Introduction
The COVID-19 pandemic has laid bare the shortcomings of current global health security arrangements, presenting an opportunity to re-examine and strengthen global governance to better protect people and societies from infectious diseases. The robust global coordination needed to prevent, prepare for, and respond to future global health security threats partly depends on revisions of current international instruments and the development of new ones.Reference Wilson1 As a new World Health Organization (WHO) intergovernmental negotiating body drafts and negotiates an instrument for pandemic prevention, preparedness, and response,2 it is important to critically consider the full range of substantive issues that should be addressed in a potential pandemic instrument to ensure adequate global readiness for the next pandemic — whether it looks like COVID-19 or a different kind of pandemic threat, like antimicrobial resistance (AMR).
To date, discussions of the pandemic instrument have primarily focused on the need for better surveillance and monitoring of emerging zoonotic infections, largely viruses like COVID-19, that are transferred from animals to humans.Reference Wilson3 This narrow approach can severely limit the world’s ability to prevent, prepare for, and respond to the full range of future global pandemic threats. Zoonoses are not the only natural source of pandemics. If governments want to be adequately prepared for the next pandemic, the COVID-19 pandemic cannot be the only point of reference. Reference Gostin, Meier and Stocking4
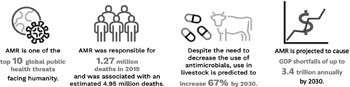
At a minimum, a future pandemic instrument should also address AMR — the natural process by which pathogens become resistant to the antimicrobial medicines intended to treat them,5 which WHO has recognized as one of the top ten global health threats facing humanity. Bacterial resistance was responsible for over 1.27 million deaths in 20196 and could result in substantial direct and indirect economic damage akin to the annual costs of the global financial crises that began in 20087 (Figure 1). If the opportunity to address AMR in a pandemic instrument is not seized, the next pandemic could be caused not by novel pathogens that have transferred from animals to humans but from existing pathogens that have become resistant to antimicrobial medicines. Even if the next pandemic starts from cross-species transmission, the treatment for many zoonoses relies on antimicrobials to reduce severity of, and death from, infections and to treat secondary infections. As rising drug resistance continues to threaten the effectiveness of antimicrobials, the negotiation of a pandemic instrument represents an unprecedented opportunity to strengthen access, conservation, and innovation of antimicrobials as part of a comprehensive global response to pandemic threats.

Figure 1 Consequence of Unabated AMR for Human Health, Animal Health, and the Global EconomyReference Murray, Ikuta, Sharara, Swetschinski and Aguilar8
If the opportunity to address AMR in a pandemic instrument is not seized, the next pandemic could be caused not by novel pathogens that have transferred from animals to humans but from existing pathogens that have become resistant to antimicrobial medicines. Even if the next pandemic starts from cross-species transmission, the treatment for many zoonoses relies on antimicrobials to reduce severity of, and death from, infections and to treat secondary infections. As rising drug resistance continues to threaten the effectiveness of antimicrobials, the negotiation of a pandemic instrument represents an unprecedented opportunity to strengthen access, conservation, and innovation of antimicrobials as part of a comprehensive global response to pandemic threats.
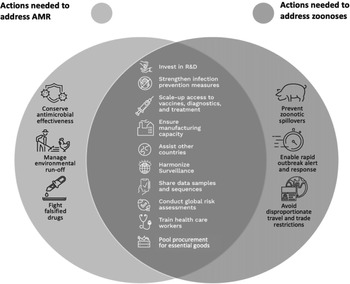
Fortunately, there is not only a strong rationale for including provisions to address the “silent pandemic” of AMR in a pandemic instrument, but this work can be done synergistically with proposed provisions to address zoonotic pandemics. Indeed, the strategies needed to support the global mechanisms required to prevent, prepare for, and respond to zoonoses and AMR overlap significantly (Figure 2).9 This means that most of the provisions needed to address AMR in a pandemic instrument would already exist or would only need to be slightly adjusted to address any unique facets of AMR. Further, any adjustments could be focused and minor since many efforts that aim to address AMR specifically can be developed after the negotiation of the main pandemic instrument if (but only if) AMR is defined within the scope of the pandemic instrument. These small adjustments would enhance global readiness for the next pandemic beyond what is currently being contemplated.
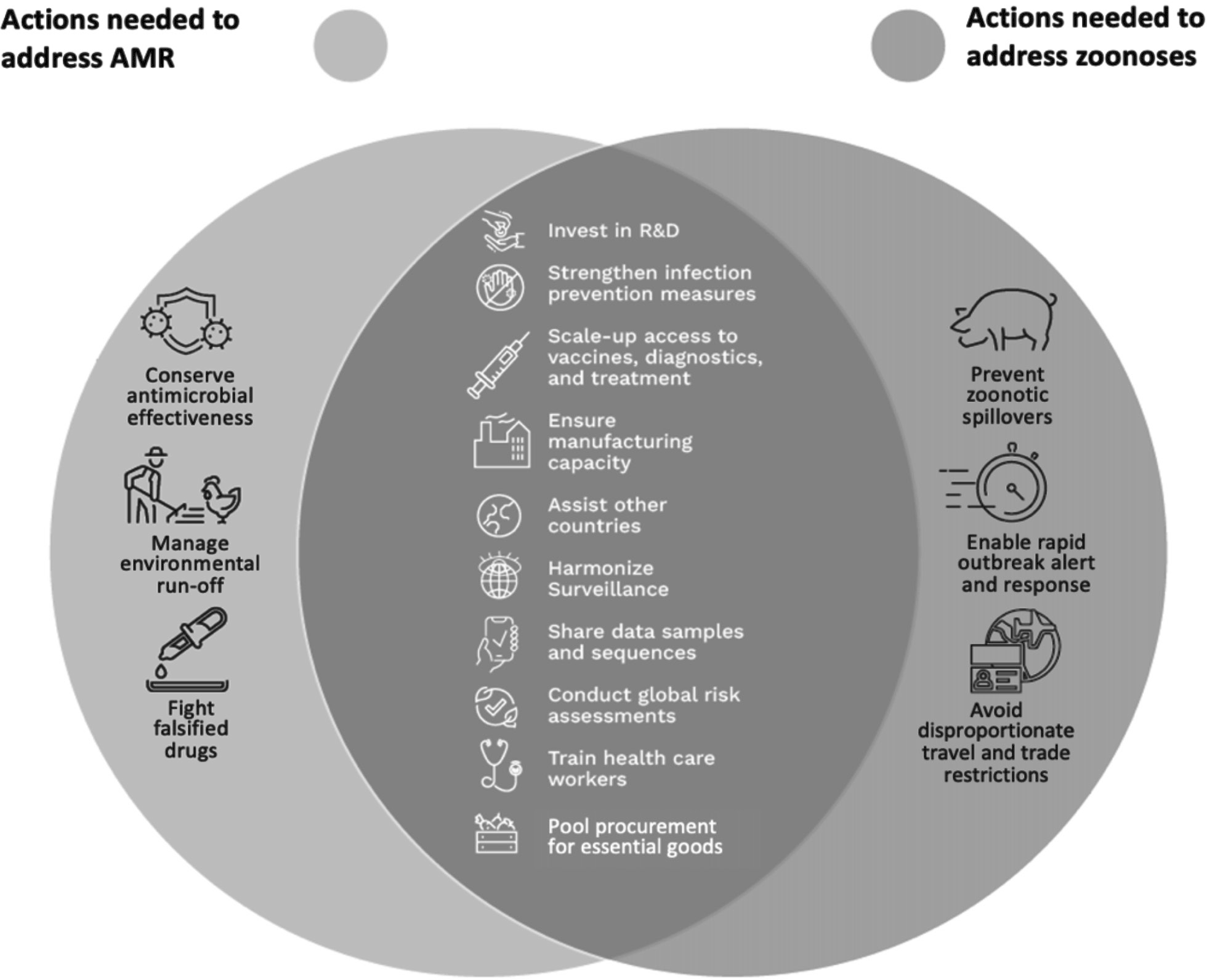
Figure 2 Overlap in Strategies Needed to Address Zoonoses and AMR10
Governments can practically and efficiently address AMR in a pandemic instrument by mapping the overlaps between the efforts needed to address zoonoses and AMR, including (a) equitable access to medical countermeasures, (b) globally integrated One Health surveillance and monitoring systems, (c) increased technical and laboratory capacity in low- and middle-income countries, and (d) a regulatory framework governing the stewardship of antimicrobials. By illustrating the clear link between the efforts to address both pandemic threats and outlining six dual-purpose provisions that could address both pandemic threats, this article makes the case that including AMR in the pandemic instrument makes the most effective use of limited time and resources to ensure the world’s best opportunity to prevent, prepare for, and respond to future global pandemics.
Zoonoses and AMR Have Overlapping Needs Which Can Be Addressed Simultaneously in a Pandemic Instrument for Greater Impact
Most of the policy responses required to address zoonoses overlap significantly with what is needed for AMR (Figure 2). This section identifies some key areas of overlap and discusses the adjustments needed to render the overall global policy response effective for both zoonoses and AMR.
Equitable Access to Medical Countermeasures
Medical countermeasures, including antimicrobials, diagnostics, personal protective equipment, and vaccines, are vital for controlling the spread of infectious diseases, like zoonoses and AMR.Reference Excler11 The extent to which they can be effective however hinges on access to them, which in turn depends on their invention, development, and validation through research, as well as their regulatory approval, manufacturing, and distribution.
A pandemic instrument could designate antimicrobials, diagnostics, personal protective equipment, and vaccines as ‘global public goods’ and create or adopt trusted mechanisms for expediting their development, ensuring their availability when needed, and funding their efficient procurement and equitable distribution. For example, a pandemic instrument could harmonize emergency regulatory approval processes and support the development of regional manufacturing hubs to produce these specific goods. In addition, through a financing mechanism or otherwise, a pandemic instrument could expand financial support for the Coalition for Epidemic Preparedness Innovations (CEPI), Combating Antibiotic-Resistant Bacteria Biopharmaceutical Accelerator (CARB-X), Global Antibiotic Research and Development Partnership (GARDP), and a permanent Access to Pandemic Tools Accelerator (like ACT-A).12 Some of these trusted mechanisms have been central to the rapid development of the COVID-19 vaccine and the global coordination of their distribution, albeit not at the ideal speed or scale to achieve equitable access.Reference Sharma, Kawa and Gomber13 Instead of re-inventing the wheel, pandemic instrument negotiators could leverage these mechanisms by officially incorporating them as part of the global pandemic response strategy and scale up financial support for them to ensure medical countermeasures are available and equitably distributed as needed in future global pandemics.
The similarities in the form of financing strategies needed for these two pandemic threats further supports the inclusion of dual-purpose provisions that simultaneously tackle both. For instance, one of the prominent mechanisms proposed to finance antimicrobial research and development (R&D) is the delinked subscription model,Reference Balasegaram, Clift and Røttingen14 which would require governments to pay an annual subscription fee for access to new antimicrobial medicines. This is similar to COVAX, an advanced market commitment and procurement mechanism used to finance the rapid scaling up of COVID-19 vaccine manufacturing,15 which required governments to pay in advance for access to future vaccines. Further, excess R&D or manufacturing capacity that might be created in anticipation of future pandemics could be efficiently used during inter-pandemic periods to develop or make global public goods related to AMR.
Expansion of Globally Integrated One Health Surveillance and Monitoring Systems
One Health approaches to the surveillance and monitoring of infectious diseases, including zoonoses and AMR, are fundamental to effective global pandemic responses.16 Like zoonoses, some new antimicrobial-resistant strains of bacteria arise at the human-animal-environment interface, especially in food and agricultural systems where antimicrobials are used in intensified agricultural practices.Reference Economou and Gousia17 Surveillance is therefore needed for early detection and notification of potential zoonoses and antimicrobial-resistant pathogens in animals, tracking emerging variants of zoonoses and the spread of resistant pathogens among humans to identify population transmission patterns, and sharing of information with researchers and policymakers at the domestic and global levels to coordinate global pandemic responses.Reference Buckeridge and Cadieux18 The effectiveness of a One Health approach to surveillance and monitoring, however, requires the systems to be globally integrated to facilitate the transfer of knowledge and data on new infections to activate public health responses, guide decision-making at the domestic and global level, and inform R&D efforts towards new global public goods.19 Indeed, the absence of these requirements has manifested as a major barrier to action on global infectious disease threats, including COVID-19.20
A pandemic instrument could mandate the implementation of One Health surveillance and monitoring capacities and develop international benchmarks for measurement of these capacities. These international benchmarks could include a risk assessment and management methodology that guides governments in identifying, assessing, and managing risks within their countries and sub-national jurisdictions. Surveillance capacities should go beyond sector-specific monitoring of established infectious disease threats to include capacity for monitoring the emergence of potential zoonoses and resistant pathogens in humans, animals, and the environment. Additionally, a pandemic instrument could mandate the development of standardized protocols on data reporting and knowledge sharing systems for potential zoonoses and AMR, including creating a mechanism for subsequent One Health regulation-making wherein an entity is created to develop technical standards related to One Health issues, including AMR. Modelled on the Food and Agricultural Organization (FAO)/WHO Codex Alimentarius, which makes non-binding regulations affecting food, agriculture, and trade,21 a new One Health regulatory entity could standardize evidence-based rules to create robust preventative measures in relation to zoonotic and AMR surveillance and monitoring practices and develop or endorse customary rules regarding the sharing of research, data, and technology for these threats at the global level.
Increased Technical and Laboratory capacity in LMICs
All countries need trained personnel and lab infrastructure, including physical space and equipment, to undertake One Health surveillance and monitoring for infectious diseases, including zoonoses and AMR.22 While many wealthier countries should already be able to fully participate in a globally integrated One Health surveillance and monitoring system, many LMICs face more barriers and constraints.23 For instance, human resources shortages have created a technical capacity gap. This manifests in the limited availability of highly qualified technical staff, such as laboratory technicians, epidemiologists, and data managers to steward surveillance systems in LMICs and enable international data sharing.24 There are also fewer clinical laboratories and diagnostic facilities in many LMICs to conduct laboratory testing for antimicrobial-resistant organisms and other infectious pathogens, exacerbating the barriers to participating in a globally integrated surveillance and monitoring system.25
To address these challenges, a pandemic instrument could promote technical assistance and knowledge sharing among countries and encourage financial support for LMICs to meet minimum One Health surveillance and monitoring capacities. All governments would also benefit from the inclusion of a mechanism for developing and sharing international best practices on laboratory testing, with support provided to LMICs to build capacity for newer technologies such as genomic sequencing and wastewater surveillance. In addition, a pandemic instrument could mandate the implementation of joint external evaluations of both surveillance and laboratory capacities as well as support to prioritize limited lab capacities during times of emergencies. These provisions are necessary to retain and increase technical capacity in LMICs to scale up surveillance and monitoring and enable full participation in the globally integrated One Health surveillance and monitoring system needed for both zoonoses and AMR.
Regulatory Framework Governing the Stewardship of Antimicrobials
Antimicrobial stewardship is core to support effective global pandemic responses to infectious diseases, including zoonoses and AMR. As AMR can develop even when antimicrobials are correctly prescribed,26 focused action is needed to promote antimicrobial stewardship and to protect the effectiveness of existing antimicrobial medicines.Reference Van Katwyk27 This is necessary to first, reduce the threat posed by AMR, and second, preserve an essential resource for responding to future pandemic threats, as the treatment for many zoonoses relies on antimicrobials to reduce severity and death from secondary bacterial infections. For instance, the majority of deaths during the 1918 Influenza pandemic likely resulted from secondary bacterial pneumonia caused by common upper respiratory tract infections;Reference Morens, Taubenberger and Fauci28 countless lives could have been saved if effective antibiotics had been available. Similarly, antibiotics have been critical to treating secondary bacterial infections in COVID-19 patients.Reference Nag and Kaur29 Given the transnational nature of AMR, the stewardship of antimicrobials must be addressed at the global level to avoid disincentivizing investment in stewardship and encourage implementation at socially optimal levels.
To safeguard the effectiveness of antimicrobials, a comprehensive pandemic instrument could mandate the development or use of a regulatory framework governing the use of antimicrobials in a sustainable, acceptable, fair, and effective manner. This regulatory framework could govern which antimicrobials should be accessed, watched, and reserved in health care (i.e., WHO’s AWaRe framework)Reference Hsia30 and which critically important antimicrobials should be limited to human use. The existing AWaRE framework could be enshrined in a pandemic instrument so that the rules governing the conservation of antimicrobials are globally harmonized. Continued antimicrobial effectiveness is likely to be important for reducing the spread and/or severity of future pandemics, which means that antimicrobial stewardship efforts should ideally be addressed in a comprehensive pandemic instrument.
Design Considerations for Dual-Purpose Provisions in a Pandemic Instrument
The clear overlaps between the efforts needed to address zoonoses and AMR illustrate the seamlessness with which provisions that simultaneously address both pandemic threats could be synergistically included within the text of a new pandemic instrument. These provisions should be designed to address zoonoses and AMR explicitly (Figure. 3), such that State Parties to the instrument have a clear framework to guide the implementation of their international obligations arising from the introduction of these provisions. For instance, designating antimicrobials and vaccines as global public goods (Figure 3) helps to ensure that State Parties have a clear idea of what their obligations are regarding R&D, distribution, and financing, and how these actions help them to meet their obligations in respect of both zoonoses and AMR as part of their overall pandemic prevention, preparedness, and response strategy. The same is true for the mandated development of a One Health mechanism with the explicit function of developing technical standards and data reporting protocols for both zoonoses and AMR, and other recommended dual-purpose provisions (Figure 3). Absent these explicit references to zoonoses and AMR, the level of action required to address AMR at the global level, especially as it relates to stewardship, might not be realised.

Figure 3 Illustrations of How AMR Can Be Addressed within the Text of a Pandemic Instrument
While the interconnectedness of zoonoses and AMR as One Health challenges also presents key entry points within the text of a pandemic instrument to address both threats under a One Health umbrella, the success of a pandemic instrument partly depends on clear, explicit rules governing access to medical countermeasures, the expansion of surveillance and monitoring, the development of technical and laboratory capacity in LMICs, and the use or development of a framework governing the stewardship of antimicrobials for both zoonoses and AMR. These provisions could be incorporated into the pandemic instrument as One Health mechanisms and successfully address AMR if the text of the instrument is explicit about the inclusion of AMR.
Conclusion
The articulated goal of a pandemic treaty is to ensure that there is a comprehensive framework to prevent, prepare for, and respond to future global pandemics. Solely addressing zoonoses and neglecting the silent pandemic of AMR would be an overly narrow approach that will not adequately prepare the world for both primary natural sources of future pandemic threats. Fortunately, not many changes would be needed to achieve this goal (Figure 3). At a minimum, AMR should be defined as being within the scope of the pandemic instrument, by including bacterial pathogens of concern in the definition of pandemic threats. Even better would be to make the small adjustments needed to ensure efforts targeting zoonoses simultaneously target AMR sources of pandemics, converting what would otherwise be a zoonotic pandemic instrument into a comprehensive pandemic instrument. Special focus should be placed on promoting antimicrobial stewardship, as such efforts are needed to sustain the effectiveness of existing antimicrobials and are unlikely to be implemented at socially optimal levels without coordinated global action such as through an international agreement like the pandemic instument.
Note
Steven J. Hoffman is the Vice-President of Corporate Data & Surveillance at the Public Health Agency of Canada (PHAC) and previously served as the Scientific Director of the Institute of Population & Public Health at the Canadian Institutes of Health Research (CIHR). The views expressed in this article are those of the authors and do not necessarily reflect those of PHAC, CIHR or the Government of Canada.
This work was supported by the Canadian Institutes of Health Research [#149542], the Social Sciences & Humanities Research Council [#895-2022-1015], and the Wellcome Trust [222422/Z/21/Z]. The funding bodies were not involved in the study design, data collection, analysis, interpretation or writing.