Introduction
Wound healing within the field of otorhinolaryngology presents significant clinical challenges due to the complex anatomical structures and the critical functional roles of the tissues involved. Successful tissue repair in the mucosal surfaces of the ear, nose and throat is essential for restoring physiological functions such as hearing, breathing and phonation. While traditional surgical techniques often achieve acceptable repair rates, the healing process can be prolonged and is frequently complicated by factors such as infection, graft failure, excessive fibrosis or persistent post-operative pain.Reference Aboelnaga, Elsharnouby, Ali, Elkamshishi and Abdelhafez1, Reference Vignesh, Nirmal Coumare, Gopalakrishnan and Karthikeyan2 These complications not only delay recovery but also significantly impact the patient’s quality of life.
Autologous platelet concentrates (APCs), including platelet-rich plasma (PRP) and platelet-rich fibrin (PRF), have emerged as promising adjunctive therapies aimed at enhancing wound healing outcomes. These preparations use the patient’s own blood to deliver a supraphysiological concentration of platelets, which, upon activation, release a substantial pool of bioactive growth factors and cytokines.Reference Sari, Karaketir, Kumral, Akgun, Gurpinar and Hanci3 Initially, the clinical rationale for using APCs focused primarily on their structural benefits specifically their ability to accelerate graft uptake and promote epithelialisation through the release of growth factors such as platelet-derived growth factor (PDGF) and transforming growth factor-beta 1 (TGF-β1).Reference Sari, Karaketir, Kumral, Akgun, Gurpinar and Hanci3, Reference Steiner, Vozel, Bozanic Urbancic, Troha, Lazar and Kralj-Iglic4
However, recent clinical observations suggest that the therapeutic utility of APCs extends beyond mere structural repair. Increasing evidence indicates that APCs possess significant immunomodulatory properties that may directly influence post-operative morbidity. Recent randomised controlled trials have highlighted a reduction in post-operative pain and mucosal oedema as critical outcome measures, suggesting a complex biological interplay.Reference Steiner, Vozel, Bozanic Urbancic, Troha, Lazar and Kralj-Iglic4, Reference Wiriyaamornchai and Santeerapharp5 Theoretically, this modulation is driven by the regulation of the inflammatory microenvironment, where APCs may help balance pro-inflammatory cytokines, such as interleukin-6 (IL-6), with tissue-remodeling enzymes like matrix metalloproteinases (MMP-9).Reference Tulaci, Yayman, Arslan, Canakci, Tulaci and Turan6 Despite the widespread clinical adoption of APCs in otorhinolaryngological surgeries, the correlation between these specific clinical improvements and their underlying biological mechanisms remains to be fully elucidated.
The current literature reveals heterogeneity in clinical outcomes, likely attributable to variations in preparation protocols and surgical applications.Reference Lipovec, Kapadia, Antonoglou, EMC, El-Sayed and Nibali7 Furthermore, the emergence of new randomised controlled trials (RCTs) in 2024 and 2025 necessitates an updated synthesis of the evidence to validate these evolving clinical perspectives.Reference Alahmadi, Lajdam, Aghashami, Hamdan, Almalki and Altalhi8, Reference Saini, Khan, Kumar, Singh and Pandey9 Therefore, this systematic review aims to comprehensively evaluate the clinical efficacy of autologous platelet concentrates on tissue regeneration outcomes in otorhinolaryngology. Additionally, it seeks to discuss the potential biological mechanisms, including immunomodulation and growth factor activity, that underlie these observed therapeutic benefits.
Methods
Literature search strategy
This systematic review was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. The review protocol focused on evaluating the clinical efficacy of APCs in enhancing tissue regeneration and reducing post-operative morbidity in otorhinolaryngological procedures.
Search Strategy A comprehensive systematic literature search was performed across three major electronic databases: PubMed, ScienceDirect and the Cochrane Library. To ensure the inclusion of the most recent evidence, the search spanned from database inception up to November 2025. The search strategy used a combination of Medical Subject Headings (MeSH) terms and free-text keywords, including: “Platelet-rich plasma” OR “PRP”, “Platelet-rich fibrin” OR “PRF”, “Autologous platelet concentrates”, “Otorhinolaryngology”, “ENT surgery”, “Tympanoplasty”, “Myringoplasty”, “Endoscopic sinus surgery”, “Tonsillectomy”, “Wound healing”, “Tissue regeneration” and “Graft uptake”. Reference lists of retrieved articles and relevant systematic reviews were also manually screened to identify additional eligible studies.
Eligibility criteria
Studies were deemed eligible for inclusion if they met the following criteria: (1) study design: RCTs and prospective comparative studies; (2) population: patients undergoing otorhinolaryngological surgical procedures, including otological, rhinological and laryngological interventions; (3) intervention: application of autologous platelet concentrates (e.g., PRP, PRF) compared with conventional treatment or placebo; and (4) outcomes: assessment of clinical wound healing parameters (e.g., graft uptake rates, epithelialisation) or post-operative morbidity outcomes, specifically pain scores and mucosal oedema. Studies were excluded if they were case reports, case series with fewer than 10 patients, reviews or editorials. Animal and in vitro studies were also excluded from the primary clinical analysis, although relevant preclinical data were consulted to support the discussion of biological mechanisms. Furthermore, studies using non-autologous or synthetic platelet products were omitted from this review.
PICO framework
The present systematic review was structured based on the PICO (Population, Intervention, Comparison, Outcome) framework to ensure a focused and systematic analysis of the available evidence. The components of the PICO framework are summarised in Table 1.
Table 1. PICO framework

Study selection
All identified records from the electronic databases were imported into a reference management software to facilitate the screening process. After the removal of duplicates, two independent reviewers screened the titles and abstracts of the retrieved records against the pre-defined eligibility criteria. Articles considered potentially relevant were retrieved in full text for a detailed assessment. The final decision on inclusion was made based on the full-text review. Any discrepancies or disagreements between the reviewers regarding study selection were resolved through discussion and consensus. This comprehensive selection process is illustrated in the PRISMA flow diagram (Figure 1).

Figure 1. PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) flow diagram of the study selection process.
Data extraction
Data extraction was performed independently by two reviewers using a pre-designed standardised form to ensure consistency and accuracy. The following variables were extracted from each included study: (1) study characteristics: first author, year of publication, country, study design and sample size; (2) intervention details: type of autologous platelet concentrate used (e.g., PRP, PRF), preparation protocols (centrifugation speed and time) and method of application; (3) clinical outcomes: primary data on structural tissue regeneration (e.g., graft uptake rates, perforation closure and epithelialisation time) and secondary data on post-operative morbidity (e.g., Visual Analog Scale [VAS] pain scores, mucosal oedema); and (4) proposed mechanisms: any reported or hypothesised biological pathways, including growth factor activity and immunomodulation. Any discrepancies in the extracted data were resolved through discussion between the reviewers until a consensus was reached.
Risk of bias and study quality assessment
The methodological quality of the included studies was assessed independently by two reviewers using tools appropriate for each study design. For RCTs, the Cochrane Risk of Bias Tool (RoB 2) was employed to evaluate domains such as randomisation process, deviations from intended interventions, missing outcome data, measurement of the outcome and selection of the reported result. For non-randomised prospective studies, the Newcastle–Ottawa Scale (NOS) was used to assess the selection of study groups, comparability of the groups and ascertainment of the outcome of interest. Any disagreements in the quality assessment were resolved through discussion and consensus.
Data synthesis
Given the significant heterogeneity across the included studies regarding study designs, types of platelet concentrates (PRP, PRF and CGF), preparation protocols and surgical applications, a statistical meta-analysis was not feasible. Consequently, a narrative synthesis was conducted to summarise the findings. The data were tabulated and categorised according to the anatomical sub-specialty (otology, rhinology and laryngology/head and neck). Clinical outcomes were synthesised descriptively, focusing on the direction of effect (positive, negative or neutral) regarding tissue regeneration and post-operative morbidity. Additionally, reported biological mechanisms were qualitatively analysed to identify common molecular pathways across the studies.
Results
Article search results
The systematic literature search conducted across three major electronic databases: PubMed (n = 142), Scopus (n = 161) and the Cochrane Library (n = 12) yielded a total of 315 records. After removing 105 duplicates, 210 records remained for screening based on titles and abstracts. During this initial screening phase, 170 records were excluded, primarily because they focused on oral and maxillofacial/dental procedures (e.g., alveolar ridge preservation, dental extraction) rather than specific otorhinolaryngological applications, or were animal and in vitro studies. Consequently, 40 full-text articles were retrieved and assessed for eligibility. Upon detailed examination, 28 articles were excluded due to reasons such as: insufficient quantitative data on specific clinical wound healing or morbidity outcomes (n = 14), retrospective or non-compliant study designs (n = 8) and the use of synthetic or non-autologous platelet products (n = 6). Ultimately, 12 studies met the inclusion criteria and were included in the qualitative synthesis.
Risk of bias assessment
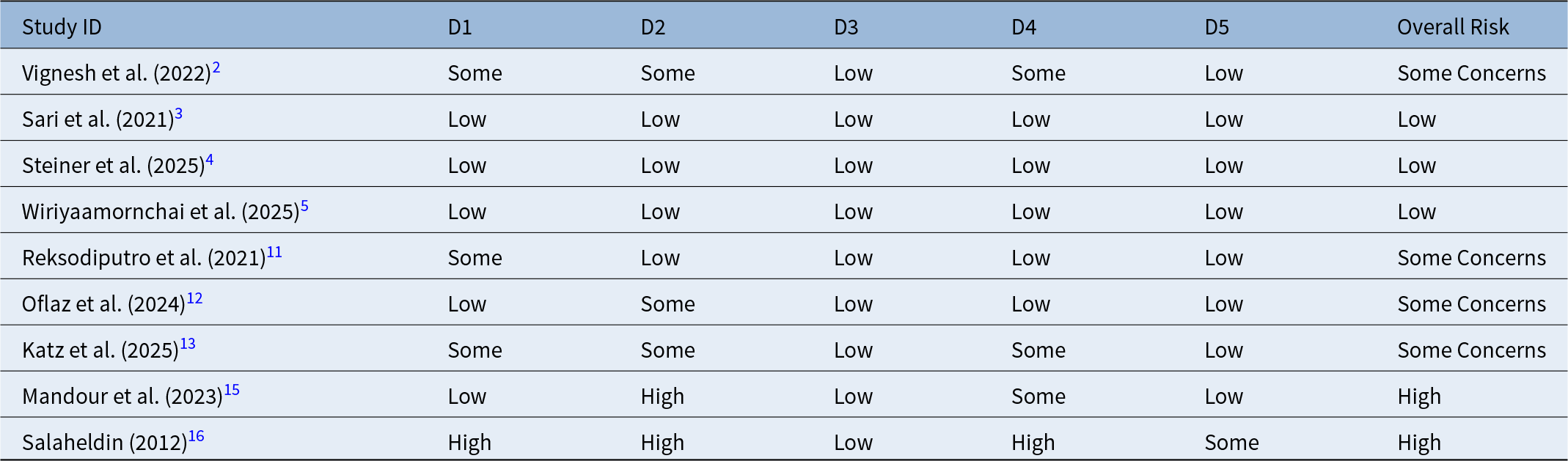
The methodological quality of the nine included RCTs was evaluated using the Cochrane Risk of Bias 2 (RoB 2) tool. The assessment revealed a variable risk profile across the studies. The domains related to “randomisation process” and “deviations from intended interventions” were the most frequent sources of bias, largely due to the inherent difficulty in blinding the surgical personnel to the intervention. However, recent trials, specifically Steiner et al. (2025) and Wiriyaamornchai et al. (2025) demonstrated a “low risk” of bias, employing rigorous double-blind protocols where both patients and outcome assessors were masked to the treatment allocation.Reference Steiner, Vozel, Bozanic Urbancic, Troha, Lazar and Kralj-Iglic4, Reference Wiriyaamornchai and Santeerapharp5 A summary of the risk of bias distribution for RCTs is presented in Table 2.
Table 2. Risk of bias assessment of included randomised controlled trials using Cochrane RoB 2 Tool

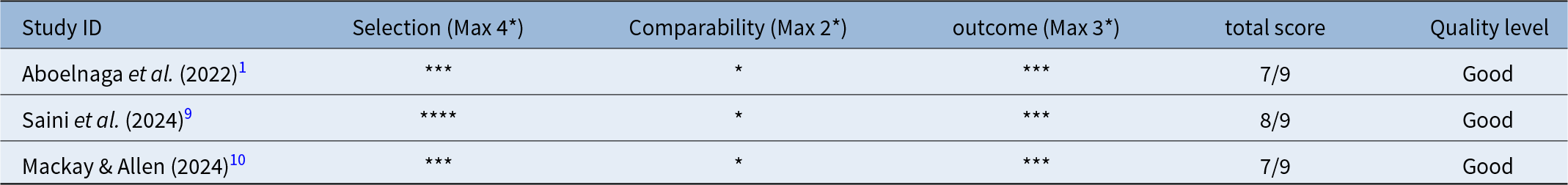
For the three non-randomised prospective studies: Aboelnaga et al., 2022; Saini et al., 2024; Mackay and Allen, 2024, quality was assessed using the NOS.Reference Aboelnaga, Elsharnouby, Ali, Elkamshishi and Abdelhafez1, Reference Saini, Khan, Kumar, Singh and Pandey9, Reference Mackay and Allen10 These studies generally achieved good quality scores (seven to eight out of nine stars), particularly in the Selection and Outcome domains, indicating representative patient cohorts and accurate follow-up. However, they scored lower in the Comparability domain due to the lack of stringent matching for confounding variables. The quality assessment for these studies is detailed in Table 3.
Table 3. Quality assessment of non-randomised prospective studies using Newcastle–Ottawa Scale

Study characteristics
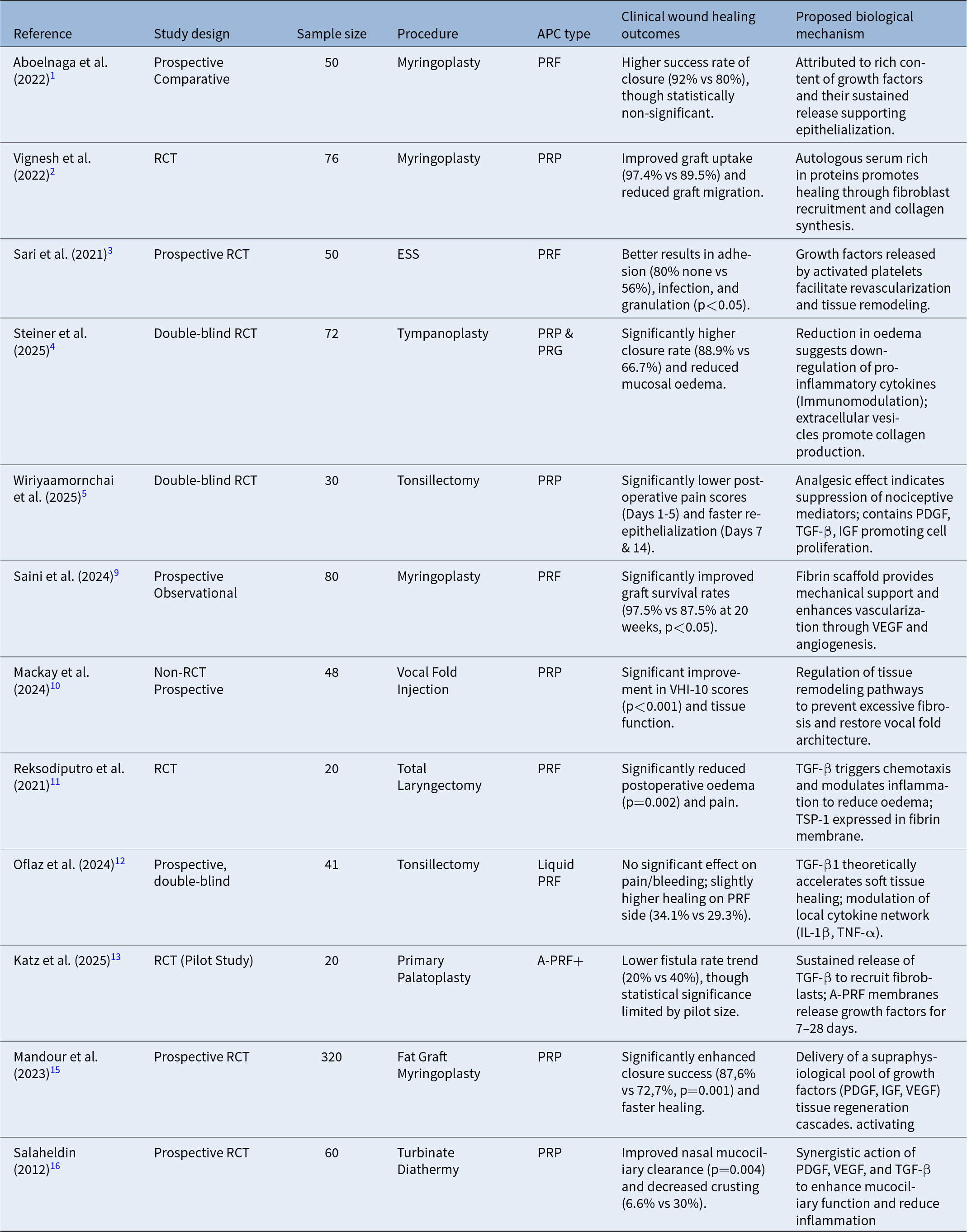
The 12 included studies varied in design, with most being prospective and randomised (e.g. Steiner et al., 2025; Wiriyaamornchai et al., 2025; Reksodiputro et al., 2021; Oflaz çapar et al., 2024).Reference Steiner, Vozel, Bozanic Urbancic, Troha, Lazar and Kralj-Iglic4, Reference Wiriyaamornchai and Santeerapharp5, Reference Reksodiputro, Hutauruk, Widodo, Fardizza and Mutia11, Reference Oflaz Çapar, Solguntekin, Kökoğlu and Şahin12 While some were non-randomised prospective studies (e.g., Mackay and Allen, 2024).Reference Mackay and Allen10 Sample sizes were heterogeneous, ranging from 20 to 320 participants, with smaller studies often serving as pilot trials and larger cohorts providing more robust clinical data.Reference Katz, Ooms, Winnand, Heitzer, Bock and Schaffrath13, Reference Elabassi, Aglan, Aske and Mandour14 The characteristics of the included studies are summarised in Table 4.
Table 4. Summary of study characteristics and clinical outcomes

APC = autologous platelet concentrates; IGF = insulin-like growth factor; IL = interleukin; PDGF = platelet-derived growth factor; PRF = platelet-rich fibrin; PRP, platelet-rich plasma; RCT = randomised controlled trial; TGF-β = transforming growth factor-beta; TNF-α = tumour necrosis factor-α; VEGF = vascular endothelial growth factor.
APCs were applied across diverse ORL procedures, including otology (myringoplasty, tympanoplasty), rhinology (endoscopic sinus surgery) and laryngology (tonsillectomy, total laryngectomy). The introduction of recent high-quality RCTs, such as those by Steiner et al. (2025) and Wiriyaamornchai et al. (2025).Reference Steiner, Vozel, Bozanic Urbancic, Troha, Lazar and Kralj-Iglic4, Reference Wiriyaamornchai and Santeerapharp5 strengthens the evidence base, particularly for outcomes related to post-operative morbidity (pain and oedema).
Clinically, APCs consistently improved key healing outcomes such as graft uptake and accelerated epithelialisation. However, the most notable and consistent clinical finding across the studies is the significant reduction in post-operative morbidity, including lower pain scores and reduced mucosal oedema.Reference Sari, Karaketir, Kumral, Akgun, Gurpinar and Hanci3, Reference Saini, Khan, Kumar, Singh and Pandey9, Reference Mackay and Allen10 While the proliferative effect of TGF-β1 remains the known regenerative foundation, the potent clinical effect on reducing pain and oedema strongly suggests a predominant role of immunomodulation in suppressing the inflammatory microenvironment.Reference Mackay and Allen10, Reference Reksodiputro, Hutauruk, Widodo, Fardizza and Mutia11 Importantly, no significant adverse effects were reported across the included cohort, underscoring the safety of these autologous preparations.Reference Aboelnaga, Elsharnouby, Ali, Elkamshishi and Abdelhafez1, Reference Vignesh, Nirmal Coumare, Gopalakrishnan and Karthikeyan2, Reference Saini, Khan, Kumar, Singh and Pandey9
Synthesis of results by otorhinolaryngology sub-specialty
Otology (myringoplasty and tympanoplasty)
The application of APCs in otological procedures, predominantly myringoplasty, demonstrated the most consistent evidence for enhancing healing. Of the included studies, six focused on tympanic membrane repair.Reference Aboelnaga, Elsharnouby, Ali, Elkamshishi and Abdelhafez1, Reference Vignesh, Nirmal Coumare, Gopalakrishnan and Karthikeyan2, Reference Steiner, Vozel, Bozanic Urbancic, Troha, Lazar and Kralj-Iglic4, Reference Saini, Khan, Kumar, Singh and Pandey9, Reference Mandour, Elsheikh, Amer, Elzayat, Barbara and Covelli15 The primary clinical outcome graft uptake was significantly improved with the use of APCs, particularly PRF, with success rates typically exceeding 90 per cent.Reference Saini, Khan, Kumar, Singh and Pandey9, Reference Mandour, Elsheikh, Amer, Elzayat, Barbara and Covelli15
More importantly, the high-quality RCT by Steiner et al. (2025) which used PRP/PRG, reported a significantly higher closure rate (88.9 per cent vs 66.7 per cent in controls) and critically, a marked reduction in mucosal oedema in the APC group during the early post-operative phase.Reference Steiner, Vozel, Bozanic Urbancic, Troha, Lazar and Kralj-Iglic4 This reduction in local inflammation strongly suggests that the benefit of APCs in the middle ear environment extends beyond mechanical regeneration, proposing an immunomodulatory role in suppressing acute inflammatory responses. No study reported differences in pure tone average (PTA) or air–bone gap (ABG) improvement that reached clinical significance, suggesting APCs primarily influence tissue healing and acute morbidity.
Laryngology (tonsillectomy and laryngeal procedures)
In the field of laryngology, the efficacy of APCs primarily relates to controlling post-operative morbidity and enhancing soft tissue recovery. The application in tonsillectomy, a procedure frequently associated with severe post-operative pain, demonstrated key benefits. Specifically, the high-quality randomised controlled trial by Wiriyaamornchai et al. (2025) using PRP, reported a significant reduction in post-operative pain scores (VAS) during the critical first five days, coupled with faster re-epithelialisation.Reference Wiriyaamornchai and Santeerapharp5 This consistent analgesic effect supports a strong immunomodulatory role for APCs in suppressing acute inflammatory mediators at the wound bed.
For complex resective surgeries, such as total laryngectomy, PRF application enhanced the overall wound healing process, showing specific efficacy in reducing both oedema and pain in the post-operative period.Reference Reksodiputro, Hutauruk, Widodo, Fardizza and Mutia11 Furthermore, in benign vocal pathologies, PRP injection showed promise in improving vocal fold tissue function.Reference Mackay and Allen10 This benefit is hypothesised to be mediated by the anti-fibrotic properties of growth factors, which regulate excessive scar formation.Reference Mackay and Allen10 Collectively, the findings in laryngology highlight the potential of APCs not just for structural tissue repair, but also for accelerating functional restoration and patient comfort.
Rhinology (endoscopic sinus surgery and nasal procedures)
In the field of rhinology, the application of APCs has demonstrated significant benefits in accelerating post-operative mucosal healing and reducing complications. Studies investigating endoscopic sinus surgery (ESS) found that the use of PRF can reduce adhesion formation, bleeding and granulation.Reference Sari, Karaketir, Kumral, Akgun, Gurpinar and Hanci3 This benefit is primarily attributed to the three-dimensional fibrin matrix of PRF, which acts as an ideal scaffold for sinonasal mucosal regeneration, a structure deemed suitable for the longer healing timeline of the sinus cavity.
Furthermore, in other nasal procedures such as inferior turbinate submucous diathermy, PRP application showed improvement in nasal mucociliary clearance and decreased the incidence of post-operative bleeding and crust formation.Reference Salaheldin and Hussein16 This effect underscores the advantage of PRP’s rapid growth factor release, which is more appropriate for procedures requiring quick surface epithelialisation. Overall, the findings in rhinology suggest that PRF is more beneficial in complex sinus cases (for mechanical support), while PRP is effective for faster superficial mucosal recovery.
Discussion
This systematic review of 12 studies demonstrates that APCs are effective therapeutic adjuncts across diverse otorhinolaryngological procedures. The included studies, which encompass high-quality RCTs from 2024 and 2025, consistently highlight two major clinical benefits: the enhancement of structural tissue regeneration and, significantly, the reduction of post-operative morbidity. While previous reviews have primarily focused on graft uptake rates, our updated analysis identifies a paradigm shift towards the use of APCs as potent agents for pain and oedema control, particularly in mucosal surgeries.Reference Steiner, Vozel, Bozanic Urbancic, Troha, Lazar and Kralj-Iglic4, Reference Wiriyaamornchai and Santeerapharp5
The variability in clinical outcomes across different procedures can be largely explained by the differential release kinetics of growth factors between PRP and PRF. Our analysis supports a dual-mechanism hypothesis. PRP is characterised by a rapid “burst release” of bioactive factors upon activation.Reference Amable, Carias, Teixeira, da Cruz Pacheco, Corrêa do Amaral and Granjeiro17 This immediate release provides a high concentration of anti-inflammatory cytokines during the critical first 24–48 hours post-surgery, which correlates perfectly with the significant reduction in acute pain scores observed in tonsillectomy patients.Reference Wiriyaamornchai and Santeerapharp5, Reference Oflaz Çapar, Solguntekin, Kökoğlu and Şahin12 In contrast, PRF contains a dense fibrin matrix that traps growth factors and allows for a slower, sustained release over 7–14 days.Reference Dohan Ehrenfest, de Peppo, Doglioli and Sammartino18 This prolonged release profile acts as a “biological scaffold,” which is mechanistically essential for the vascularisation and integration of tympanic grafts in myringoplasty, where the healing process requires sustained support rather than immediate symptom relief.Reference Aboelnaga, Elsharnouby, Ali, Elkamshishi and Abdelhafez1, Reference Saini, Khan, Kumar, Singh and Pandey9
Beyond growth factor release, the rapid suppression of mucosal oedema reported in recent trials strongly suggests a dominant immunomodulatory role for APCs. The observed reduction in oedema and post-operative pain challenges the traditional view that APCs function solely through proliferation. Instead, the evidence suggests that APCs actively reprogram the local inflammatory microenvironment.Reference Reksodiputro, Hutauruk, Widodo, Fardizza and Mutia11 This is hypothesised to involve the localised downregulation of pro-inflammatory cytokines, such as IL-6, which are typically elevated in acute surgical trauma.Reference Steiner, Vozel, Bozanic Urbancic, Troha, Lazar and Kralj-Iglic4 Furthermore, the accelerated epithelialisation observed in mucosal defects suggests a balanced regulation of MMP-9, preventing excessive tissue degradation and facilitating a smoother transition from the inflammatory to the proliferative phase.Reference Mackay and Allen10 Preclinical models corroborate this, showing that APC application significantly reduces inflammatory cell infiltration and oxidative stress in nasal mucosa.Reference Tulaci, Yayman, Arslan, Canakci, Tulaci and Turan6
The aggregate data from six studies confirms that APCs, particularly PRF, significantly improve graft uptake rates (up to 97.5 per cent) compared to conventional methods.Reference Saini, Khan, Kumar, Singh and Pandey9, Reference Mandour, Elsheikh, Amer, Elzayat, Barbara and Covelli15 The fibrin matrix appears to stabilise the graft in the avascular environment of the tympanic membrane, consistent with recent meta-analytic findings.Reference Alahmadi, Lajdam, Aghashami, Hamdan, Almalki and Altalhi8 In Rhinology, the use of APCs in endoscopic sinus surgery has been shown to reduce adhesion formation and bleeding, attributed to the enhancement of mucosal re-epithelialisation.Reference Sari, Karaketir, Kumral, Akgun, Gurpinar and Hanci3 Meanwhile, in Laryngology, specifically tonsillectomy, the primary benefit is analgesic, with PRP significantly lowering pain scores and accelerating the return to normal diet.Reference Wiriyaamornchai and Santeerapharp5
Despite these promising findings, a critical limitation identified in the current literature is the scarcity of direct molecular analysis alongside clinical outcomes. As highlighted by our systematic search analysis of 40 relevant records, there remains a substantial evidence gap regarding biomarker quantification in otorhinolaryngological APC studies. Specifically, systematic data indicate that only 12.5 per cent of studies measured IL-6, merely 5 per cent quantified TGF-β and none (0 per cent) directly measured MMP-9 levels in patient tissues. Consequently, the biological mechanisms discussed herein are theoretically inferred from clinical phenotypes rather than confirmed by direct quantification in the included human subjects. Furthermore, the lack of standardisation in preparation protocols (e.g., centrifugation speed and time) remains a barrier to reproducibility, as variations in G-force can significantly alter the leukocyte and growth factor content of the final product.Reference Lipovec, Kapadia, Antonoglou, EMC, El-Sayed and Nibali7
What is already known on this topic
• Autologous platelet concentrates (APCs), including platelet-rich plasma (PRP) and platelet-rich fibrin (PRF), are increasingly used in otological, rhinological, and laryngological surgery to enhance graft uptake and mucosal healing
• Existing clinical studies mainly focus on structural outcomes (e.g., graft closure, epithelialization), with heterogeneous preparation protocols and limited systematic evaluation of post-operative pain, oedema or mechanistic biomarkers
What this paper adds
• This systematic review of 12 prospective and randomised studies demonstrates that autologous platelet concentrates (APCs) consistently improve tissue regeneration and, importantly, reduce post-operative morbidity, particularly pain and mucosal oedema after myringoplasty, tympanoplasty and tonsillectomy
• The synthesis supports a procedure-specific role of PRF for sustained graft support and PRP for rapid symptom control, and proposes a predominant immunomodulatory mechanism involving downregulation of interleukin-6 (IL-6) and regulation of matrix metalloproteinase-9 (MMP-9) activity
• The review identifies critical gaps in standardized APC preparation and biomarker quantification, defining clear priorities for future translational trials in otorhinolaryngology
Conclusion
This systematic review establishes that APCs are effective adjunctive therapies in otorhinolaryngology, offering significant benefits not only for structural tissue regeneration but also for reducing post-operative morbidity. The evidence supports a procedure-specific application strategy: PRF is optimal for otological and complex sinus procedures requiring sustained scaffolding and prolonged growth factor release to ensure graft integration. Conversely, PRP demonstrates superior utility in mucosal surgeries, such as tonsillectomy and turbinate reduction, where its rapid “burst release” of bioactive factors provides immediate analgesic and anti-inflammatory effects.
Furthermore, the consistent reduction in acute mucosal oedema and pain observed in recent high-quality trials suggests that APCs function through a potent immunomodulatory mechanism, likely involving the downregulation of pro-inflammatory cytokines like IL-6 and the regulation of MMP-9 activity. While the safety profile of APCs is excellent, future clinical practice and research must prioritise the standardisation of preparation protocols and the systematic quantification of biomarkers to maximise therapeutic reproducibility and validate these molecular pathways.
Acknowledgement
The authors express gratitude to Tanti Agustina for her assistance in organizing the literature data.
Competing interests
The authors declare that they have no conflicts of interest.
PROSPERO registration statement
This systematic review has been registered in the International Prospective Register of Systematic Reviews (PROSPERO) under the registration number CRD420251137161.
Funding
Article processing charge are covered by Universitas Brawijaya.