Introduction
Antarctica supports a low diversity of arthropod species compared to the rest of the world (Convey & Biersma Reference Convey, Biersma and Scheiner2024), and its highest species richness is typically found at sites in the relatively mild and wet Maritime Antarctic region. The South Shetland Islands host 14 springtail species (Collembola), including 8 native species and 6 exotics (Greenslade Reference Greenslade2010, Greenslade et al. Reference Greenslade, Potapov, Russell and Convey2012). During a biological survey on Byers Peninsula, Livingston Island as part of a wider study of the impact of penguin-derived nutrients on arthropod diversity patterns during February 2017 (Bokhorst et al. Reference Bokhorst, Convey and Aerts2019), where vegetation samples were heat extracted, we noticed an unusual springtail species (Fig. 1) to be present in 18 of the 240 vegetation samples (n = 64 individuals, 1–11 individuals/sample) obtained in that campaign. The springtail’s morphology resembles that of the known Maritime Antarctic-native species Folsomotoma octooculata (Willem, 1901), but it clearly differed from this species by having 1+1 ocelli instead of the 4+4 ocelli configuration of F. octooculata (Convey et al. Reference Convey, Greenslade, Arnold and Block1999, Fanciulli et al. Reference Fanciulli, Leo, Convey, Frati and Carapelli2018), and in most cases individuals are shorter than F. octooculata. Following the taxonomic key of Heckman (Reference Heckman2001) for South American aquatic insects, this springtail was identified as Isotoma punctata (Wahlgren 1906). The genus Isotoma contains many subgenera, amongst which Folsomotoma was raised to full genus by Greenslade (Reference Greenslade1986). There are currently four recognized Folsomotoma species with 1+1 ocelli: Folsomotoma boerneri, Folsomotoma subflava, Folsomotoma marionensis and Folsomotoma punctata (Fanciulli et al. Reference Fanciulli, Leo, Convey, Frati and Carapelli2018), of which F. punctata has the closest geographical distribution to the South Shetland Islands, being native in southern South America (Heckman Reference Heckman2001) and also being recorded as native from sub-Antarctic South Georgia and other sub-Antarctic islands (Convey et al. Reference Convey, Greenslade, Arnold and Block1999).

Figure 1. Stereomicroscope image of previously unrecorded springtail species found in vegetation samples obtained on Byers Peninsula, Livingston Island. The springtail on the far right is the native Folsomotoma octooculata, which is commonly found in this region. The middle springtail is most probably a juvenile, whereas the two on the left represent adults. The size of the new species is smaller than that of F. octooculata, but the overall appearance is similar with the exception of the number of ocelli. The lower images show close-up magnifications (×400) of the head and 1+1 ocelli of the unrecorded species.
Results
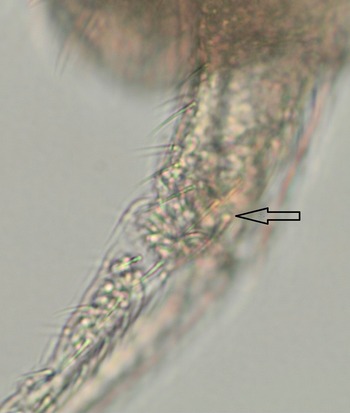
We were unable to quantify the setae on the corpus of the retinacle, a distinguishing factor within F. boerneri, F. subflava, F. marionensis and F. punctata, but there appear to be 1+1 ampoule setae on the manubrium (Fig. 2), reducing the possibilities to F. marionensis and F. punctata. DNA was extracted from two springtail individuals using the Qiagen tissue protocol, followed by polymerase chain reaction (PCR) with universal cytochrome oxidase I (COI) primers COI-LCO1490F and COI-HCO2198R, and also a custom 18S rRNA primer based on F. punctata (GenBank DQ365777). No amplification products were obtained, probably due to low or absent DNA yield, primer mismatch or insufficient sequence similarity.

Figure 2. Close-up image of the manubrium of the putative Folsomotoma punctata found on Byers Peninsula, Livingston Island. The arrow indicates the presence of an ampoule setae, of which F. punctata has 1+1 on the manubrium according to Fanciulli et al. (Reference Fanciulli, Leo, Convey, Frati and Carapelli2018). We were unable to bring both ampoule setae into focus within one picture.
Specimens of F. punctata were obtained on Byers Peninsula from the mosses Sanionia uncinata (number of samples with F. punctata: n = 5) and Andreaea regularis (n = 10), the lichen Sphaerophorus globosus, the grass Deschampsia antarctica and an unidentified lichen (Fig. 3). All springtail communities were dominated by Cryptopygus antarcticus, and F. punctata was, in almost all instances, found together with F. octooculata, indicating preference for the same habitat (Fig. 4 & Table I). Other dominant microarthropod species were present across Byers Peninsula, except for the oribatid mites Alaskozetes antarctica and Halozetes belgicae, which are generally coastal species and were absent from the interior of the peninsula (Fig. 4). Species abundances across the different habitat types sampled are shown in Table I. Overall, there were no significant species abundance differences between moss, lichen and grass habitats, except for Globoppia loxolineata, which was 3.5 times more abundant (F 1,24 = 8.0, P = 0.009) amongst lichen (2.6 ± 0.7 ind. g−1) compared to moss (0.7 ± 0.2 ind. g−1) habitats.

Figure 3. Sampling locations where Folsomotoma punctata was found on Byers Peninsula, Livingston Island, during February 2017. Different symbol types and colours represent different habitat types. F. punctata (64 individuals in total) was also found in an unidentified lichen sample at the same location as that of Sphaerophorus globosus. Open circles represent sample sites (n = 29) at which multiple vegetation samples were collected. The inset shows the location of the South Shetland Islands relative to the north-west coast of the Antarctic Peninsula.

Figure 4. Arthropod species presence across Byers Peninsula, Livingston Island. Each symbol represents a location where lichens, mosses or plants were sampled and microarthropod species were extracted. All sampling locations are shown in Fig. 3. Total individuals collected from 240 samples: Cryptopygus antarcticus = 30 112; Folsomotoma octooculata = 1703; Friesea antarctica = 491; Tullbergia mixta = 705; Alaskozetes antarcticus = 3307; Halozetes belgicae = 1064; Gamasellus racovitzai = 217; Stereotydeus villosus = 6316; Globoppia loxolineata = 120; and 3244 unidentified very small prostigmatid and astigmatid mites, which may also include juveniles of various mite species.
Table I. Arthropod abundances across vegetation habitats on Byers Peninsula, Livingston Island. Arthropod species abundances (individuals per gram dry substrate) across different lichen and moss species and those associated with the grass Deschampsia antarctica are shown. Vegetation samples were obtained across Byers Peninsula as part of transect sampling from the coast to inland sites (Fig. 3), with a focus on dominant moss and lichen species.

n = number of samples; NA = not applicable; SE = standard error.
Discussion and conclusion
The most recent survey of the arthropod community on Byers Peninsula dates back to the early 1990s (Richard et al. Reference Richard, Convey and Block1994), with five springtail species being identified in that survey (Convey et al. Reference Convey, Greenslade, Richard and Block1996). The observation of a new species most probably reflects a more extensive sampling regime across both the northern and southern beaches of the peninsula as well as inland sampling (Fig. 3), whereas Richard et al. (Reference Richard, Convey and Block1994) mainly collected along the western beaches. F. punctata was recorded on Deception Island, but this observation remained unpublished in a thesis (Enriquez Reference Enriquez2017), and we thank the reviewers for bringing this to our attention. The morphology and description of setae match with the specimens from Byers Peninsula, and F. punctata was consistently observed together with F. octooculata and C. antarcticus on Deception Island, as was also found here. It is possible that F. punctata is a more recent arrival from South America, perhaps via geothermally heated sites on nearby Deception Island, where other springtails have been introduced to the region (Greenslade et al. Reference Greenslade, Potapov, Russell and Convey2012). The South Shetland Islands provides some of the mildest conditions anywhere in the Maritime Antarctic and so are particularly vulnerable to the establishment of non-native springtails (Hughes et al. Reference Hughes, Pescott, Peyton, Adriaens, Cottier-Cook and Key2020). It is also possible that the springtail may have been transported by migratory birds, or vagrant birds from southern South America that might have been blown off course. However, given the wide distribution of F. punctata across Byers Peninsula (Fig. 3) and the typically slow geographical dispersal of small soil-dwelling organisms (Ojala & Huhta Reference Ojala and Huhta2001), it seems improbable that this springtail species is a recent arrival that has already migrated several kilometres from the vegetated southern beaches, across mostly unvegetated habitats of the centre of Byers Peninsula to the northern beaches. Regardless of its origin, this finding represents a new species for the South Shetland Islands and the Maritime Antarctic region, highlighting the need for more thorough and high-spatial-resolution systematic surveys across different vegetation types and habitats in order to better understand terrestrial biodiversity patterns in Antarctica.
Acknowledgements
The authors are grateful for the logistical support given by the British Antarctic Survey and the Spanish Antarctic Program during the fieldwork. All samples are stored at VU Amsterdam. The paper was improved by the constructive comments of anonymous reviewers and Luis Pertierra.
Author contributions
SB: fieldwork, species identification and writing. JM: molecular approaches and review. PC: review and writing.
Financial support
This work was funded by a grant from the Netherlands Polar Programme (NPP-NWO 851.20.016) to SB, and PC is supported by NERC core funding to the British Antarctic Survey’s ‘Biodiversity, Evolution and Adaptation’ team.
Competing interests
The authors declare none.