Introduction
Since 2021, highly pathogenic avian influenza (HPAI) A(H5N1) clade 2.3.4.4b has caused the largest and most severe global epizootic event in poultry, captive and wild birds witnessed to date (Kuiken and Cromie Reference Kuiken and Cromie2022; Pohlmann et al. Reference Pohlmann, King, Fusaro, Zecchin, Banyard and Brown2022; Wille and Barr Reference Wille and Barr2022; Gamarra-Toledo et al. Reference Gamarra-Toledo, Plaza, Gutiérrez, Luyo, Hernani and Angulo2023). Until 2021, avian influenza viruses in Europe occurred seasonally, with the majority of infections being reported between autumn and spring with no recognised infections over the summer months (EFSA et al. Reference Adlhoch, Fusaro and Gonzales2022c; Pohlmann et al. Reference Pohlmann, King, Fusaro, Zecchin, Banyard and Brown2022). While in winter 2020/21 subtype HPAI H5N8 dominated the epizootic and led to severe mortality among Barnacle Geese Branta leucopsis and Greylag Geese Anser anser (EFSA et al. Reference Adlhoch, Fusaro and Gonzales2020, Reference Adlhoch, Fusaro and Gonzales2021a, Reference Adlhoch, Fusaro and Gonzales2021b), the subtype HPAI H5N1 clade 2.3.4.4b dominated in wild birds across Europe from late spring 2021 onward. Infections were detected sporadically throughout the summer and early autumn of 2021, causing mass mortality in Great Cormorants Phalacrocorax carbo in Estonia in May 2021 (Bregnballe et al. in prep.) and in Great Skuas Stercorarius skua in the United Kingdom (UK) in July 2021 (Banyard et al. Reference Banyard, Lean, Robinson, Howie, Tyler and Nisbet2022). However, in autumn and winter 2021/22, Europe witnessed an unprecedented outbreak of HPAI H5N1 in both wild birds and poultry (Pohlmann et al. Reference Pohlmann, King, Fusaro, Zecchin, Banyard and Brown2022). The virus was detected in at least 62 wild bird species, of which 17 were waterfowl and 12 were raptor species (EFSA et al. Reference Adlhoch, Fusaro and Gonzales2022c). The most severe cases were the losses of more than 4,500 Barnacle Geese of the Svalbard breeding population that died on the Solway Firth (UK) and the associated decline of the wintering population by 38% (EFSA et al. Reference Adlhoch, Fusaro and Gonzales2022a), the death of 5,000 Common Cranes Grus grus in northern Israel, and 2,000 Dalmatian Pelicans Pelecanus crispus in Greece (Alexandrou et al. Reference Alexandrou, Malakou and Catsadorakis2022; Scientific Task Force on Avian Influenza and Wild Birds 2022; Wille and Barr Reference Wille and Barr2022).
By spring 2022, HPAI H5N1 clade 2.3.4.4b had become enzootic, meaning that it did not disappear during the summer months, and resulted in the most severe outbreak in wild birds and poultry that Europe had ever witnessed (EFSA et al. Reference Adlhoch, Fusaro and Gonzales2022a, Reference Adlhoch, Fusaro and Gonzales2022b). The virus subsequently spread to the Americas, including South America, where outbreaks had not been reported previously (Caliendo et al. Reference Caliendo, Lewis, Pohlmann, Baillie, Banyard and Beer2022; Miller Reference Miller2022; Stokstad Reference Stokstad2022; Wille and Barr Reference Wille and Barr2022; Gamarra-Toledo et al. Reference Gamarra-Toledo, Plaza, Gutiérrez, Luyo, Hernani and Angulo2023). In Europe, almost 2,500 outbreaks among poultry with 47.7 million birds culled between October 2021 and September 2022 signified an enormous economic loss and pressure on the food chain (EFSA et al. Reference Adlhoch, Fusaro and Gonzales2022d). Between March and September 2022, the virus was detected in 80 wild avian species (EFSA et al. 2022b, 2022d), with some colonially nesting seabirds being affected the most. Great Skuas, Northern Gannets Morus bassanus, Great Cormorants, Common Terns Sterna hirundo, and Sandwich Terns Thalasseus sandvicensis all experienced major die-offs (Camphuysen and Gear Reference Camphuysen and Gear2022; EFSA et al. Reference Adlhoch, Fusaro and Gonzales2022d; Rijks et al. Reference Rijks, Leopold, Kühn, In ‘t Veld, Schenk and Brenninkmeijer2022; Pohlmann et al. Reference Pohlmann, Stejskal, King, Bouwhuis, Packmor and Ballstaedt2023; Lane et al. Reference Lane, Jeglinski, Avery-Gomm, Ballstaedt, Banyard and Barychka2023).
Sandwich Terns are highly gregarious seabirds, with the majority of the European population breeding in a small number of large coastal colonies (Stienen Reference Stienen, Keller, Herrando, Voříšek, Franch, Kipson and Milanesi2020). As Sandwich Terns are a conservation priority (listed in Annex I of the EU Birds Directive), these colonies are monitored annually and often extensively. This allowed the spread and the impact of HPAI in this wild avian species to be closely monitored. The European Sandwich Tern breeding population consists of three flyway populations (Møller Reference Møller1981). Of these, the north-western European population was worst affected by HPAI in 2022.
Sandwich Terns nest at densities of up to seven breeding pairs (BP)/m² with an average distance between nests of 30–35 cm (Veen Reference Veen1977). Contrary to other European tern species, adults defecate around the nest while breeding, to an extent that vegetation growth is hampered by toxic amounts of nutrients (Cullen Reference Cullen1960; Veen Reference Veen1977; Soots and Landin Reference Soots and Landin1978; Courtens et al. Reference Courtens, Verstraete, Vanermen, van de Walle and Stienen2017). Adults are highly mobile, moving within and between colonies, both during and between breeding seasons (Fijn et al. Reference Fijn, Wolf, Courtens, Verstraete, Stienen and Iliszko2014; Knief and Haupt Reference Knief and Haupt2018; Courtens and Fijn Reference Courtens and Fijn2020; Spaans et al. Reference Spaans, Leopold and Loos2021). Viral transmission via the faecal–oral route probably predominates among wild birds (Fouchier and Munster Reference Fouchier and Munster2009). The Sandwich Tern’s behaviour promotes airborne and direct-contact transmission routes and may put the species at high risk for an HPAI epizootic once the virus has entered the population (Boulinier Reference Boulinier2023).
Sandwich Terns are long-lived, with an adult annual survival rate of 0.92 (Courtens et al. Reference Courtens, van Daele, Brenninkmeijer, Leopold, Lutterop, Delta, van Bemmelen, Courtens, Collier and Fijn2022) and a recorded maximum age of 31 years and 5 months in the north-western European population (Fransson et al. Reference Fransson, Kolehmainen, Moss and Robinson2023). Their reproductive output is usually low (less than one fledgling per BP and year) (Thorup and Koffijberg Reference Thorup and Koffijberg2016), which makes adult survival of paramount importance in population dynamics (Boulinier Reference Boulinier2023). In this paper, we therefore focus predominantly on the impact of HPAI on adult mortality. We describe the spatiotemporal patterns of infection across all Sandwich Tern colonies in north-western Europe and evaluate containment, preventive and mitigating measures that were pursued during the breeding season in 2022.
Methods
Relevant population
European-breeding Sandwich Terns are separated into three flyway populations: the north-western (also referred to as “Northern and Western”) European population that predominantly winters along the coasts of western and southern Africa; the Mediterranean and Black Sea population, which is partially synhiemic with the north-western European population but winters to some extent also in the Black and Mediterranean Seas; and the totally allohiemic Caspian Sea population (Møller Reference Møller1981; del Hoyo et al. Reference del Hoyo, Elliott and Sargatal1996; Bauer et al. Reference Bauer, Fiedler and Bezzel2005; Wetlands International 2021; Spina et al. Reference Spina, Baillie, Bairlein, Fiedler and Thorup2022). According to 2015 assessments by BirdLife International, the north-western European population then amounted to 160,000–186,000 individuals (1% = 1,700 individuals) or 45,600–55,300 BP, the Mediterranean and Black Sea population amounted to 62,000–221,000 individuals (1% = 1,100 individuals), and the Caspian Sea population amounted to 110,000 individuals (1% = 1,100 individuals) (BirdLife International 2015). We gathered data on all colonies from the north-western European population, and from the westernmost colonies in the Mediterranean Sea, which are sometimes included in the north-western European population. There were no indications that the Mediterranean colonies were infected with HPAI. Throughout the paper, we refer to the north-western European population excluding the Mediterranean colonies unless otherwise stated, because they may belong to different flyway populations.
Breeding data
For the 2022 breeding season, we collated data on all north-western European Sandwich Tern breeding colonies (N = 67 colonies with 63,116 BP) (see Supplementary material Table S1) by contacting local administrators, site managers, NGOs, scientists, and stakeholders, who together form the European Sandwich Tern Research Group (see author list and acknowledgements for names and affiliations). Specifically, we requested data on the location of colonies, the number of BP, and estimates of breeding success. We furthermore received data from the Mediterranean colonies in Spain (N = 3 colonies, 2,468 BP), southern France (N = 4 colonies, 3,652 BP), and Italy (N = 4 colonies, 2,214 BP). Some colonies in Belgium, the Netherlands, Germany, Denmark, Sweden, and Ireland with 3,484 BP in total formed only late in the season, possibly by 3–4-year-old birds arriving late on the breeding grounds and by birds that lost their first clutch elsewhere. These birds were included in the north-western population data, which means they contributed to the 63,116 BP.
HPAI data
For each colony, we ascertained whether it was affected by HPAI. A colony was classified as being affected by HPAI if adult or juvenile Sandwich Terns were dying in unusual numbers or were observed with the typical clinical signs of HPAI infection, which include debilitation, lethargy, disorientation, loss of flight, opisthotonos, torticollis, and other abnormal behaviours (Rijks et al. Reference Rijks, Leopold, Kühn, In ‘t Veld, Schenk and Brenninkmeijer2022) that lead to death within hours after the onset of clinical signs (N = 39 colonies affected out of 65 colonies with HPAI status data) (Table S1). We further recorded whether dead birds tested positive for HPAI and, if so, for which virus subtype. Where a colony was classified as being affected by HPAI, we recorded the date of first fatality among adults (defined as the onset of the infection in a colony; available for N = 37 out of 39 affected colonies), and the total numbers of dead adult and juvenile Sandwich Terns found. We calculated the percentage of dead adults reported relative to the total number of breeding adult birds and used this as a measure of the severity of the HPAI outbreak. For a subset of colonies (N = 11), we also repeatedly collected (longitudinal) data to reconstruct fatality curves, which represent the cumulative number of adult Sandwich Terns found dead and dying. In some countries, dead adult Sandwich Terns were also counted along the shorelines (Belgium, the Netherlands, and western Germany) and we used these data to extrapolate the number of birds that died outside the colonies (see below for statistical details).
Containment and mitigation strategy data
For affected colonies, we noted whether containment and mitigation measures were implemented, and what these were. The only strategy implemented in colonies with known infection start dates (N = 37) was carcass removal (N = 27) (Table S1). However, the implementation of carcass removal varied between colonies. At some colonies, dead adults were removed every day or every other day, whereas at others, a week or more separated successive carcass removal efforts. Moreover, at some colonies, dead chicks were removed in addition to dead adults. For the final analyses, we dichotomised carcass removal, lumping all carcass removal schemes into one category. While this may seem overly simplistic, it is the most objective way of assessing the impact of carcass removal on survival probability in a colony in general, because accurate data on the extent and timing of carcass removal in all colonies were missing. The observed effect of carcass removal, however, is likely underestimated for the following reasons. (1) It is possible that colonies without carcass removal were not monitored as comprehensively as those with carcass removal, potentially leading to underestimation of total mortality at the former. By the end of the breeding season, when carcasses were counted and cleared at these colonies, many carcasses could have already decomposed, lost in the overgrowing vegetation or been scavenged. (2) In some cases, carcass removal may only have begun once a colony was heavily affected. The positive effect of carcass removal could then have been limited due to the increased mortality and spread prior to implementation, although these colonies would still have been included in the carcass removal treatment group. (3) Where only dead adults were removed, dead chicks may have been another source of infection leading to higher mortality. (4) More regular carcass removal is likely to have had a greater effect on reducing mortality.
Colony-specific environmental data
Specific environmental parameters may be associated with the severity of an HPAI outbreak (e.g. Roche et al. Reference Roche, Lebarbenchon, Gauthier-Clerc, Chang, Thomas and Renaud2009; Domanska-Blicharz et al. Reference Domanska-Blicharz, Minta, Smietanka, Marché and van den Berg2010; Ramey et al. Reference Ramey, Reeves, Drexler, Ackerman, De La Cruz and Lang2020; Camphuysen and Gear Reference Camphuysen and Gear2022; Furness et al. Reference Furness, Gear, Camphuysen, Tyler, de Silva and Warren2023; Pohlmann et al. Reference Pohlmann, Stejskal, King, Bouwhuis, Packmor and Ballstaedt2023). We collected data on the predominant soil type in the colony (six categories), the predominant vegetation density at the beginning of June (six categories), and the distance of the colony to standing brackish or fresh water that is regularly visited by ducks, geese, gulls, and Sandwich Terns for washing or preening. For soil type, we established the categories: (1) gravel; (2) plant material washed ashore; (3) coarse sand or very sandy soil; (4) saltmarsh or clay soil; (5) rock or concrete; (6) other soil types. These categories are ordinal with regard to water permeability. For vegetation density, we used the categories: (1) bare ground; (2) plant material washed ashore; (3) patchy or sparse vegetation; (4) short vegetation; (5) in and around tussocks of long grass (e.g. Lyme Grass Leymus sp. or Marram Grass Ammophila sp.); (6) other vegetation. These categories are ordinal with regard to vegetation density. To help people judge vegetation density and to increase inter-observer agreement, we handed out drone photographs of typical vegetation density categories (Figure S3, Table S2). However, soil type and vegetation density can vary within a colony (and within a season), and we included only a single category per colony that represented the majority within a colony at the time of the outbreak. Of the initially anticipated six categories for each of these variables, some (soil type: plant material washed ashore; vegetation: plant material washed ashore and other vegetation) were not observed. The final number of categories was therefore five for soil type and four for vegetation density.
Data analyses
All statistical analyses were performed in R (v4.2.0) (R Core Team 2022). Spatially explicit models were fitted using the sdmTMB package (v0.1.0; Anderson et al. Reference Anderson, Ward, English and Barnett2022), which allows for the control of spatial covariation between colonies by fitting a spatial random field. To construct the random field, we used geographic coordinates in the equidistant Mercator projection and the R package INLA (v22.12.16; Lindgren and Rue Reference Lindgren and Rue2015). As Sandwich Terns rarely move inland, we included the European coastline (PBSmapping v2.73.2; Schnute et al. Reference Schnute, Boers and Haigh2022) with a buffer of 10 km as a barrier in our spatial random field. Model fit was assessed via visual inspection of the distribution of residuals and using the DHARMa package (v0.4.6; Hartig Reference Hartig2022). We performed sensitivity analyses of the spatial random field to different meshes with varying triangle edge length and extension distance.
We calculated the distance of each colony to the centroid of all colonies using the rgeos package (v0.6-1; Bivand and Rundel Reference Bivand and Rundel2022) and the geosphere package (v1.5-18; Hijmans Reference Hijmans2022).
We assessed whether the probability of a colony becoming infected with HPAI was dependent on the size of that colony (log-transformed number of BP), the distance to the centroid of all colonies (N = 65 colonies with HPAI status information), and the distance to the first three outbreaks, which were most likely independent virus entry events (colonies at St John’s Pool in Scotland, at Langenwerder in Germany, and at Platier d’Oye in France). To do this, we fitted a Generalised Linear Mixed-effects Model (GLMM) with a binomial error structure, taking infection status of a colony (0 = not infected, 1 = infected with HPAI, irrespective of colony size) as the dependent variable, and colony size, distance to the centroid, and distance to first outbreaks as three covariates.
Next, we tested whether the severity of an HPAI outbreak (defined by level of adult mortality) was explained by the onset of the infection in a colony or by the carcass removal containment strategy. To do this, we restricted the data set to HPAI-affected colonies with known infection start date and carcass removal information (N = 37). We fitted a spatially explicit binomial GLMM, using the probability of survival at each colony as our dependent variable. We calculated probability of survival by first subtracting the number of adults reported dead from the total number of breeding individuals (= 2 × BP) to obtain the number of adult birds not reported dead, then dividing this by the total number of breeding individuals (= 2 × BP). In order to incorporate differences in colony size, we included the number of breeding birds per colony (= 2 × BP) as a weights argument. We fitted the infection start date as a covariate and carcass removal as a factor with two levels (yes or no) as our predictors. We transformed both predictors using the rescale function from the arm package (v1.13-1; Gelman and Su Reference Gelman and Su2022), which sets the mean to 0 and divides by two standard deviations to ensure comparability (cf. Gelman Reference Gelman2008). We also fitted an observation-level random effect to account for overdispersion.
Lastly, we assessed whether the severity of an HPAI outbreak was associated with colony-specific environmental variables. To do this, we again restricted the data set to those 37 colonies in which HPAI infection was confirmed and the starting date of the outbreak was documented. We fitted a spatially explicit binomial GLMM with the probability of survival at each colony as our dependent variable, and included the number of breeding birds per colony (= 2 × BP) as a weights argument. As the infection start date and carcass removal were significantly associated with survival probability, we fitted these two predictors and added either soil type (five levels) or vegetation density (four levels) as further explanatory variables. In separate models, we fitted soil type and vegetation density as covariates (using one degree of freedom each). Including an observation-level random effect prevented the model from converging, so this was dropped from these models.
As the infection start date may be mis-specified when a colony is not visited on the day of the first HPAI fatality case, we made use of repeatedly collected (longitudinal) data from 11 colonies to fit logistic curves to the cumulative number of dead adults collected in a colony over time (see Figure 3). For each colony, we estimated the asymptotes and the inflection points of the curves using nonlinear least-squares regression. We used these two variables in a subsequent linear model to assess whether the asymptotes (which represent the severity of the outbreaks) depended on the inflection points (i.e. when half of the total adult mortality had occurred).
In order to predict the number of adult Sandwich Terns that died unreported outside the colonies, we fitted a major axis regression using the R package smatr (v3.4-8; Warton et al. Reference Warton, Duursma, Falster and Taskinen2012). For a subset of countries (N = 3 out of 12 countries in total), we obtained data on the number of Sandwich Terns found dead outside the colonies (see above). We used these data as our dependent variable, and the number of birds found dead inside the colonies as our sole predictor. Subsequently, for all countries, we predicted the number of birds found dead outside the colonies from the number of dead birds inside the colonies. For the subset of countries with data on the number of Sandwich Terns found dead outside the colonies available, the sum of predicted and observed values was the same.
Sero-surveillance and monitoring of HPAI infections in a late-established Sandwich Tern colony
The sero-surveillance work was undertaken as part of the wildlife HPAI disease surveillance programme conducted by the Flemish Government in Belgium. A late breeding colony was established near Zeebrugge (Belgium) in June, which consisted of breeders that had abandoned other HPAI-affected colonies and younger (3–4-year-old) first-time breeders. This was confirmed by sightings of colour rings and head moult patterns: 25 out of the 42 ringed and resighted birds in the colony were 3–4 years old. Out of the 109 birds that were found dead in or near the colony, 36 had a black cap and were presumably young birds, and 73 had white feathers in the cap and were presumed to be older birds (Nehls Reference Nehls1971). On 18 July, when birds were still raising chicks at this colony, 35 adults were caught there with mist-nets at night on the adjacent beach. It is possible that this sample of birds could have included some birds that were prospecting, dispersing or used Zeebrugge as a stopover during migration. Approximately 300 μl of blood was taken from the brachial vein by a trained veterinarian and subsequently the serum was separated from clotted blood by centrifugation (1,227 g for five minutes). Sera were screened by the AI National Reference Laboratory of Belgium for influenza A nucleoprotein-specific antibodies using the ID Screen® NP competition enzyme-linked immunosorbent assay (ELISA) (IDVet, Montpellier, France) according to the manufacturer’s guidelines. The NP-ELISA test is considered positive with competition <45%. NP-ELISA positive samples were subjected to haemagglutination inhibition (HAI) testing with different sets of H5-antigens, which allows the differentiation of Eurasian H5- from an Asian GsGd-clade H5-immune response. The HAI test conformed to Swayne and Brown (Reference Swayne and Brown2022). Reference antigens were diluted at four HA-units and tested with a 0.5 serial dilution of sera under investigation. HAI-values >3 × log2(1/8) were considered as positives. Sensitivity and specificity of NP-ELISA have not been ascertained in wild birds and vary among chickens and ducks (chickens: 95% confidence interval [CI] sensitivity of 0.72–1.00 and 95% CI specificity of 0.91–1.00; ducks: 95% CI sensitivity of 0.72–1.00 and 95% CI specificity of 0.72–1.00). Here, we use the widest CI ranges to obtain confidence intervals (see below).
For the same 35 birds, the presence of avian influenza virus shed via the oral–cloacal route was assessed by the AI National Reference Laboratory of Belgium. Oropharyngeal and cloacal swabs were collected from each bird and were immediately placed in viral transport medium and analysed in pools of a maximum of five individuals. Briefly, viral RNA was extracted from 200 μl swab fluid with the semi-automated Indimag Pathogen Kit® on the IndiMag 48s (Indical Bioscience, Leipzig, Germany). Viral RNA detection was performed by TaqMan® real-time (RT)-polymerase chain reaction (PCR) (ThermoFisher Scientific, Waltham, MA, USA) using a universal oligoset, which detects the highly conserved M-gene, diagnostic for influenza A viruses (Borm et al. Reference Borm, Steensels, Ferreira, Boschmans, De Vriese and Lambrecht2007; Swayne and Brown Reference Swayne and Brown2022). The AgPath-IDTM One-Step RT-PCR kit (ThermoFisher Scientific) was used for amplification of the viral RNA according to the manufacturer’s instructions on the LightCycler® 480 RT-PCR system (Roche, Basel, Switzerland). Sample quality and extraction efficacy were evaluated by the detection of the beta-actin endogenous control (cf. Spackman et al. Reference Spackman, Senne, Myers, Bulaga, Garber and Perdue2002).
We analysed the sero-surveillance data using sensitivity and specificity values asserted in ducks, and estimated the prevalence of seropositive individuals with 95% CIs using the bootComb R package (v1.1.2; Henrion Reference Henrion2021).
Surveys at major roosts
Sandwich Terns show post-breeding dispersal before migrating to their wintering grounds along the western and southern African coasts (Møller Reference Møller1981). Ring recovery data indicate that birds breeding along the shores of the southern North Sea (but also some that breed around the Baltic Sea and, rarely, birds breeding in the UK) gather in Danish North Sea waters in late summer. It has been estimated that 10,000 birds roost on the island Fanø alone every year (Vergin et al. Reference Vergin, Fox, Fischer, Sterup and Bregnballe2024), because of high local prey abundance. This makes Fanø a representative sample of birds breeding along the shores of the southern North Sea, which were most heavily affected by HPAI. From 2020 to 2022, the number of adults and first-year birds (i.e. juvenile birds hatched in the respective year) roosting on Fanø were recorded between July and September.
The percentage of first-year birds at Fanø was analysed with binomial GLMMs in the R package lme4 (v1.1-32; Bates et al. Reference Bates, Mächler, Bolker and Walker2015). The proportion of first-year birds was fitted as the dependent variable, and the number of birds observed as a weights argument. Scaled date was fitted as a quadratic and linear covariate (using the poly() R function) in interaction with year (factor with three levels: 2020, 2021, 2022). Location was included as a random intercept. CIs, marginal effects, and contrasts were estimated using the ggeffects package (v1.2.1; Lüdecke Reference Lüdecke2018).
Results and Discussion
Severity of the HPAI outbreak
We collected data from 67 colonies, comprising a total of 63,116 BP. This corresponds to estimates of the entire known north-western European breeding population (BirdLife International 2015; Buijsman Reference Buijsman2020). HPAI was confirmed in 39 out of 65 colonies (60%) (Figure 1), comprising 74% of BP (N = 46,701). In 32 colonies, dead birds were tested for HPAI infection, and in every instance, H5N1 was confirmed. In total, 16,873 adult Sandwich Terns were reported dead inside or near the affected colonies. This represents 18.1% of breeding adults at affected colonies and 13.4% of breeding birds at all colonies. For a subset of regions, dead adults were also counted along the shorelines away from colonies (Belgium: 59; the Netherlands: 1,600; Lower Saxony in Germany: 598; Schleswig-Holstein in Germany: 196). These were likely birds that died away from their colonies, either on the shoreline or at sea while foraging and subsequently washed up. Using these data, we estimated that an additional 3,658 adult Sandwich Terns died across north-western Europe outside the colonies. We therefore estimated that at least 16.3% (= [16,873 + 3,658] / [2 × 63,116]) of the entire adult north-western European breeding population of Sandwich Terns died during the breeding season of 2022. This figure is likely to be an underestimate of actual total mortality (see below), but nevertheless represents an order of magnitude higher than the normal baseline (pre-HPAI) mortality rate, which has been calculated as ~1.3% (= 8% yearly mortality / 12 months × 2 months of breeding season) (cf. Courtens et al. Reference Courtens, van Daele, Brenninkmeijer, Leopold, Lutterop, Delta, van Bemmelen, Courtens, Collier and Fijn2022). Some birds attempted to breed for a second time after losing their first clutch (cf. Fijn and van Bemmelen Reference Fijn and van Bemmelen2023). Colonies with a total of N = 3,484 BP formed late in the season 2022. Assuming that the majority of these birds attempted to breed a second time, the total number breeding in Europe reduces to 59,632 (= 63,116 – 3,484 BP), and with this number the mortality rate increases to >17%. As many birds were likely lost at sea, beached without being reported, or died on land away from colonies (Cunningham et al. Reference Cunningham, Amandine Gamble, Hart, Humphreys, Philip and Tyler2022), the actual mortality rate is likely to have been substantially higher. Supporting evidence for this comes from standardised seawatching observations conducted in the Netherlands during autumn migration, where passing rates of Sandwich Terns were 70–80% lower compared with previous years (Rijks et al. Reference Rijks, Leopold, Kühn, In ‘t Veld, Schenk and Brenninkmeijer2022). There was also a much-reduced percentage of first-year birds at a major north-western European roost in autumn 2022 (percentages in 2020/21 = 37.2% vs 6.9% in 2022; estimated contrast = 31.0% [95% CI = 25.2%–36.8%], P <0.001) (Figure S1). This is likely due to the fact that most chicks died at affected colonies, either from HPAI infection itself or because at least one of their parents died in the outbreak.

Figure 1. Distribution of Sandwich Tern colonies and mortality rates of adult breeding birds due to HPAI in north-western Europe. Dot size reflects colony size and pie charts represent the percentages of adults with an unknown survival fate (i.e. either survived or died elsewhere; blue) and reported dead (red) in a colony. IE = Ireland, UK = United Kingdom, FR = France, BE = Belgium, NL = the Netherlands, DE = Germany, DK = Denmark, SE = Sweden, PL = Poland, EE = Estonia.
Colony risk factors of getting infected
The HPAI outbreak did not affect all Sandwich Tern colonies in north-western Europe. The risk of a colony being infected increased with its size (β = 0.74 [95% CI = 0.019–1.45], P = 0.044) and with its position relative to the centre of the species’ breeding range in north-western Europe (β = 4.86 [95% CI = 1.33–8.38], P = 0.007) (Figure 1). Specifically, colonies towards the periphery of this range in the Baltic Sea (eastern Sweden and Estonia), in the Irish Sea (western UK and Ireland), and the Atlantic (south-western France) were not affected, whereas the large colonies along the shores of the southern North Sea (northern France, Belgium, the Netherlands, western Germany and south-eastern UK) suffered major losses. The distance to first outbreaks showed a trend in the expected direction (smaller distances corresponded with higher probability of a colony becoming infected (β = 2.41 [95% CI = -0.80–5.62], P = 0.14). Overall, this suggests that adult Sandwich Terns transmitted the virus between colonies, as larger and more central colonies are expected to attract more visitors than smaller, more peripheral ones. Indeed, ring recovery data suggest a high visiting rate of birds from other colonies in a large, central colony (Spaans et al. Reference Spaans, Leopold and Loos2021), and that British and Irish colonies are partially isolated from the continental population (Møller Reference Møller1981; Spina et al. Reference Spina, Baillie, Bairlein, Fiedler and Thorup2022).
Spatiotemporal description of the HPAI outbreak
HPAI was likely introduced into the Sandwich Tern breeding population in 2022 at least three times independently, as there were two simultaneous outbreaks that occurred at the end of April/beginning of May in eastern Germany and in northern Scotland, and a third outbreak in the middle of May in northern France (Figure 2). This was supported by genetic analyses of whole viral genomes (Rijks et al. Reference Rijks, Leopold, Kühn, In ‘t Veld, Schenk and Brenninkmeijer2022; Pohlmann et al. Reference Pohlmann, Stejskal, King, Bouwhuis, Packmor and Ballstaedt2023): the east German outbreak seemed to be caused by a single virus variant (cluster 2 in Pohlmann et al. Reference Pohlmann, Stejskal, King, Bouwhuis, Packmor and Ballstaedt2023), whereas the French and Dutch outbreaks were caused by two variants of HPAI H5N1 clade 2.3.4.4.b (clusters 2 and 3 in Rijks et al. Reference Rijks, Leopold, Kühn, In ‘t Veld, Schenk and Brenninkmeijer2022; Pohlmann et al. Reference Pohlmann, Stejskal, King, Bouwhuis, Packmor and Ballstaedt2023). The variants causing the outbreak in northern Scotland were not determined.
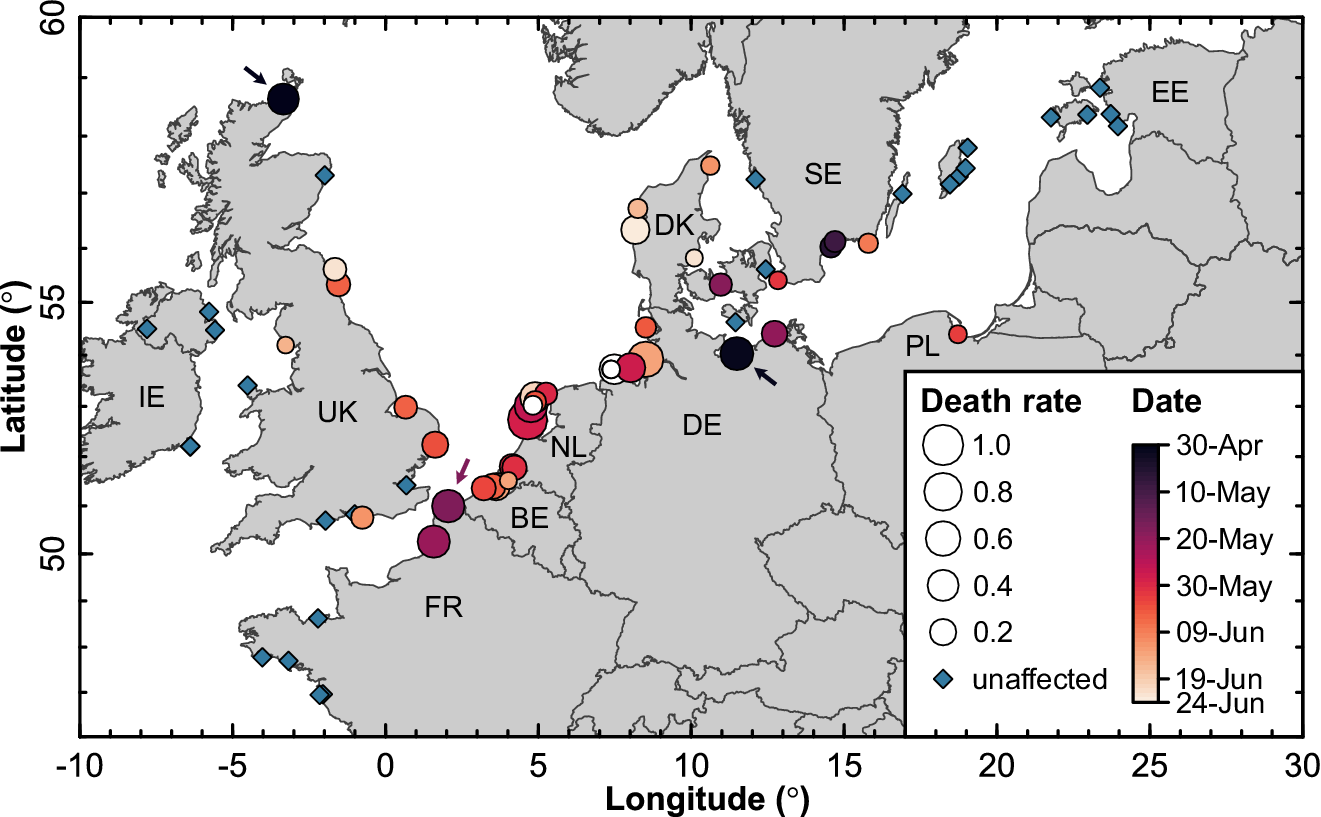
Figure 2. Temporal spread of HPAI in Sandwich Tern colonies across north-western Europe. Dot size reflects the severity of the outbreak (i.e. the percentage of breeding adults that were reported dead in a colony) and colours represent the date of the first dead adult noticed (white: no date recorded). Arrows mark three initial entry points. IE = Ireland, UK = United Kingdom, FR = France, BE = Belgium, NL = the Netherlands, DE = Germany, DK = Denmark, SE = Sweden, PL = Poland, EE = Estonia.
Both variants circulating among Sandwich Terns had previously been isolated from geese and gulls (Rijks et al. Reference Rijks, Leopold, Kühn, In ‘t Veld, Schenk and Brenninkmeijer2022; Pohlmann et al. Reference Pohlmann, Stejskal, King, Bouwhuis, Packmor and Ballstaedt2023). In Scotland and Germany, dead geese were found on site around two weeks prior to the outbreak in Sandwich Terns. The geese in Germany tested positive (those in Scotland were not tested) and this specific variant had been previously isolated from geese (Pohlmann et al. Reference Pohlmann, Stejskal, King, Bouwhuis, Packmor and Ballstaedt2023). In northern France, infected Herring Gulls Larus argentatus were found just before the first cases of Sandwich Terns. As salinity levels of the water bodies surrounding all three colonies are low, contaminated environment is a likely route of introduction of HPAI into the Sandwich Tern population (Domanska-Blicharz et al. Reference Domanska-Blicharz, Minta, Smietanka, Marché and van den Berg2010; Nazir et al. Reference Nazir, Haumacher, Ike, Stumpf, Böhm and Marschang2010; Ahrens et al. Reference Ahrens, Selinka, Mettenleiter, Beer and Harder2022).
In May 2022, the spread of HPAI remained limited to five colonies in the vicinity of the initial two outbreaks in Germany and France. It seems that the outbreak in Scotland did not spread any further, possibly because the colony was isolated and small (N = 100 BP). Genetic data suggest that the eastern German variant circulated among colonies in the southern Baltic Sea for a while and only later infected colonies in northern Denmark (Pohlmann et al. Reference Pohlmann, Stejskal, King, Bouwhuis, Packmor and Ballstaedt2023). This was also confirmed by the temporal data, where Danish colonies were affected only early June (Figure 2). From late May onwards, the spread of the virus accelerated, such that almost all colonies in the Netherlands, Belgium, and Germany were infected within two weeks. Phylogenetic analysis of the variants suggests that this expansion emanated from the French and Dutch outbreaks (Pohlmann et al. Reference Pohlmann, Stejskal, King, Bouwhuis, Packmor and Ballstaedt2023). As it spread along the southern shores of the North Sea, the virus extirpated entire colonies within a week. Early-to-mid June, the wave of infection subsequently reached the eastern coasts of the UK and only receded from the north-western European population in July (Figures 2 and 3), roughly coinciding with the departure of birds from colonies. No mortality was noted in Mediterranean breeding colonies.

Figure 3. Cumulative fatality curves for 11 Sandwich Tern colonies. Depicted are the raw data and parameter estimates with 95% confidence bands derived from a logistic growth model. To aid comparability between colonies of different sizes, the number of adults reported dead per colony was standardised to the total number of breeding birds in a colony. SE = Sweden, NL = the Netherlands, FR = France, DE = Germany, BE = Belgium, UK = United Kingdom.
Seasonal effects on HPAI outbreak dynamics
The severity of the outbreak (measured as the percentage of adult birds that were reported dead within a colony) decreased as the season progressed, with the most apparent decline starting from around the middle of June (β = 1.46 [95% CI = 0.41–2.50], P = 0.006) (Figures 2 and 4). While it is difficult to establish infection start dates retrospectively, we obtained the same result across colonies for which we had sufficient longitudinal data to estimate the inflection points and asymptotes of the fatality curves (which we used as our independent and dependent variables, respectively, N = 11 colonies, P = 0.027) (Figure 3). Among the many variables changing across the breeding season, increasing UV radiation and temperatures towards the summer may have limited the environmental persistence of the virus and thereby reduced the possibility of infections through contaminated environment (Domanska-Blicharz et al. Reference Domanska-Blicharz, Minta, Smietanka, Marché and van den Berg2010; Nazir et al. Reference Nazir, Haumacher, Ike, Stumpf, Böhm and Marschang2010; Ahrens et al. Reference Ahrens, Selinka, Mettenleiter, Beer and Harder2022). Furthermore, the transmission rate might have been limited due to decreasing densities of birds in the colonies, because once the chicks grow older, parents lure them away from the nesting sites (Stienen and Brenninkmeijer Reference Stienen and Brenninkmeijer1999) into potentially less contaminated areas. This behaviour has been interpreted previously as a mechanism to avoid infectious diseases (Cullen Reference Cullen1960).

Figure 4. The effects of carcass removal and the onset of infection on the survival probability within an affected colony. Depicted are the raw data (coloured dots) with dot size reflecting the number of breeding pairs in a colony. Estimated effects of carcass removal (no removal in red, removal in blue) and infection start date with their 95% confidence intervals are derived from a Generalised Linear Mixed-effects Model with a binomial error structure that controls for spatial autocorrelation between colonies and overdispersion.
Effectiveness of carcass removal
While controlling for the start date of the infection (see above) and spatial autocorrelation between colonies (cf. Figure 1), we found that removal of carcasses was associated with reduced adult mortality in affected colonies (β = 1.28 [95% CI = 0.29–2.27], P = 0.011) (Figure 4). This reduction in mortality is quantified at 15% on average. The exact transmission route of the virus from carcasses to living birds is not entirely clear, yet the viral load and infectivity remain high for prolonged times in carcasses (Yamamoto et al. Reference Yamamoto, Nakamura and Mase2017). In 2022, Sandwich Terns were observed actively interacting with dead conspecifics, for example, by pecking or copulating with them (cf. Swift and Marzluff Reference Swift and Marzluff2018), and carcass fluids may also infect soil or water bodies nearby (Keeler et al. Reference Keeler, Dalton, Cressler, Berghaus and Stallknecht2014; Ahrens et al. Reference Ahrens, Selinka, Mettenleiter, Beer and Harder2022).
Environmental effects on HPAI outbreaks
Changes to the breeding habitat may serve as a preventive and mitigation strategy (El-Hacen and Common Wadden Sea Secretariat Reference El-Hacen2022). We evaluated whether the permeability of the soil, vegetation density or the distance to fresh or brackish water had an effect on the severity of the outbreak in affected colonies. None of these explanatory variables had a significant effect (all P > 0.1) (Figure S2).
Testing for immunity
Nine out of the 35 individuals blood sampled at a late established colony in Belgium tested seropositive for Asian H5 antibodies (prevalence = 25.7% [95% CI = 3.1–44.0%]). These results suggest that these birds had gained immunity from an earlier infection. The increasing presence of seropositive individuals in the population may have contributed to the decreasing mortality observed during the progress of the summer. The qPCR test targeting a highly conserved region of the viral genome, diagnostic for influenza A viruses, confirmed the absence of active influenza A virus infections in the examined individuals, meaning that the individuals tested were not shedding virus at the time of sampling and the seropositive individuals were therefore no longer infectious.
Chick mortality
Across all affected colonies monitored during the breeding season in north-western Europe, with the exception of two smaller ones, less than 50% of the chicks survived. Fledging success was mostly below 10%, with more than 95% of all chicks dying (many still in the nest) in 18 infected colonies. This included colonies where only a smaller fraction of breeders was reported dead and adults continued to be present in the colonies. This observation suggests that HPAI easily propagated to chicks, rapidly and most likely airborne from chick to chick, and that chicks had no immunity against the virus.
Age distribution of dead adult birds
Herrmann et al. (Reference Herrmann, Fiedler and Geiter2022) published data on the age distribution of Sandwich Terns reported dead in Germany in 2022. All age classes (birds aged 1–27 years) were present among N = 403 ringed birds, forming the expected age distribution pyramid for this species, i.e. the youngest birds are largely missing because they stay at the wintering grounds in Africa (Møller Reference Møller1981).
Recommendations to breeding-site managers
There are no national or international structures in place that record the total numbers of wild birds dying due to an infection with avian influenza, as this disease is still treated mainly as an economic, agricultural, and human-health related problem, rather than a severe threat to protected wildlife (Kuiken and Cromie Reference Kuiken and Cromie2022). Thus, an active monitoring and surveillance strategy of the virus and associated mortality in wild birds should be implemented to allow for rapid intervention once HPAI is detected in a Sandwich Tern breeding colony or any other wild bird population of conservation concern.
Removing carcasses seems to be an effective containment strategy that should at least be implemented in those colonies that are intensively monitored and managed. The advantages and disadvantages of carcass removal are reviewed in Bregnballe et al. (Reference Bregnballe, Meise and Packmor2023), Scottish Government (2023), and Natural England (publication in progress). Removal schemes should be fully documented (including records of the number and age of the birds found dead and metal- and colour-ring codes). To maximise effectiveness, removal should start as early as possible to reduce the risk of infection from carcasses and to keep disturbance to a minimum. At certain phases of the breeding cycle, entering and disturbing a colony for prolonged periods of time can lead to increased aggression between birds, reduced breeding success, and can cause abandonment of entire colonies. Thus, increased movement and aggression could increase transmission rates, while any infected birds that abandon the colony as a result of disturbance could attempt to breed again elsewhere (Fijn and van Bemmelen Reference Fijn and van Bemmelen2023), thereby spreading HPAI to previously unaffected sites. Thus, also for this reason, carcass removal should start as early as possible.
Breeding colonies must be intensively monitored to detect any early signs of HPAI and access must be granted to those people and organisations looking after the breeding sites in case of an outbreak to be able to monitor impacts and implement containment strategies. It is of vital importance for people working in the colonies to wear adequate personal protective equipment (PPE) to minimise the risk of viral spillover to humans. Only people vaccinated against human flu should work in bird flu-infected colonies to reduce the risk of a recombination event between the mammalian and avian influenza virus. Furthermore, the human influenza vaccination also appears to promote restricted protection against avian influenza (Oshansky et al. Reference Oshansky, Wong, Jeevan, Smallwood, Webby and Shafir2014).
A detailed risk assessment is currently being conducted by an expert group under the direction of the Common Wadden Sea Secretariat and the Friedrich-Loeffler-Institute. Furthermore, an expert group under the direction of the Common Wadden Sea Secretariat has developed guidelines for mitigation and data collection for avian influenza in bird colonies in the Wadden Sea (Bregnballe et al. Reference Bregnballe, Meise and Packmor2023).
Recommendations for further studies
For a better understanding of why Sandwich Terns were particularly susceptible to HPAI, more research on the sources and modes of infection, incubation times, effective containment, and immunity is urgently needed. This includes: (1) population ecology studies on movements of Sandwich Terns between breeding sites (cf. Fijn and van Bemmelen Reference Fijn and van Bemmelen2023; Jeglinski et al. Reference Jeglinski, Lane, Votier, Furness, Hamer and McCafferty2023); (2) detailed survival analyses and demographic modelling using ring recovery data (cf. Boulinier Reference Boulinier2023); (3) the extent of survival and immunity should be assessed in larger samples and more detail in live birds captured at the breeding grounds; (4) containment strategies (e.g. carcass removal) should be documented well (e.g. including experimental designs) to enable a better evaluation of the effects of these measures. In conclusion, the research on the natural history of this species should be extended, which means breeding colonies should remain accessible to scientists.
Conclusions
The impact of the 2022 HPAI H5N1 outbreak across an entire flyway population of a colonially nesting seabird, the Sandwich Tern, is unprecedented. We estimate total mortality of adult Sandwich Terns at >17% of the north-western European breeding population. There were at least three separate introductions of the H5N1 virus into the north-western European Sandwich Tern population during May 2022. Subsequently, the infection spread from two of these entry points and expanded through intraspecific contact within and between breeding sites. Mortality peaked in June and subsequently decreased as the breeding season progressed, possibly due to increased UV radiation and temperature in the summer (decreasing the presence of infectious viral load in the environment), decreasing density of individuals in the colonies (preventing transmission of the virus between individuals), and increasing levels of immunity in the population. Removing carcasses from colonies was an effective containment strategy that lowered the total mortality rate by on average 15%. Assuming a population growth rate of 1.7% per year (Buijsman Reference Buijsman2020), and no further outbreaks, it will take the north-western European population decades to recover from the 2022 HPAI outbreak.
Acknowledgements
We are enormously grateful to all people and organisations contributing data on the HPAI outbreak in the Sandwich Tern breeding season 2022: Antoine Arnaud, Thomas Blanchon, Marco Basso, Amy Burns, Bernard Cadiou, Philippe Carruette, Olivier Enjalbert, Jitske Esselaar, Duncan Halpin, Robin Harvey, Bernd Heinze, Mikael Hellman, Yann Jacob, Luke Johns, Rebecca Jones, Rémi Jullian, Alain Le Dreff, Meelis Leivits, Adeline Leray, Mhairi Maclauchlan, Murray Ochard, Parc Natural del Delta de l’Ebre (Departament de Acció Climàtica, Alimentació y Acció Rural, Generalitat de Catalunya), Patrice Peron, Jean-Roger Perrot, Kathleen Perrot, Laurie Pescayre, Dominique Robart, Carolin Rothfuß, Vincent Rotureau, Philippe Sauvaget, Daryl Short, Alexandre Sibille, Hugh Thurgate, Roberto Tinarelli, Rémi Tiné, Jens Umland, Marc Van de Walle, Nicolas Vanermen, Hilbran Verstraete, Guillaume Villette, Izzy Williamson, Chris Wynne, and Barry Yates. Jan Baert, Lowie Lams, and Annemarie Waerendorff assisted in the serology sampling and Sciensano (Federal Research Institute for Public and Animal Health in Belgium) kindly conducted serological analyses. Sean Anderson provided invaluable support in sdmTMB. We further thank the Common Wadden Sea Secretariat for organising workshops on avian influenza in colonial seabirds. This work was funded by the Common Wadden Sea Secretariat to UK; the Ministry of Infrastructure and Water Management of the Netherlands to RF and RvB; the Ministry of Agriculture, Nature and Food Quality of the Netherlands to SK and ML; OFB, DREAL Occitanie, DREAL PACA to OS; EU LIFE (LIFE on the edge: LIFE19NAT/UK/964) to WS; serological sampling and analyses were financed by the Flemish Agency for Nature and Forests in Belgium.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S0959270923000400.
Author contribution
IA, MB, TB, AB, SB, AC, WC, JD, EE, RF, BH, MH, VH, CH, RtV, EK, UK, MK, SK, KL, RL, NL, ML, SL, LL, RM, HM, WM, PM, SN, PO, FP, KTP, CR, FSc, FSe, OS, LS, AS, JSm, WS, JSt, ES, VS, RGV, RvB, JV, EW, MW collected HPAI data in the field. MV performed serological tests. KF collected data on Danish roosts. UK, WC, and TB designed the study. UK summarised and analysed the data with input from WC. UK and WC wrote the first draft of the manuscript and prepared the final manuscript with input from all authors. All authors approved the final manuscript. Blood samples were taken under the wildlife HPAI disease surveillance of the Flemish Government in Belgium. Data and analyses scripts are available in the Supplementary material and through the Open Science Framework (doi: 10.17605/OSF.IO/Y89K6).





