Introduction
In Australia, approximately 440 people per day are diagnosed with cancer, and more than 1 million people are living with a diagnosis of cancer (cancer survivors) (Australian Institute of Health and Welfare 2022). Globally, by 2040, an estimated 26 billion people will be cancer survivors (Shapiro Reference Shapiro2018), with up to 50% experiencing pain (Haumann et al. Reference Haumann, Joosten and Everdingen2017; Shapiro Reference Shapiro2018). The etiology of pain in cancer patients is diverse, relating to the disease, its treatment, or an unrelated comorbidity (Glare et al. Reference Glare, Davies and Finlay2014; Van Den Beuken-Van Everdingen et al. Reference Van Den Beuken-Van Everdingen, Hochstenbach and Joosten2016). Pain may have a nociceptive mechanism (e.g., due to ongoing tissue damage), or may have neuropathic or mixed contributions, sustained by aberrant somatosensory processing in the peripheral or central nervous systems (Falk et al. Reference Falk, Bannister and Dickenson2014; Rasmussen et al. Reference Rasmussen, Madeleine and Arroyo-Morales2023).
While opioids have been established as the cornerstone of pain management for patients with moderate to severe cancer pain (Organization Reference Organization2018), they have limited efficacy and there are concerns of opioid-induced hyperalgesia (Yi and Pryzbylkowski Reference Yi and Pryzbylkowski2015; Higgins et al. Reference Higgins, Smith and Matthews2019), other opioid-related adverse effects (Glare et al. Reference Glare, Davies and Finlay2014), and potential roles in tumor progression (Singleton et al. Reference Singleton, Moss and Karp2015). As the prognosis of cancer improves, patients are living longer, raising concerns about prolonged use (tolerance, addiction, accidental overdose) as in nonmalignant pain populations (Farquhar-Smith and Brown Reference Brown and Farquhar-Smith2017). Therefore, there is an increasing shift toward a multimodal approach to managing cancer pain, including utilization of interventional pain procedures (Glare et al. Reference Glare, Davies and Finlay2014; Lee et al. Reference Lee, Roy and Laszlo2021).
Despite the potential benefits of these interventional procedures, clinician practice, including the timing of referral and referral pathways, appears to vary significantly. Furthermore, outcomes from such procedures are not routinely collected and published. While oncology supportive care services serve as an emerging model to address the issue of fragmented symptom care (Hui et al. Reference Hui, Hoge and Bruera2021), little attention has been paid to interventional pain services within the model. As such, we have established a pilot cancer pain intervention database at 2 tertiary centers in Sydney, Australia. Our vision is that data from this registry will guide and assist decision making in the clinical pathway for the treatment of cancer pain.
The purpose of this study was to use this pilot cancer pain intervention database to assess the feasibility of developing a registry for cancer pain intervention. Such a database will allow timely evaluation of clinical outcomes and practice at a local level, thus helping to improve future patient care.
Methods
This was a pilot, prospective, longitudinal clinical registry of patients undergoing interventional procedures to manage cancer pain at 2 centers in Sydney (Chris O’Brien Lifehouse and Royal Prince Alfred Hospital). The study was conducted between October 2021 and March 2023. The study conformed to the principles outlined in the Declaration of Helsinki and was approved by the Sydney Local Health District Human Research Ethics Committee, Australia (2021/ETH11313).
Registry development
The pilot registry, including variables to be included, was developed by a pain specialist (YCL) and an anesthetist/biostatistician (AW) in conjunction with cancer care providers working in palliative care and pain medicine at Chris O’Brien Lifehouse and Royal Prince Alfred Hospital. The assessment instruments were chosen for their relevance in cancer populations, ease and practicality of use, and validity in this setting. Registry data were collected and managed using Research Electronic Data Capture (REDCap) Software hosted at Sydney Local Health District (Harris et al. Reference Harris, Taylor and Thielke2009, Reference Harris, Taylor and Minor2019). REDCap is a secure, web-based software platform designed by Vanderbilt University to support data capture for clinical research. It provides an intuitive interface for validated data capture; audit trails that allow tracking of data manipulation and export procedures; automated export procedures to facilitate seamless data downloads to common statistical packages; and procedures for importing data from external sources. Participants either completed a paper form or were emailed a link that allowed them to enter the data directly into REDCap. Information on the paper forms was entered into REDCap by the study staff.
Participants
All patients undergoing interventional procedures for the management of pain associated with cancer or cancer treatment at the 2 participating centers were included in the registry under an “opt-out” consent process. Due to the nature of the registry, an opt-out approach to consent was taken to maximize data capture, minimize biased sampling, while still ensuring each participant had the time to consider if they did not wish to have their data collected. All potential participants were provided with written information about the registry during a routine clinic visit by one of their treating clinicians.
Registry data collection (variables)
Data collection for an individual patient commenced at the time of scheduling their interventional procedure. At baseline, defined as anytime before the intervention (usually within 2 weeks), information was collected on the patient’s demographics, cancer-related characteristics (type, stage, duration, treatment, recurrence, and Australia-modified Karnofsky Performance Status [AKPS]) (Abernethy et al. Reference Abernethy, Shelby-James and Fazekas2005); pain-related attributes (duration, type of pain, severity, distress, disability, and psychological mediators of distress and disability); medication use; symptom burden (using the Patient-Reported Outcome Measurement Information System – Pain Intensity and Pain Interference questionnaire [PROMIS-PI]; Amtmann et al. Reference Amtmann, Cook and Jensen2010; Khanna et al. Reference Khanna, Krishnan and Dewitt2011; Habibi et al. Reference Habibi, Karia and Ward2023); and quality of life (using the European Organization for Research and Treatment of Cancer Quality of Life Questionnire-Core-15-Palliative Care [EORTC QLQ-C15-PAL]; Fayers et al. Reference Fayers, Aaronson and Bjordal2001; Karen et al. Reference Karen, Mogens and Morten Aagaard2006).
The AKPS is an amalgam of the original Karnofsky Performance Status (KPS) and the Thorne-modified KPS, which is commonly used in inpatient and community palliative care settings to assist with clinical decision making (Abernethy et al. Reference Abernethy, Shelby-James and Fazekas2005). The AKPS is a categorical scale with 11 levels, which range from 100 – normal; no complaints; no evidence of disease to 0 – dead (Abernethy et al. Reference Abernethy, Shelby-James and Fazekas2005).
PROMIS-PI measures the self-reported consequences of pain on a person’s life, including pain intensity and pain interference.
EORTC QLQ-C15-PAL is a 15-item survey with 4-item Likert responses, from 1 (not at all) to 4 (very much) (Groenvold et al. Reference Groenvold, Petersen and Aaronson2006). The last question asks about quality of life and is scored on a 7-item Likert scale from 1 (very poor) to 7 (excellent) (Groenvold et al. Reference Groenvold, Petersen and Aaronson2006).
Information on the interventional pain management procedure; any complications; medication use; symptom burden; self-assessment of treatment (using the self-assessment of treatment [SAT] tool; Wyrwich et al. Reference Wyrwich, Kawata and Thompson2012); and quality of life was collected at various time-points after the procedure, including one immediately post-procedure (within 2 weeks) and one after 3 months if appropriate. The SAT is a 5-item simple question scale using a 5-point Likert scale, where the lower option (−2) indicated a negative response, the middle option (0) indicated a neutral response and the higher option (+2) positive response (Wyrwich et al. Reference Wyrwich, Kawata and Thompson2012). Efforts were made to align the timing of the data collection with patients’ routine clinical follow-up.
Statistical methods
The data were summarized descriptively. No imputation was used for missing data. PROMIS scores were converted to T-scores as per its instrument scoring manual, and EORTC QLQ-C15-PAL scores were transformed to a 0 to 100 scale as per its scoring procedure. Changes from baseline in outcomes of interest were tested univariately by regressing against the time when the measurement occurred (baseline or 1 week), and then using a multivariate linear mixed effect model adjusted for age, sex, and baseline AKPS score, and accounted for within-procedure variations. For both univariate and multivariate models, information was included at the procedure (not patient) level. p-Values of <0.05 were considered statistically significant. Analyses were conducted using R Statistical Software (version 4.2.2; R Core Team 2021).
Results
Patients
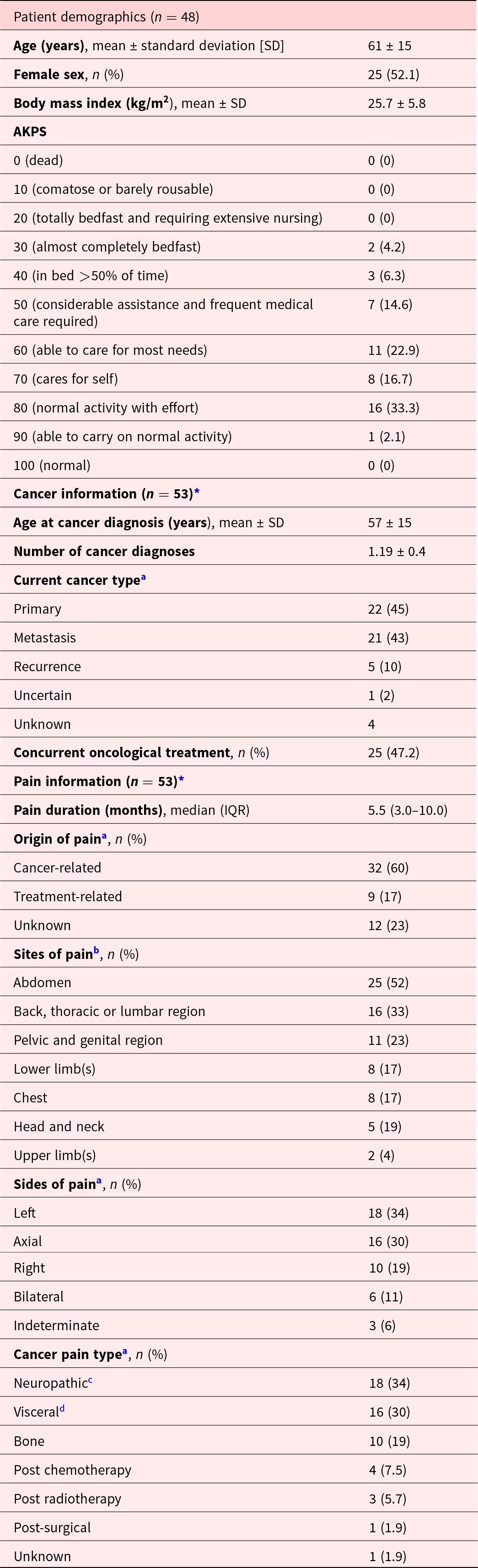
Between October 2021 and March 2023, 48 patients were entered into the registry, with information on 55 procedures being captured, as some patients had multiple procedures. Participant demographics and baseline clinical characteristics are reported in Table 1. Included patients typically had good performance status (approximately 50% had AKPS scores of 70 or better), and had been experiencing pain, typically cancer-related pain, for more than 8 months. The most common site of pain was the abdomen.
Table 1. Patient demographics and baseline clinical characteristics

AKPS = Australia-modified Karnofsky Performance Status.
a Total number here is lower than the total procedures performed (n = 55) due to missing data.
b Does not sum to 100% as patients could have more than one pain location.
c Thoracic tumor with brachial plexus invasion or pelvic cancer affecting lumbosacral plexus or spinal cord compression.
d Liver metastasis, pancreatic tumor.
* Patients could have more than one cancer type.
Procedures
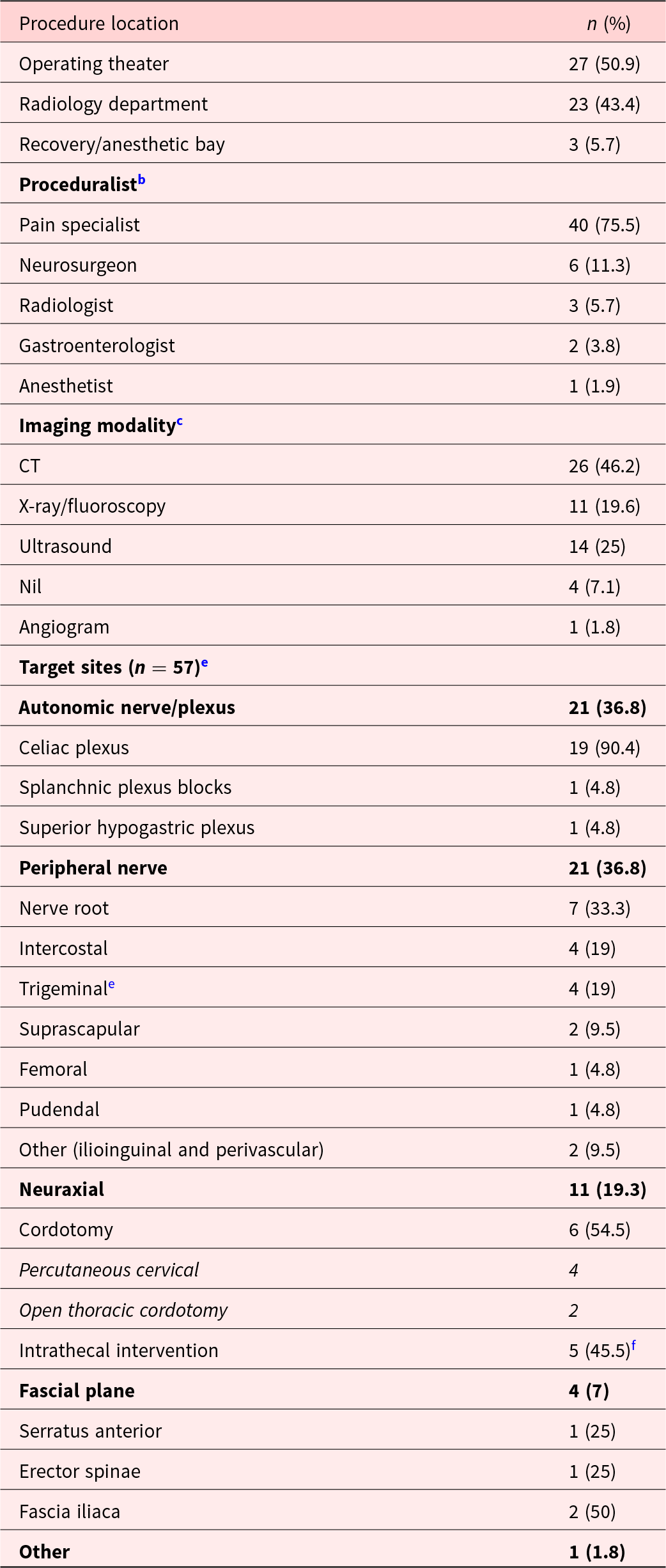
The registry collected information on 55 procedures (Table 2). Most procedures were performed in an operating theater (50.9%) by pain specialists (75.5%: Table 2). Most procedures required imaging guidance, typically by computed tomography (CT – 46.2%) or ultrasound (25%). The most common procedure performed was celiac plexus neurolysis (33.3%).
Table 2. Cancer pain intervention procedures (n = 55)a

a Two patients on the registry did not receive intervention.
b n > 55 as some procedures involved more than one proceduralist.
c n = 56 as one procedure involved both fluoroscopy and ultrasound.
d n = 57 as some procedures included more than 1 target site.
e Trigeminal nerves include mental, mandibular, supratrochlear and supraorbital nerves.
f Four intrathecal neurolysis (saddle block) and 1 intrathecal catheter placement (as a temporalizing measure to bridge and facilitate cordotomy).
Outcomes
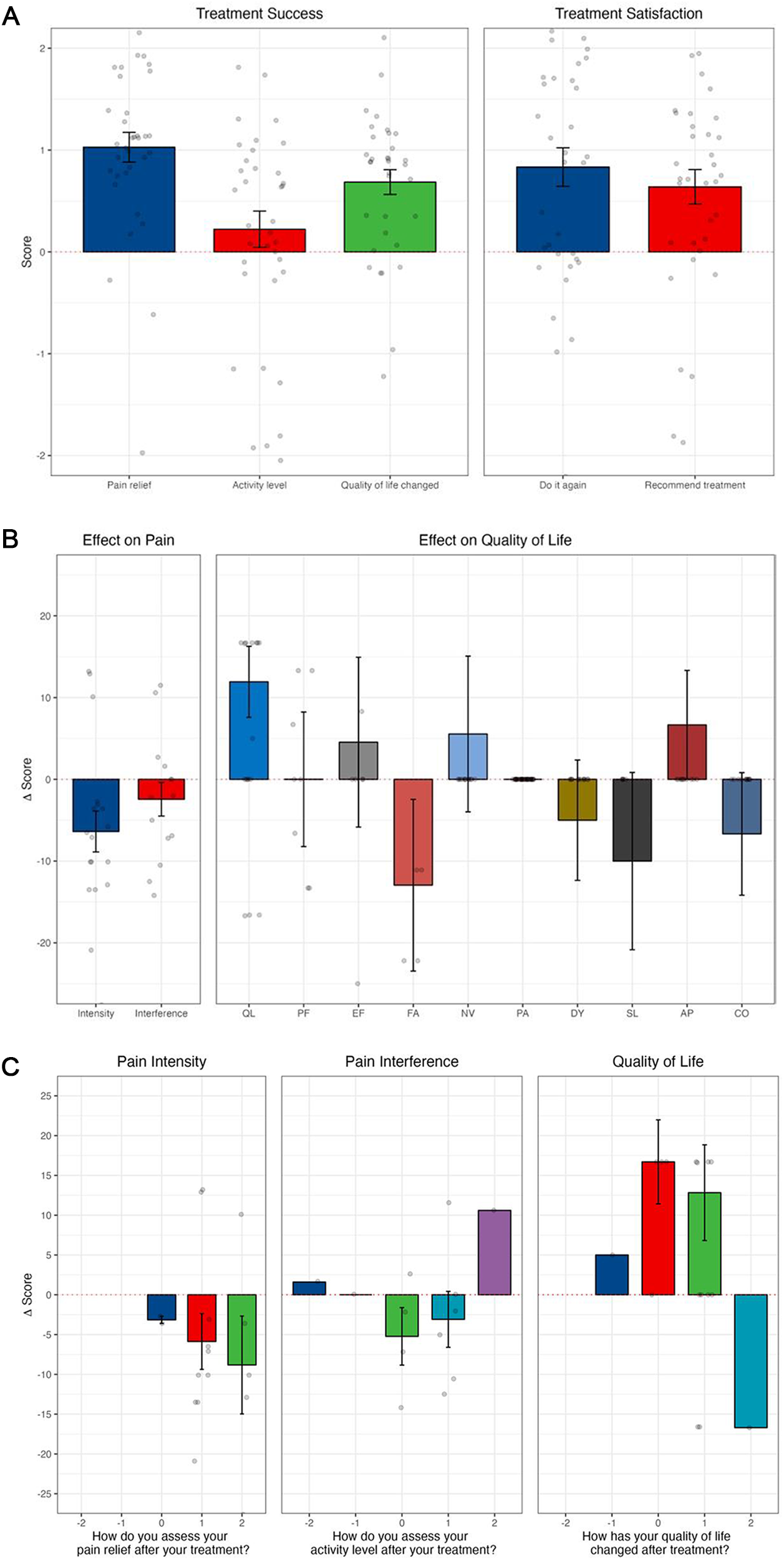
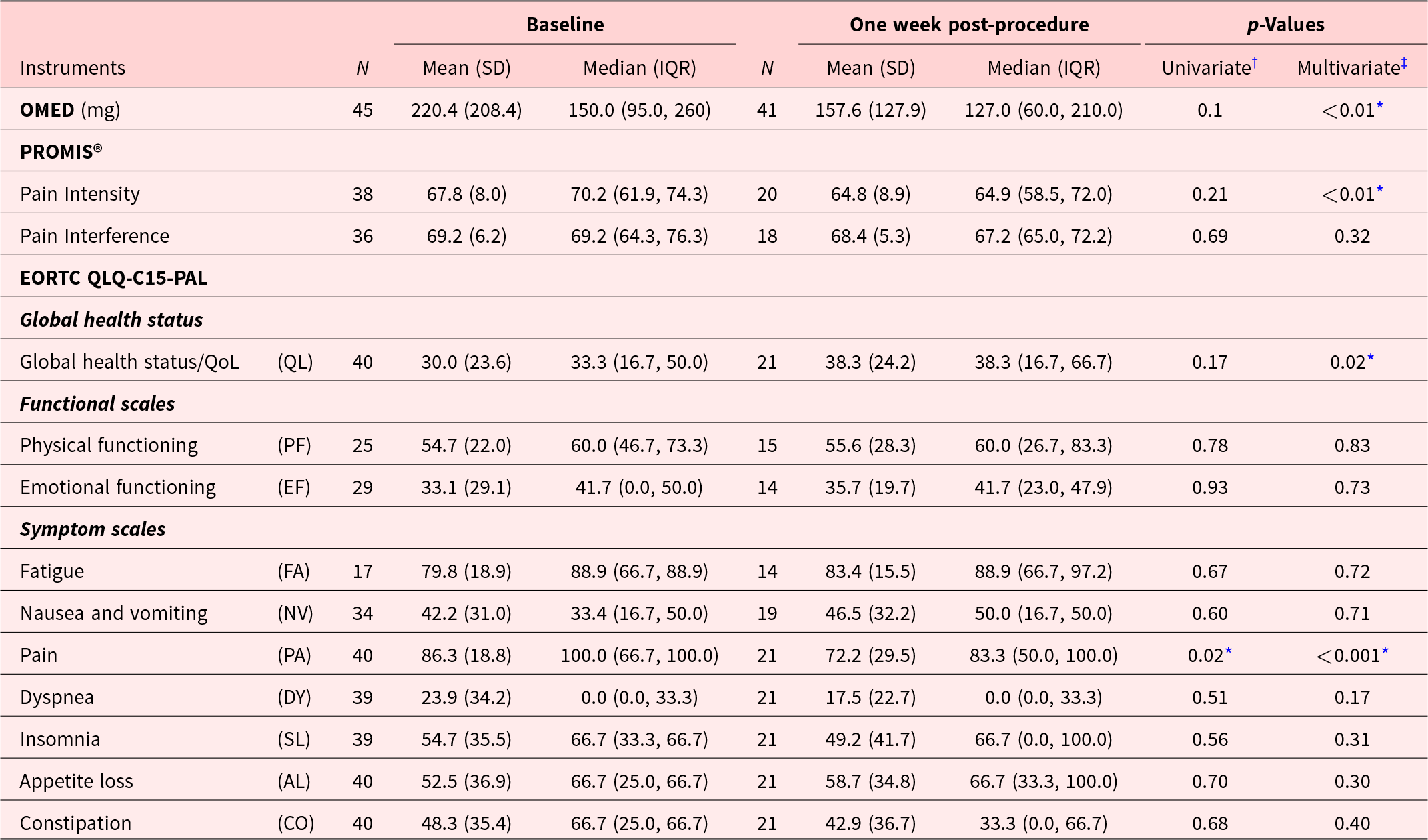
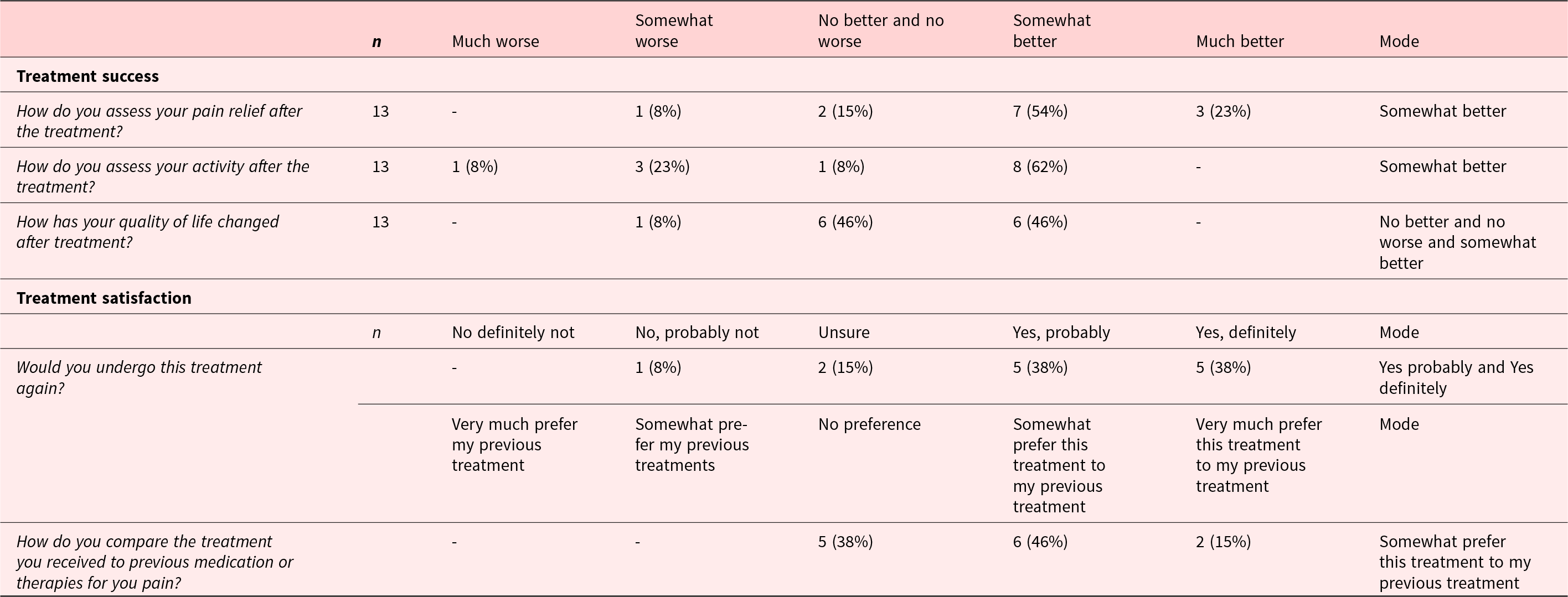
There was a clinically and statistically significant reduction in adjusted (by age, sex, and baseline AKPS) morphine equivalent dose requirements (Table 3), pain intensity (PROMIS) and pain (EORTC-QLQ-C15-PAL), and global health status observed between day 1 and 14 post-procedure (Figure 1). There was an overall significant reduction in pain intensity (p < 0.01), a decrease in opioid consumption, and an improvement in quality of life (Table 4; Figure 1).

Figure 1. Outcomes following all interventional pain procedures: (A) treatment success and satisfaction by SAT; (B) PROMIS-pain intensity, PROMIS-pain interference, and EORTC QLQ-C15-PAL; (C) results from PROMIS and EORTC QLQ-C15-PAL correlating to subjective questions of significance from SAT. (A) The SAT is a 5-item simple question scale using a 5-point Likert scale, where the lower option (−2) indicated a negative response, the middle option (0) indicated a neutral response and the higher option (+2) positive response. (B) Effect on pain intensity and interference (PROMIS) and effect on subdomains of quality of life (EORTC QLQ-C15-PAL). EORTC QLQ-C15-PAL subdomains include AP appetite; CO constipation; DY dyspnea; EF emotional functioning; FA fatigue; NV nausea and vomiting; PA pain; PF physical functioning; QL quality of life; and SL sleeping difficulties. (C) Changes from baseline in pain intensity and pain interference from PROMIS, and changes in quality of life measured on EORTC QLQ-C15-PAL. Categories on the x-axis refer to SAT responses for pain relief, activity level, and quality of life as much worse (−2), somewhat worse (−1), no better and no worse (0), somewhat better (1), or much better (2). Change scores on the y-axis relate to changes in the overall quality of life score reported on PROMIS (pain intensity and pain interference) or EORTC QLQ-C15-PAL (quality of life).
Table 3. Oral morphine equivalent dose and patient-reported outcome measures at baseline and 1 week after procedure

IQR = inter-quartile range; OMED = oral morphine equivalent dose; SD = standard deviation. PROMIS® scores were converted to T-scores as per the instrument scoring manual. EORTC QLQ-C15-PAL scores were transformed to 0–100 as per the EORTC QLQ scoring procedure;
*: statistical significance. p-Value from the † univariate linear model compared the averages of baseline to 1-week post-procedure and ‡multivariate linear mixed effect model adjusted for age, gender, and baseline AKPS scores, and accounted for within-patient variations.
Table 4. SAT item responses at 2 days and 1 week post-procedure

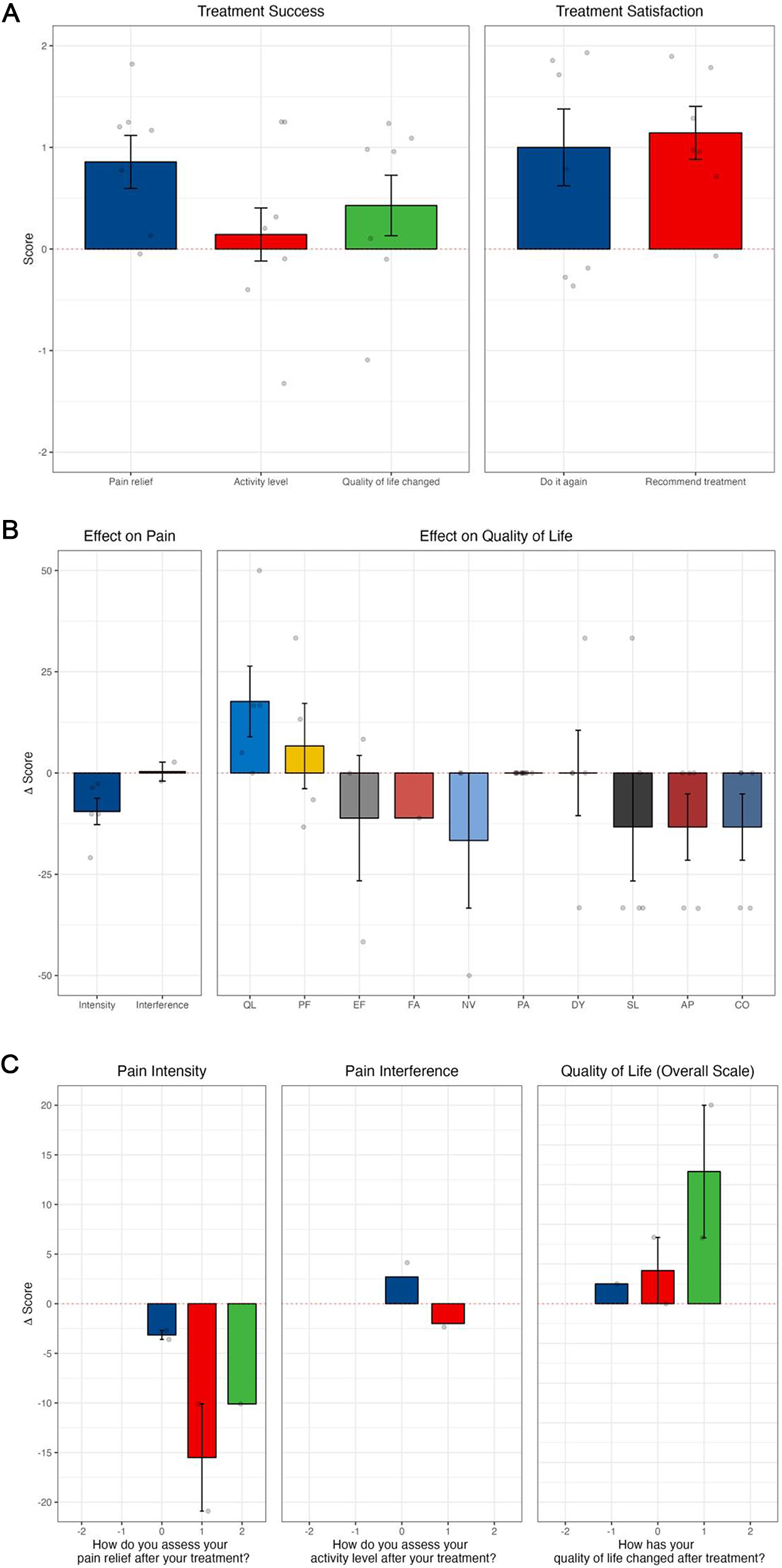
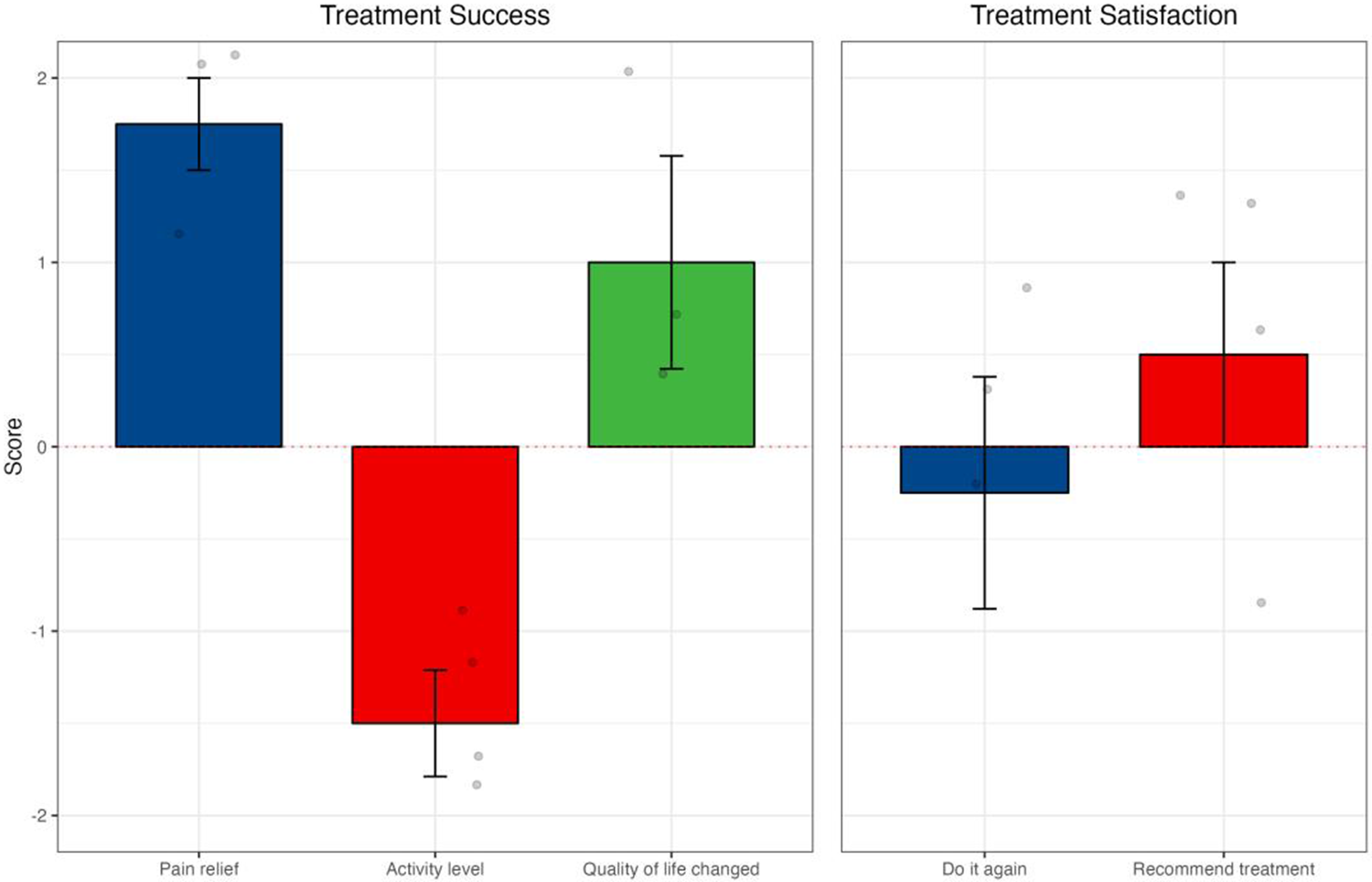
Similarly, when analyzing celiac plexus neurolysis in isolation, there were positive mean scores from SAT in all dimensions, and improvement of quality of life (Figure 2(A) and (B)). With cordotomy, despite a reduction in activity levels post-procedure, there was a positive change in pain relief and quality of life and reported treatment satisfaction (Figure 3).

Figure 2. Outcomes following celiac plexus neurolysis (A) SAT; (B) PROMIS-pain intensity, PROMIS-pain interference, and EORTC QLQ-C15-PAL; and (C) results from PROMIS and EORTC QLQ-C15-PAL correlating to subjective questions of significance in SAT. Changes from baseline in pain intensity and pain interference from PROMIS, and changes in quality of life measured on EORTC QLQ-C15-PAL. Categories on the x-axis refer to SAT responses for pain relief, activity level, and quality of life as much worse (−2), somewhat worse (−1), no better and no worse (0), somewhat better (1), or much better (2). Change scores on the y-axis relate to changes in the overall quality of life score reported on PROMIS (pain intensity and pain interference) or EORTC QLQ-C15-PAL (quality of life).

Figure 3. Outcomes following cordotomy: (A) SAT from cordotomy procedures; (B) PROMIS-pain intensity, PROMIS-pain interference, and EORTC QLQ-C15-PAL; and (C) results from PROMIS and EORTC QLQ-C15-PAL correlating to subjective questions of significance.
In terms of adverse events, 3 patients who underwent celiac plexus neurolysis had transient fever, which improved within a few days. One patient developed bilateral lower limb weakness following bilateral thoracic cordotomy, which resolved spontaneously within 1 week.
Mortality data
Twelve patients (25%) in the registry have since passed away. In this group, the mean survival time was 82.5 ± 70.62 days (range 25–233 days).
Practicality of instruments included in the registry
The SAT showed reasonable acceptability (mean 1 week post-procedure pain relief was 1.1, suggesting “somewhat better”) with a completion rate of 50% at 1 week post-procedure (n = 24/48). However, less than half of the patients (n = 21/48; 43.7%) in the registry completed the post-procedure quality of life surveys (EORTC QLQ-C15-PAL).
Discussion
This study was designed as a prototype to facilitate further development of a more widely applicable cancer pain intervention registry. It highlights the multidisciplinary approach to cancer pain management, including but not limited to general practitioners, oncologists, palliative medicine specialists, specialist pain medicine physicians, anesthetists, interventional radiologists, neurosurgeons, and gastroenterologists. Our study has used a pragmatic approach that serves both as a pilot implementation project for an interventional pain registry, while also collecting important clinical outcomes.
Patient outcomes following the interventional pain procedures were generally positive; however, a significant proportion of patients only benefited from it for a short period of time or for the remainder of their lives. Further work into optimizing the timing of the procedure, such as at the earlier stage of disease, may be considered. The registry only captured patients who had procedures undertaken, and not those patients who may have been appropriate for interventional pain procedures but were not referred for the service.
This registry captured a wide range of procedures, from peripheral nerve block to emerging treatments such as trans-arterial embolization. Previously, it has been difficult to access such integrated information through a single source or platform. The findings highlight the importance of collaborative care from the diverse specialties in managing cancer pain. Establishing the registry has served as a quality assurance process for the intervention practice as it has allowed direct and timely feedback to the clinical team, which will assist in improving future patient care. The registry has also shown signals of positive and negative clinical outcomes (clinical efficacy as measured by medication use, change of pain severity, and adverse events), which continues to generate additional information for systematic evaluation.
The most common interventional pain procedure reported in the registry was celiac plexus neurolysis, primarily for pancreatic cancer, with good evidence of support in the literature (Arcidiacono et al. Reference Arcidiacono, Calori and Carrara2011; Shwita et al. Reference Shwita, Amr and Okab2015). However, there was relatively limited information for different types of cancer, such as stomach cancer or cancer with metastasis into the liver and other upper gastrointestinal structures with this procedure. Moreover, there was a scarcity of data available on direct comparison for different approaches, including fluoroscopy, computed tomography, or endoscopic techniques. It is not clear whether this was due to a lack of availability or referral for these services, or because they were not considered appropriate in the individual patient context. Further details on the barriers and facilitators of interventional pain management for cancer pain from the perspective of clinicians were explored in a qualitative sub-study by Erciyas et al. (Reference Cornall, Zhao and Luckett2024), which found that limited clinician knowledge may be one of the major barriers for patients accessing interventional pain management (Erciyas et al. Reference Cornall, Zhao and Luckett2024).
The relatively poor completion of some survey instruments in the registry highlights the need for more suitable instruments to maximize completion rate without compromising validity. For example, slightly greater proportions of patients (50%) completed the SAT, while quality of life measures were reported by only 43.7% of patients post-procedure. Others have reported the need to be flexible in the timing and method of data collection in this setting (Chatland et al. Reference Chatland, Harvey and Kelly2023), as questionnaire burden remains a barrier to completion of questionnaires by people undergoing largely palliative-based care (Dy et al. Reference Dy, Al Hamayel and Hannum2017). Not surprisingly, the completion rate in this study was comparable to that shown in a study specifically assessing patient-reported outcomes in palliative care units (Müller et al. Reference Müller, Mayer-Steinacker and Gencer2023). In our study, the collection of data relied heavily on existing clinical staff, which may further explain the suboptimal collection of information. This also identifies the need for additional resources to facilitate implementation of the registry on a larger scale; or consideration to alternative methods (such as patient self-report via electronic means). In the future, survey instruments that take less time and effort for patients to complete, especially for those who are more advanced in their treatment journey, will be explored, such as the PEG, a 3-item scale to assess pain intensity (Krebs et al. Reference Krebs, Lorenz and Bair2009).
As is common in studies of advanced cancer, our study was limited by the amount of missing data in the post-procedure outcome measures. As described above, alternative measures for the collection of data, and the number of instruments and time-points included, should be considered. We also note there is heterogeneity in the population, and potentially outcomes should be stratified by predicted prognosis (e.g., patients with expected longer-term survival versus those with very limited prognoses) (Chow et al. Reference Chow, Abdolell and Panzarella2008). Given the small number of centers involved, it is unclear how generalizable these findings are to the broader patient population in Australia, or indeed elsewhere. In particular, the challenges identified in these 2 adjacent centers may not fully reflect feasibility when extending to different sites. Additionally, the data on the range of procedures available, referral pathway, and clinical outcomes may be skewed due to differing resource and practice patterns. Obtaining more tailored information on them will be crucial before introducing it to new centers.
Conclusion
This is a vital first step in creating a more widely applicable registry evaluating cancer pain interventions. It provided a systematic evaluation on the range of cancer pain intervention procedures available at a local level, as well as data on outcome measures using validated instruments. This allowed a timely review of clinical practice, which may lead to improvements in future patient care. Further expansion of the registry to other Australian hospitals is warranted.
Acknowledgments
The authors thank Belinda Butcher PhD CMPP for providing medical writing support by assisting with editing the draft and incorporating author comments. Medical writing support was funded by Chris O’Brien Lifehouse SurFebruary Cancer Research Fund in accordance with Good Publication Practice (GPP2022) guidelines (https://www.acpjournals.org/doi/10.7326/M22-1460).
Author contributions
All authors contributed to conception and design, or acquisition of data, or analysis and interpretation of data; drafting the article or revising it critically for important intellectual content; and read and approved the version to be published.
Funding
This project is supported by Chris O’Brien Lifehouse SurFebruary Cancer Research Fund for employment of a part-time research assistant over a 2-year period.
Ethical approval and consent to participate
This study was approved by the Sydney Local Health District Human Research Ethics Committee, Australia (2021/ETH11313).
All participants were given a participant information sheet. This study utilized an opt-out approach to consent approved by the ethics committee.