift and subsidence, leading to a complex pattern of marine incursions that led to marine deposition across the basin (Supplementary Fig. S1.10). This complex tectonic history is evident in the radial drainage pattern observed in the region. Besides, the pre-Eocene uplift of the Saurashtra Peninsula during the initial stages of the India-Asia collision led to significant erosion and non-deposition during the subsequent Eocene and Oligocene epochs, resulting in a hiatus in the sedimentary record (Pandey et al. Reference Pandey, Kondo, Jain, Bahadur and Pradhan2008; Sciunnach & Garzanti, Reference Sciunnach and Garzanti2012) (Supplementary Fig. S1.11). Earlier, Sriram et al. (Reference Sriram, Gupte, Kothari, Bisen, Waraich and Mehta2006) reported that at the end of the middle Eocene, the Saurashtra Arch subsided below the photic zone, resulting in the cessation of carbonate sedimentation. Besides, the Eocene epoch was characterized by relatively lower global sea levels compared to the Miocene (Lincoln & Schlanger, Reference Lincoln and Schlanger1991; Pandey et al. Reference Pandey, Kondo, Jain, Bahadur and Pradhan2008). Recent research findings by Miller et al. (Reference Miller, Schmelz, Browning, Rosenthal, Hess, Kopp and Wright2024) suggest that the Eocene, often characterized as an ice-free period, was punctuated by multiple episodes of significant sea-level fall (∼20–40 m), potentially driven by the growth and decay of continental ice sheets. These combined effects resulted in the preservation of just Miocene sediments within the Dwarka Basin. The significant climatic fluctuations, including global warming events during this early to middle Miocene period, are well reflected in the fossil record, particularly in the assemblages of benthic foraminifera like Ammonia sp., LBF like Archaias sp. and gastropods families such as Strombidae, Naticidae and Conidae.
LBFs have the ability to adapt their reproductive strategies based on environmental factors. LBFs primarily exhibit characteristics of K-strategists, including slow growth and extended lifespans. Nevertheless, these organisms can demonstrate opportunistic traits in shallow marine settings, swiftly multiplying when conditions are favourable (Murray, Reference Murray2006). Our study highlights the high species diversity in the Nandana and Kuranga members, suggesting a stable and favourable environment during the time these formations were deposited. However, a clear declining trend in faunal diversity is observed moving from the Ranjitpur Member to the Dabla Mandir Member (Fig. 3). This decrease may indicate changing environmental and climatic conditions, which correlate with the Middle Miocene Climatic Optimum (MMCO), which became less suitable for a wide range of species. The benthic foraminifera genus Ammonia is the most commonly observed taxon across all members of the studied sequence. A notable abundance of Ammonia is documented from the Nandana Member to the Kuranga Member, indicating favourable environmental conditions for its proliferation. However, a significant decline in Ammonia populations is observed from the Ranjitpur to the Dabla Mandir Member. Rising sea surface temperatures and increased thermal stress during this period created less favourable conditions, leading to the decline of species populations. The small benthic foraminiferal genus Ammonia is particularly indicative of this trend, showing substantial populations in earlier members but a significant reduction in abundance in the later members. This reduction coincides with increasing thermal stress and environmental changes, likely linked to elevated sea surface temperatures during MMCO (Murray, Reference Murray2006; Saraswat et al. Reference Saraswat, Nigam and Pachkhande2011; Titelboim et al. Reference Titelboim, Rothwell, Lord, Harniman, Melbourne and Schmidt2024).
The chamber size of Ammonia exhibits a noticeable trend across the studied members (Supplementary Fig. S1.12). From the Nandana to the Kuranga Member, there is a progressive increase in chamber size, with the Kmb Unit displaying the largest chambers. This increase reflects favourable environmental conditions, higher carbonate saturation and stable carbonate chemistry conducive to calcification during deposition at the pre-MMCO interval (Supplementary Fig. S1.12). However, following the MMCO, a marked reduction in chamber size is observed, particularly in the Kmc Unit (Supplementary Fig. S1.12). Following this unit, Ammonia disappears completely from the record until the Lowrali Member. This reduction and disappearance align with the thermal stress and environmental changes associated with the MMCO, and this is due to warming-induced dwarfism (Titelboim et al. Reference Titelboim, Rothwell, Lord, Harniman, Melbourne and Schmidt2024). From the Dma Unit onward, Ammonia size began to increase, and it correlates with optimal warm, stable oligotrophic conditions after MMCO (Hallock, Reference Hallock1987; Boudaugher-Fadel, Reference Boudaugher-Fadel2018) (Supplementary Fig. S1.12). While Ammonia size is reduced during MMCO, the LBF here, Archaias sp., showed increased test sizes during the MMCO (Supplementary Fig. S1.12). Carbonate saturation and photosymbiotic enhancement during pre-MMCO and MMCO, due to warm, oligotrophic conditions, led to larger tests in these LBFs, a trend opposite to that observed in smaller benthic foraminifera (Renema et al. Reference Renema, Bellwood, Braga, Bromfield, Hall, Johnson, Lunt, Meyer, McMonagle, Morley, O’Dea, Todd, Wesselingh, Wilson and Pandolfi2008). During the last stage and post-MMCO cooling, improved environmental and nutrient conditions promoted ecosystem recovery, leading to a population increase in Archaias sp. through enhanced reproduction rather than test size growth. Our observations confirm that these environmental changes were not merely regional but reflect a global impact of MMCO, as also emphasized by Foster et al. (Reference Foster, Lear and Rae2012), who attributed the warming during this interval to be primarily driven by elevated pCO₂ based on boron isotope data.
The MMCO had a profound impact on coral ecosystems, also. Fossil assemblages reveal a significant abundance of corals during earlier phases, particularly within the Kuranga Member. However, following this period, a sharp decline in coral populations is evident, with coral abundance drastically reduced in the overlying units. Elevated sea surface temperatures impaired coral growth and calcification, as thermal stress inhibits the biomineralization processes critical for calcium carbonate deposition (Hoegh-Guldberg, Reference Hoegh-Guldberg1999; Pandolfi & Kiessling, Reference Pandolfi and Kiessling2014). Here, coral disappearance during and after the MMCO was likely driven by the combined effects of elevated temperatures, increased terrigenous input linked to tectonic tilting and regional controls, and reduced aragonite saturation, collectively creating conditions unfavorable for reef recovery. A similar pattern observed in phylloid and coralline algae further supports the interpretation of a gradual environmental shift during this phase. Another interesting observation is a clear trend of declining body size in gastropods (Strombidae, Naticidae, Conidae) from the Charkala Member through the Lowrali Member, followed by a notable size increase in the Dabla Mandir Member. This size decline follows the temperature–size rule, with peak MMCO warmth maintaining smaller sizes and post-MMCO cooling enabling recovery through reduced thermal stress and better environmental conditions consistent with observations reported by previous workers for this interval (Shevenell et al. Reference Shevenell, Kennett and Lea2004; Harzhauser & Piller, Reference Harzhauser and Piller2007). Besides, microfacies associations and petrographic studies reveal that during the pre-MMCO, the deposition of evaporites/halite in different units indicates arid and warm climatic conditions, often associated with high evaporation rates. These observations strongly indicate an environmental shift from the base of the Charkala Member to the base of the Dabla Mandir Member (∼ 17.5–15.9 Ma), confirming that the signature of the MMCO is well preserved in the sedimentary archive of the Gaj Formation (Supplementary Fig. S1.1)
5. Conclusions
Utilizing multiple proxies, this study provides a comprehensive reconstruction of the paleoclimatic and paleodepositional scenarios of the Gaj Formation in the Dwarka Basin.
• Foraminiferal biostratigraphic analysis identifies the Gaj Formation spanning from Aquitanian to Langhian, with a significant part corresponding to the Burdigalian stage.
• Microfacies analysis indicates that the Gaj Formation predominantly represents deposition in shallow marine environments, with depositional settings influenced by regional tectonic activities and climatic fluctuations.
• Fossil evidence highlights the pronounced impact of the warming event, with warming-induced morphological adaptations and size changes observed in the marine organisms.
• The integration of biostratigraphy, microfacies and paleoclimatic signals underscores the dynamic interaction between global climatic events and regional depositional processes in shaping the sedimentary history of the Gaj Formation during the Miocene.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S0016756825100356.
Acknowledgements
The authors are grateful to the Central Research Facility, Department of Applied Geology of IIT (ISM) Dhanbad, for providing the necessary laboratory facilities. SC, KB and SSD acknowledge the Geological Studies Unit, Indian Statistical Institute (ISI), Kolkata, for providing funding and necessary logistical support. This work was supported by the Indian Statistical Institute [5427D for the year 2019–2020] and the Science and Engineering Research Board, Department of Science and Technology, Government of India [EMR/2017/000328]. KB also acknowledges the Department of Earth Sciences, IIT Bombay, for providing infrastructural facilities. The authors are thankful to Prof. Pratul Kumar Saraswati, IIT Bombay, and Prof. Jahnavi Punekar, IIT Bombay, for the identification of some of the foraminifera species. The authors extend their sincere thanks to Tapas Kar, ISI Kolkata, for his invaluable assistance in preparing thin sections and providing rock-cutting support. The authors are thankful to S. Vinodbhai and Sunil Parmar for their local assistance in fieldwork. The authors are grateful to editor Dr Bas Van de Schootbrugge, handling editor and two anonymous reviewers, whose comments and queries helped to improve the quality of this work.
Competing interests
The authors declare no conflict of interest.