Introduction
Bovine herpesvirus-4 (BoHV-4) is a double-stranded DNA virus belonging to the subfamily of gammaherpesvirinae. Isolation of BoHV-4 from calves with respiratory and keratoconjunctivitis in Europe in the 1960s [Reference Bartha, Juhasz and Liebermann1] and later from the USA in the 1970s [Reference Mohanty, Hammond and Lillie2] marked the beginning of understanding of its potential pathogenic role in cattle disease. Although it has been isolated from healthy animals as well as from a range of clinical diseases in infected animals [Reference Thiry and Wittmann3], to date the role of BoHV-4 as a causal agent of specific diseases has not been conclusively established, signifying the need for a continued investigation. Interestingly, its isolation from a wide range of diseased cattle including ocular discharges, conjunctivitis, skin infections, respiratory infections and abortion [Reference Thiry4] consolidates an increasingly held opinion regarding its pathogenicity.
For instance, Bellino et al. [Reference Bellino5] recently suggested BoHV-4 as a potential pathogenic agent of dermatitis, pyrexia and haemorrhagic syndrome in cattle from Italy. Isolation of BoHV-4 from suppurative and ulcerative endometritis in cows [Reference Frazier6] is an additional indication of its increasing role in the pathogenesis of animal diseases. This suggests the need for conducting well-designed causal inference studies to generate sufficient evidence-based information to ascertain whether BoHV-4 is a causal agent of specific diseases. Knowledge on how host, agent and environmental factors contribute to the occurrence of the virus helps investigators design and implement specific and appropriate control and prevention strategies.
The role of BoHV-4 in reproductive diseases of dairy cows such as post-partum metritis requires further investigation. Post-partum metritis affects approximately 40% of dairy cattle causing significant infertility and economic loss to the dairy industry [Reference Sheldon and Dobson7]. The presence of BoHV-4 infection in cows with post-partum metritis might further complicate control and prevention programmes. BoHV-4 is a ubiquitous virus and the majority of BoHV-4 affected animals exhibit persistent and asymptomatic infection [Reference Thiry4, Reference Franceschi8], which highlights the intricacy of controlling and preventing diseases caused by the virus.
The dairy industry is a vital sector in and a major contributor to California's economy [Reference Hatamiya9]. California leads the nation in dairy production with its contribution of over 20% of the national dairy products [Reference Hatamiya9]. California is also a leading exporter of dairy products with over 40% of the milk produced and processed in California exported to other parts of the country and around the world [Reference Hatamiya9, Reference Sumner, Azuara and Coughlin10]. According to the California Department of Food and Agriculture [11], over 40.4 billion pounds of milk (average production per milk cow = 22 968 pounds) were produced in the state (19.0% of the country's milk production), from nearly 1.8 million dairy cows making it top ranked among all the states. To ensure sustainability of such dairy productivity, it is essential to pay special attention to dairy herd health. As a result of the association of BoHV-4 with persistent infections and diseases including abortion, metritis, pneumonia, diarrhoea and other reproductive diseases such as vaginitis and mammary skin infection [Reference Thiry and Wittmann3, Reference Castrucci12–Reference Dubuisson15], its isolation should be of major concern due to significant impacts on productivity in dairy herds.
Several strains of BoHV-4 have been isolated and identified from clinical samples of naturally infected animals with a range of clinical signs. However, only a few studies have documented the genomic analysis of potentially pathogenic field isolates of the virus. To date, no studies have been performed to identify dominant genotypes of BoHV-4 that are associated with disease in dairy cows in California. Therefore, this is the first preliminary study to document predominant BoHV-4 genotypes and their association with post-partum metritis in dairy cows. We hypothesised that: (1) BoHV-4 infection is strongly and positively associated with clinical post-partum metritis, stage of lactation and other co-infecting viruses; and (2) multiple genotypes of BoHV-4 are circulating in California dairy herds. Therefore, the aims of this study were to: (1) determine the prevalence and associated risk factors for BoHV-4 infection; and (2) identify and document the genetic characteristic and predominant genotypes of BoVH-4 in dairy herds in California.
Methods
Study design
A cross-sectional multi-stage sampling technique leading to hierarchical or clustered structure of study population, where farms were considered as a group variable [Reference Austin and Merlo16] and individual animals as observational units [Reference Austin and Merlo16], clustered in groups was used. The use of this multi-stage sampling technique helped investigate the effects of individual animals clustering at farm level on the distribution of BoHV-4 between and within farms. Selection of farms was based on convenience, while individual animal selection was random.
Study area, study population and sample collection
The study was conducted over a period of 12 months from May 2015 to May 2016, on client-owned dairy farms located in Yolo and Tulare counties, California. Additionally, some data records and samples collected as part of routine disease surveillance activities of the California Animal Health and Food Safety (CAHFS) laboratory system were also included as part of the study. Samples were collected when cows were restrained in head locks during post-partum evaluation by the investigators or farm veterinarians. Individual cow's information (breed, lactation number and days in milk) and the presence or absence of metritis was recorded. Post-partum metritis was defined as a cow with one or more of the following: (1) abnormally enlarged uterus with fetid watery red brown discharge, (2) systemic illness (including dull mentation, decreased milk production and appetite, other signs of toxaemia) and (3) fever (rectal temperature >103.1°F (39.5 °C)) [Reference Sheldon17].
Prior to the collection of samples, the rectal temperature was evaluated using a rectal thermometer. The vaginal area was then cleaned with a non-sterile gauze followed by insertion of a sterile, saline-moistened polyester tipped applicator swab (Puritan Medical Products, Guilford, ME, USA). The swab was then rolled 5–10 times, clockwise and anti-clockwise, until saturated with discharge (uterine/vaginal). The swabs were then placed in red top tube and transported on ice to the CAHFS laboratories in Tulare and Davis for analysis.
Diagnosis of BoHV-4 and other co-infecting viruses
Detection of BoVH-4 and other co-infecting viruses including bovine viral diarrhoea virus (BVDV) [Reference Cvetojevic18], bovine herpes virus-1 (BoHV-1), bovine herpes virus-2 (BoHV-2) and parapox virus (PPV) in uterine/vaginal samples was confirmed by real-time (RT) PCR at the CAHFS laboratory, Davis. The assay targeted three open reading frames [Reference Altamiranda19] of the viral genome (ORF 3 – encoding thymidine kinase, ORF 8 – encoding glycoprotein B and ORF 22 – encoding glycoprotein H). Fifty microlitres of swab fluid or cell culture was extracted (Max 96 Viral RNA Isolation kit (AM 1836-5), Life Technologies, Carlsbad, CA, USA) to prepare samples for RT PCR testing following the recommendations of the manufacturer. RT PCR was performed using a primer set kindly provided by Dr K. Kurth (WVDL, Madison, WI, USA) utilizing Path-ID™ Multiplex One-Step RT-PCR Kit (Life Technologies, Carlsbad, CA, USA). Reactions were performed on an Applied Biosystems 7500 Fast Real-Time PCR System (Life Technologies, Carlsbad, CA, USA) with the following PCR thermocycler settings: stage 1 at 50 °C for 10 min; stage 2 at 95 °C for 10 min; and stage 3 at 95 °C for 15 s; followed by stage 4 at 60 °C for 1 min; stages 3 and 4 were repeated for a total of 40 cycles.
Isolation of BoVH-4 virus, DNA extraction and purification
Twenty-three uterine samples that tested positive for BoHV-4 (13 from post-partum metritis and 10 from non-metritis cows) were further analysed for viral isolation. Virus isolation was performed in Madin Darby bovine kidney (MDBK) cells supplemented with 2% foetal bovine serum (FBS)/minimum essential media (MEM, Cell Grow, #10-009-c). A 10% swab suspension was inoculated onto 70–90% confluent cells and incubated at 37°C for 1 h. Inoculum was removed, and the monolayer was rinsed once using 2% FBS/MEM maintenance media. Cell culture was refed (2% FBS/MEM) and observed for cytopathic effects. The DNA was then isolated and purified using the DNeasy Blood & Tissue Kits according to the manufacturer's instructions (QIAGEN, Westburg, The Netherlands).
Phylogenetic analysis of BoHV-4 isolates
For molecular phylogenetic characterisation of the 10 BoHV-4 field strains, sequence analysis was performed using standard PCR procedures along with primers previously described [Reference Altamiranda19–Reference Verna21], with the exception of using a Path-ID Multiplex qPCR master mix (Life Technology, Carlsbad, CA, USA). Standard PCR assays targeting three open reading frames [Reference Altamiranda19] of the viral genome were evaluated. These included ORF 3 (encoding thymidine kinase), ORF 8 (encoding glycoprotein B) and ORF 22 (encoding glycoprotein H). Amplicons were verified by gel electrophoresis in a 3% agarose gel, and DNA bands were visualised using an ethidium bromide stain. The PCR amplicons were cleaned up using an Amicon Ultra Centrifuge Spin column (Millipore, Billerica, MA, USA) according to the recommendations of the manufacturer. Sequencing was performed by a commercial sequencing facility and the data were analysed at the CAHFS laboratory, Davis, using Geneious 10.2.3, molecular biology and NGS analysis tools (Biomatters Inc, Newark, USA).
Forward and reverse sequences generated from each amplicon were aligned and manually trimmed and a consensus sequence was generated using the program Geneious (Version 9.1.5). Consensus sequences from all 10 BoHV-4 isolates and several published reference sequences were aligned using the Geneious alignment algorithm, and the Neighbour-Joining tree building method with 1000 bootstrap replicates was then used to assess the phylogenetic relationships among isolates.
Statistical analysis
Infection status of BoHV-4 was a response variable with binary outcome (infected/not infected). Predictor variables were all categorical and included number of lactation [Reference Bartha, Juhasz and Liebermann1], stage of lactation (early: 0–120 days, mid-lactation: 120–240 days and late lactation: >240 days), breed (Holstein, Jersey or cross-bred), season (summer, winter, fall or spring), post-partum metritis (positive/negative) and location (Yolo or Tulare).
Distribution of the various categories of variables with respect to the status of BoHV-4 was presented in a tabular form. The association between recorded variables and BoHV-4 was initially explored using univariate standard logistic regression model. All predictor variables were included in the final model regardless of the level of significance with univariate analysis (based on their presumed association with BoHV-4). Because of the multi-stage sampling design of the study that led to the hierarchical structure of the data, in addition to the binary outcome of the response variable, use of logistic mixed-effect model was deemed appropriate. Such model increases precision of parameter estimates by incorporating cluster-specific random parameters that account for correlation of the data [Reference Austin and Merlo16]. The following is a model equation for multilevel logistic mixed-effect regression model incorporating cluster-specific random effects to account for the within-cluster correlation of BoHV-4 infection:
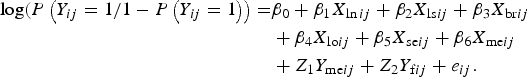 $$\eqalign{{\rm log}(P\left( {Y_{ij} = {\rm 1}/{\rm 1} - P\left( {Y_{ij} = {\rm 1}} \right)} \right) = & \beta _0 + \beta _{\rm 1}X_{\ln ij} + \beta _{\rm 2}X_{{\rm ls}ij} + \beta _{\rm 3}X_{{\rm br}ij} \cr & + \beta _{\rm 4}X_{{\rm lo}ij} + \beta _{\rm 5}X_{{\rm se}ij} + \beta _{\rm 6}X_{{\rm me}ij} \cr & + Z_{\rm 1}Y_{{\rm me}ij} + Z_{\rm 2}Y_{{\rm f}ij} + e_{ij}.}$$
$$\eqalign{{\rm log}(P\left( {Y_{ij} = {\rm 1}/{\rm 1} - P\left( {Y_{ij} = {\rm 1}} \right)} \right) = & \beta _0 + \beta _{\rm 1}X_{\ln ij} + \beta _{\rm 2}X_{{\rm ls}ij} + \beta _{\rm 3}X_{{\rm br}ij} \cr & + \beta _{\rm 4}X_{{\rm lo}ij} + \beta _{\rm 5}X_{{\rm se}ij} + \beta _{\rm 6}X_{{\rm me}ij} \cr & + Z_{\rm 1}Y_{{\rm me}ij} + Z_{\rm 2}Y_{{\rm f}ij} + e_{ij}.}$$e ij~N(0,σ 2e) = Variance of level 1 (individual animal) random term (residual error),
Z j~N(0,σ 2z) = Variance of level 2 (farm) random term.
Let:
• Y ij = 1 denotes positive test result for BoHV-4 infection, while Y ij = 0 denotes negative test result for BoHV-4 infection.
• P(Y ij = 1) = Probability of being BoHV-4 infection positive for i th cow with in j th farm,
• X Lnij = Lactation for i th cow in j th farm,
• X Lsij = Stage of lactation for i th cow in j th farm,
• X brij = Breed of i th cow in j th farm,
• X Loij = Geographic location of i th cow in j th farm,
• X seij = Season of lactation for i th cow in j th farm,
• X meij = Metritis status of i th cow in j th farm,
• Y 1meij = Individual cow-specific metritis status for i th cow within j th farm (random slope),
• Y 2fij = Status of BoHV-4 infection for i th cow within j th farm (cluster variable),
• β 0 = Intercept for fixed-effect component of the model,
• β 1 = Regression coefficient for explanatory variables,
• Z 1 = Regression coefficient for random variable,
• Z 2 = Coefficient for cluster variable.
Multilevel logistic mixed model was fit with maximum likelihood estimation (adaptive Gauss–Hermite Quadrature). An introduction of random effect allows the variation in the level of association between BoHV-4 and individual cows across farms [Reference Austin and Merlo16], thus accounting for within-farm correlation of BoHV-4 for better estimation of measure of association and underlying heterogeneity at individual levels. Significance of clustering effect due to farm was calculated by computing likelihood ratio test statistic (LR) as two times the difference in the log likelihood values between two-level model (that incorporated farm) and single-level model (individual cow's level). Larger value of the difference in the log-likelihood ratio of the two models (compared with reference value from χ 2 table, df = 1, P < 0.05) was considered statistically significance (between-farm variance is non-zero). Furthermore, to account for difference in the prevalence of metritis between the farms (due to variation in farm management and veterinary care), a random slope for metritis was incorporated in the model and tested for its significance using LR. Intra-cluster coefficient [Reference Castrucci12] was estimated as the proportion of total variation observed at individual level in an outcome variable that is attributable to between-cluster variation (the ratio of the between-cluster variance to the total variance) [Reference Austin and Merlo16]. The intra-cluster coefficient (ICC) allows comparison of heterogeneity in the risk of exposure of individual cows to BoHV-4 within farms compared with between farm. Large value of ICC indicates similarity in the risk of exposure to BoHV-4 for cows within farms. For all statistical tests, P < 0.05 was considered statistically significant. The data were analysed using R statistical computing software (Version 3.3.2) (R Foundation for Statistical Computing).
Results
One hundred and forty-eight dairy cows (115 from Yolo county and 33 from Tulare county) with mean age of 4.04 years were examined from 11 dairy farms. Breed composition showed 73.0% (108/148) Holstein, 16.9% (25/148) Jersey and the rest 10.1% (15/148) cross-bred. Mean rectal temperature for cows with and without post-partum metritis, when available were 104.91°F (40.5 °C) and 100.1°F (37.83 °C), respectively (convert to degrees Celsius as that is the metric unit).
Table 1 represents frequency distribution of the various categories of recorded variables with respect to BoHV-4 infection. Overall prevalence of BoHV-4 infection in the study population was 22.3% (33/148), while post-partum metritis was 33.8% (48/142). Univariate standard logistic regression analysis showed a significant level of association between BoHV-4 and number of lactation (P < 0.05), stage of lactation (P < 0.05) and post-partum metritis (P < 0.05). However, no significant association was found between BoHV-4 and breed (P ⩾ 0.05), geographic location (P ⩾ 0.05) or season (P ⩾ 0.05). The odds of BoHV-4 infection were higher among cows in the fourth (OR = 4.44, 95% CI 1.12–18.54; P = 0.033) and fifth lactation (OR = 5.92, 95% CI 1.63–23.67, P = 0.008) compared with cows in the first lactation. Cows in the early stage of lactation had higher odds for BoHV-4 infection (OR = 8.97, 95% CI 2.44–58.16, P = 0.004) compared with cows in the late stage of lactation. Similarly, a strong association was found between BoHV-4 and metritis infection (OR = 4.45, 95% CI 1.98–10.32; P = 0.00036).
Table 1. Frequency distribution of the various levels of recorded variables with respect to BoHV-4 infection status
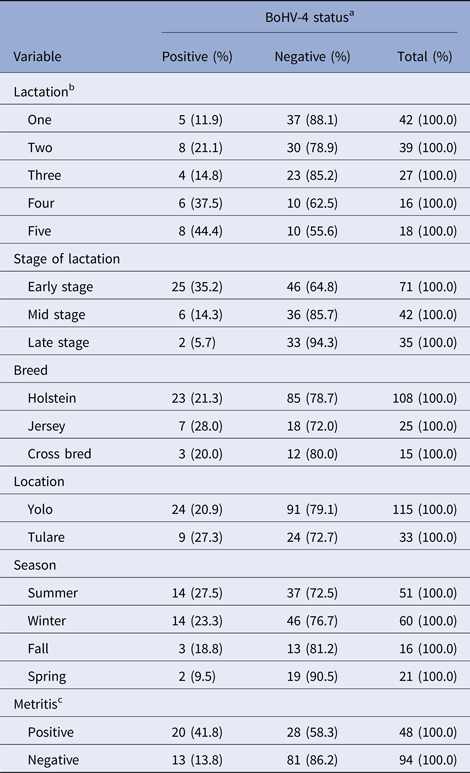
a One observation missing.
b Seven observations missing.
c Six observations missing.
Table 2 summarises estimates of crude (ORcrude) and adjusted odds ratio (ORadjust) from univariate standard logistic regression and multilevel logistic mixed model, respectively, showing the relationship between recorded variables and BoHV-4 infection in post-partum dairy cows. Similarly, results from the multilevel logistic mixed model showed strong association between BoHV-4 infection and lactation number, lactation stage and metritis. When other variables were held constant, the odds of being positive for BoHV-4 infection was 6.79 (95% CI 1.19–38.55, P = 0.03) times higher for cows in the fifth lactation compared with cows in the first lactation. Similarly, cows in the fourth lactation were at significantly increased risk (OR = 6.47; 95% CI 1.17–35.92, P = 0.032) of being positive for BoHV-4 infection compared with cows in the first lactation. When post-partum metritis-affected cows in the same farm, geographic location, lactation number and same breed were considered, BoHV-4 infection was strongly associated with the stage of lactation; with the risk being 8.27 (95% CI 1.43–47.94, P = 0.019) times more likely among cows at the early stage of lactation (0−120 days) compared with those in the late stage of lactation (>240 days). When other variables were held constant, for cows on the same farm, the odds of infection with BoHV-4 was 4.51 times more likely (95% CI 1.27–16.02, P = 0.019) for cows with post-partum metritis compared with those without post-partum metritis. We found no significant effect of breed, geographic location and season on BoHV-4 prevalence.
Table 2. Estimated crude (ORcrude) and adjusted odds ratio (ORadjust) from univariate standard logistic regression and multilevel logistic mixed model, respectively, showing the effects of recorded variables on BoHV-4 in post-partum dairy cows in California
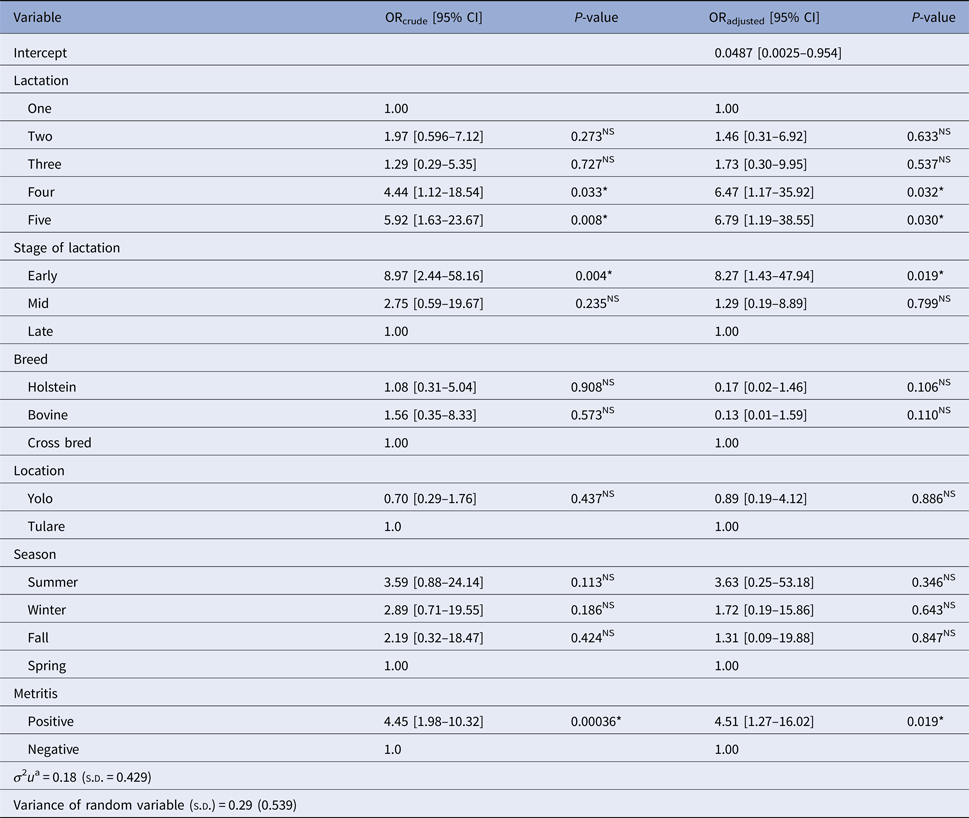
*, Significant;NS, Non-significant.
a Variance of cluster variable (between farm) variance).
LR for cluster effect due to farm was 5.36 indicating significant evidence that between-farm variance was non-zero (df = 1; P < 0.05). Test of random slope for metritis (the difference in the log likelihood values between the model with and without the random slope for metritis at farm level) showed its significant variability across the farms (χ 2 = 4.6; df = 1; P < 0.05). Estimated value of ICC was 0.396 (39.6% variability in BoHV-4 between individual cows was due to farm difference).
Co-infecting viruses
RT PCR analysis of uterine/vaginal samples revealed simultaneous presence of PPV and BoHV-4 in five samples, all from post-partum metritis-affected cows. BoHV-1 was detected in one uterine/vaginal sample collected from metritis-affected cows with no BoHV-4 infection. RT PCR did not reveal the presence of BoHV-2 in any tested samples. Simultaneous presence of BVD and BoHV-4 was detected in one uterine/vaginal sample collected from metritis-affected cow.
Phylogenetic analysis of BoHV-4 isolates
Of the 23 uterine/vaginal discharge samples analysed for viral isolation and sequencing, only 13 samples yielded positive results for the isolation of the virus, and all these samples originated from post-partum metritis-affected cows. However, none of the samples collected from metritis-negative cows yielded positive results for viral isolation.
After nucleotide sequence alignment and trimming of terminal alignment of columns containing missing data, our dataset for all 10 BoHV-4 field strains contained 531 positions of TK gene, 593 positions of gB gene and 543 positions of the gH gene [Reference Sumner, Azuara and Coughlin10].
Figure 1 presents a phylogenetic analysis of BoHV-4 field isolates based on sequencing of the ORF-3 gene that encodes for TK gene. Six single nucleotide polymorphisms (SNPs) across the TK gene alignment separated the BoHV-4 isolates into two distinct groups, which we designate as TK genotype 1 and TK genotype 2 (Fig. 1). TK genotype 1 was a unique group comprising four isolates: D1611430-1.88, D1609492-1.6, D1300426-1.1 and D1611430-2.48.
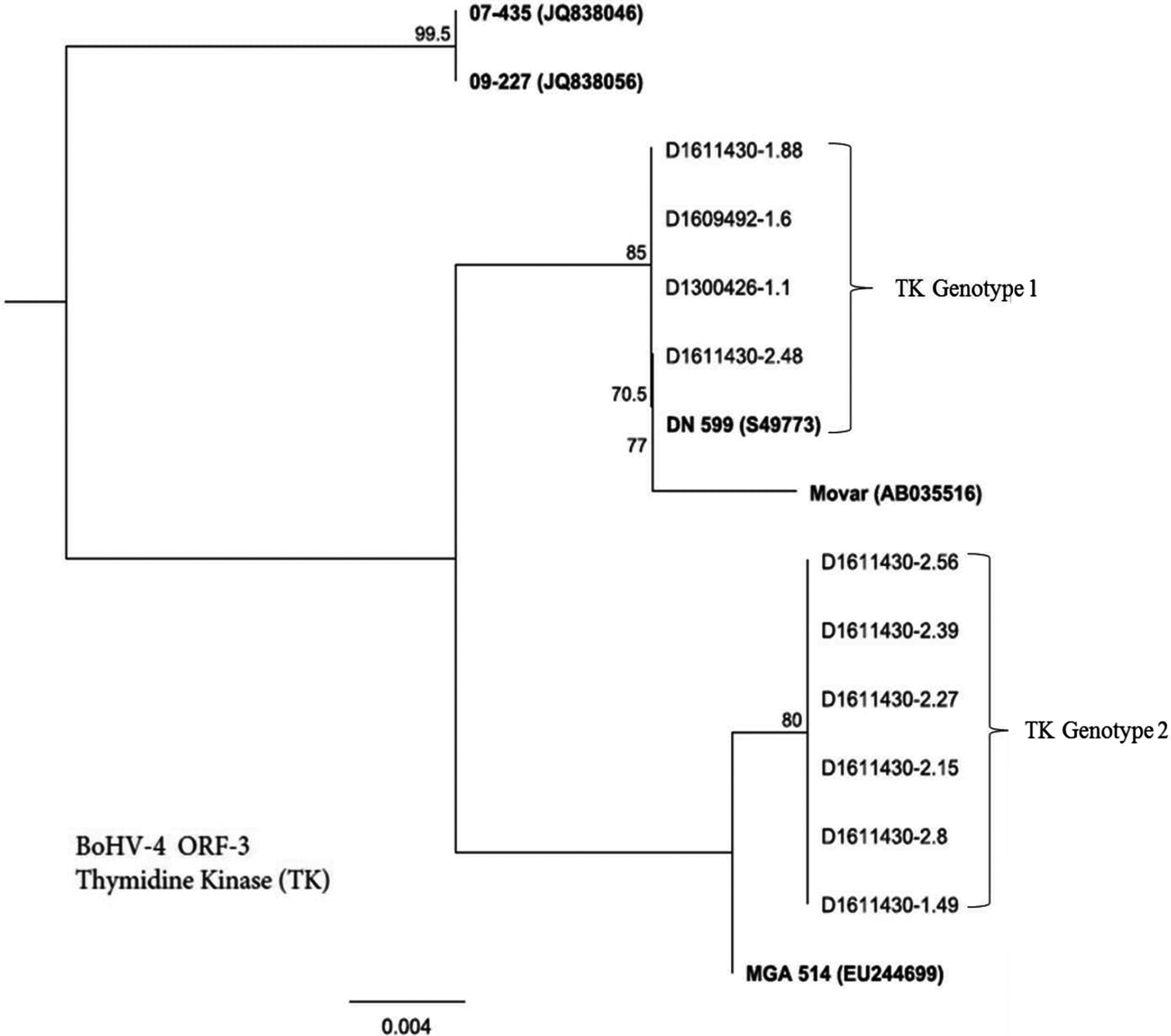
Fig. 1. Genetic relatedness of BoHV-4 isolates (n = 10) obtained from uterine/vaginal discharge samples of California dairy cows with post-partum metritis. Phylogenetic tree was constructed using Neighbour-Joining method from sequencing of ORF-3 gene that encodes for thymidine kinase (TK). Movar and DN599 are European and American prototypes of BoHV-4 strains, respectively.
Whereas TK genotype 2 included six isolates: D1611430-2.56, D1611430-2.39, D1611430-2.27, D1611430-2.15, D1611430-2.8 and D1611430-1.49.
A translation of the TK gene alignment showed two amino acid differences between the two genotypes. Members of TK genotype 1 were closely related to previously described European (DN 599-S49773) and American (Movar-AB035516) reference strains. Whereas TK genotype 2 isolates were more closely related to MGA514 (EU244699) strain than DN 599 and Movar. On the other hand, neither of TK genotype 1 and TK genotype 2 were closely related to 07-435 (JQ838046) and 09-227(JQ838056) – the two genetically analogous strains, described from Argentina [Reference Altamiranda19].
Figure 2 presents phylogenetic analysis of BoHV-4 field isolates based on sequencing of gB coding for ORF-3. Nucleotide and amino acid differences across the gB gene were more pronounced than TK gene. Across 593 nucleotide positions, 36 unique SNPs and 1 3 bp insertion/deletion (indel) were present. These corresponded to 22 amino acid substitutions and one amino acid indel. Phylogenetic analysis of the gB gene revealed three distinct groups: gB genotype 1, gB genotype 2 and gB genotype 3 among the field strains (Fig. 2). The gB genotype 1 comprised six isolates: D1611430-2.56, D1611430-2.39, D1611430-2.27, D1611430-2.15, D1611430-2.8 and D1611430-1.49. Whereas, gB genotype 2 included one isolate: D1300426-1.1. The gB genotype 3 included three isolates: D1609492-1.6, D1611430-2.48 and D1611430-1.88. The only difference with TK gene was that strain D1300426-1.1 was clustered with strains D1609492-1.6, D1611430-2.48 and D1611430-1.88 in the TK analysis and with the remaining field strains in the gB analysis. The gB genotype 3 was more ancestral in evolutionary process than gB genotype1 and gB genotype 2.
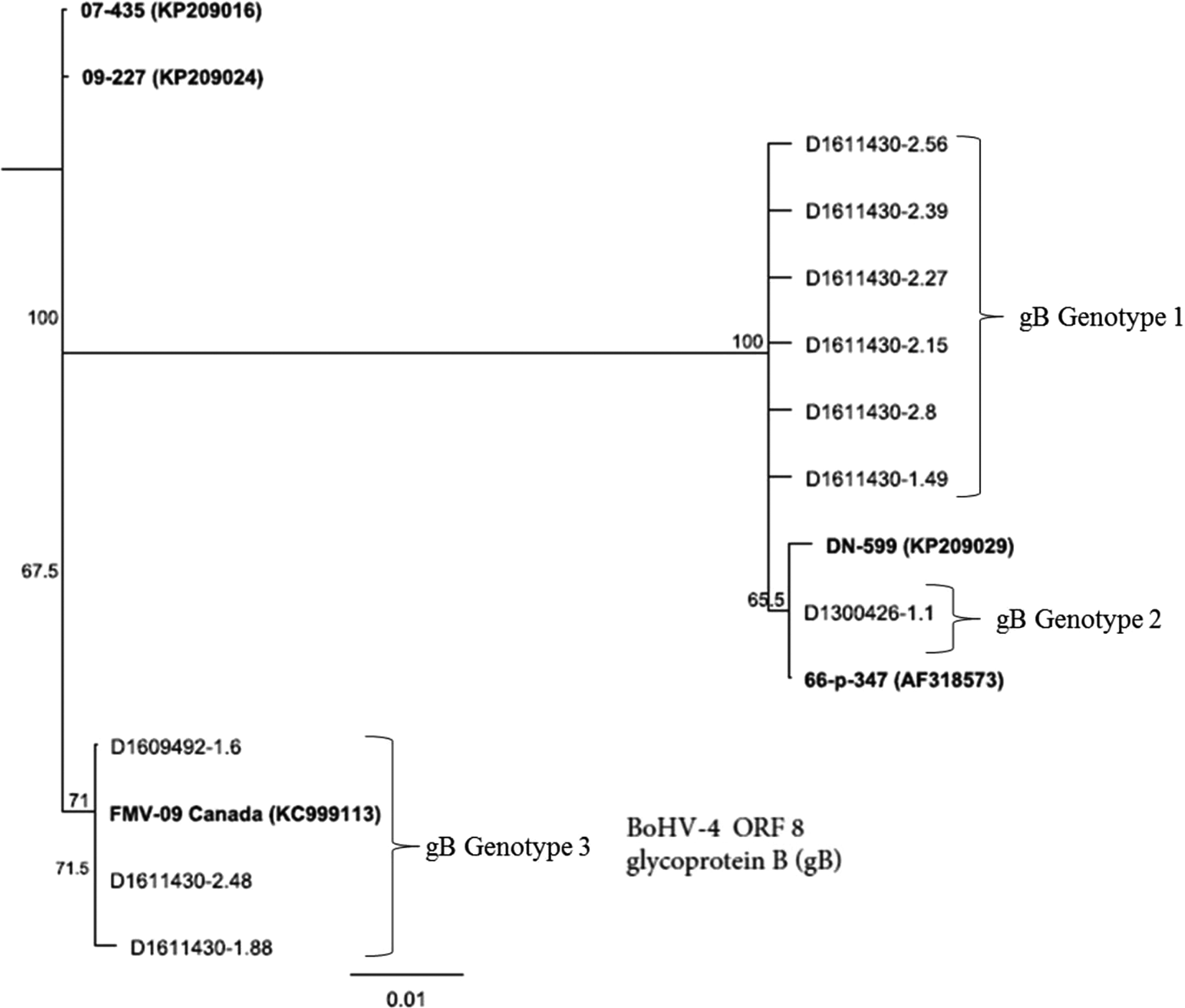
Fig. 2. Genetic relatedness of BoHV-4 isolates (n = 10) obtained from uterine/vaginal samples of California dairy cows with post-partum metritis. Phylogenetic tree was constructed using Neighbour-Joining method from sequencing of the ORF-8 gene that encodes for glycoprotein (gB).
Additionally, in contrast to the TK gene, the evolutionary relationships based on the gB gene analysis showed that the three strains D1609492-1.6, D1611430-2.48 and D1611430-1.88 were more closely related to FMV-09 (KC999113) strain than they were to the other field strains or reference strains (DN-599 and 66-p-347(AF318573)).
Figure 3 presents genetic resemblance of BoHV-4 isolates (n = 10) based on sequencing of ORF-22, a gene that encodes for gH. Three district groups (genotypes) were recognised: gH genotype 1, gH genotype 2 and gH genotype 3. Members of gH genotype 1 included three isolates D1611430-2.48, D1611430-1.88 and D1609492-1.6. Whereas gH genotype 2 members encompassed six isolates: D1611430-2.56, D1611430-2.39, D1611430-2.27, D1611430-2.15, D1611430-2.8 and D1611430-1.49. Only one isolate (D1300426-1.1) was included in gH genotype 3. The gH genotype 1 appears to be a unique group that did not relate to either the reference strain (07-435) or the rest of field isolates.
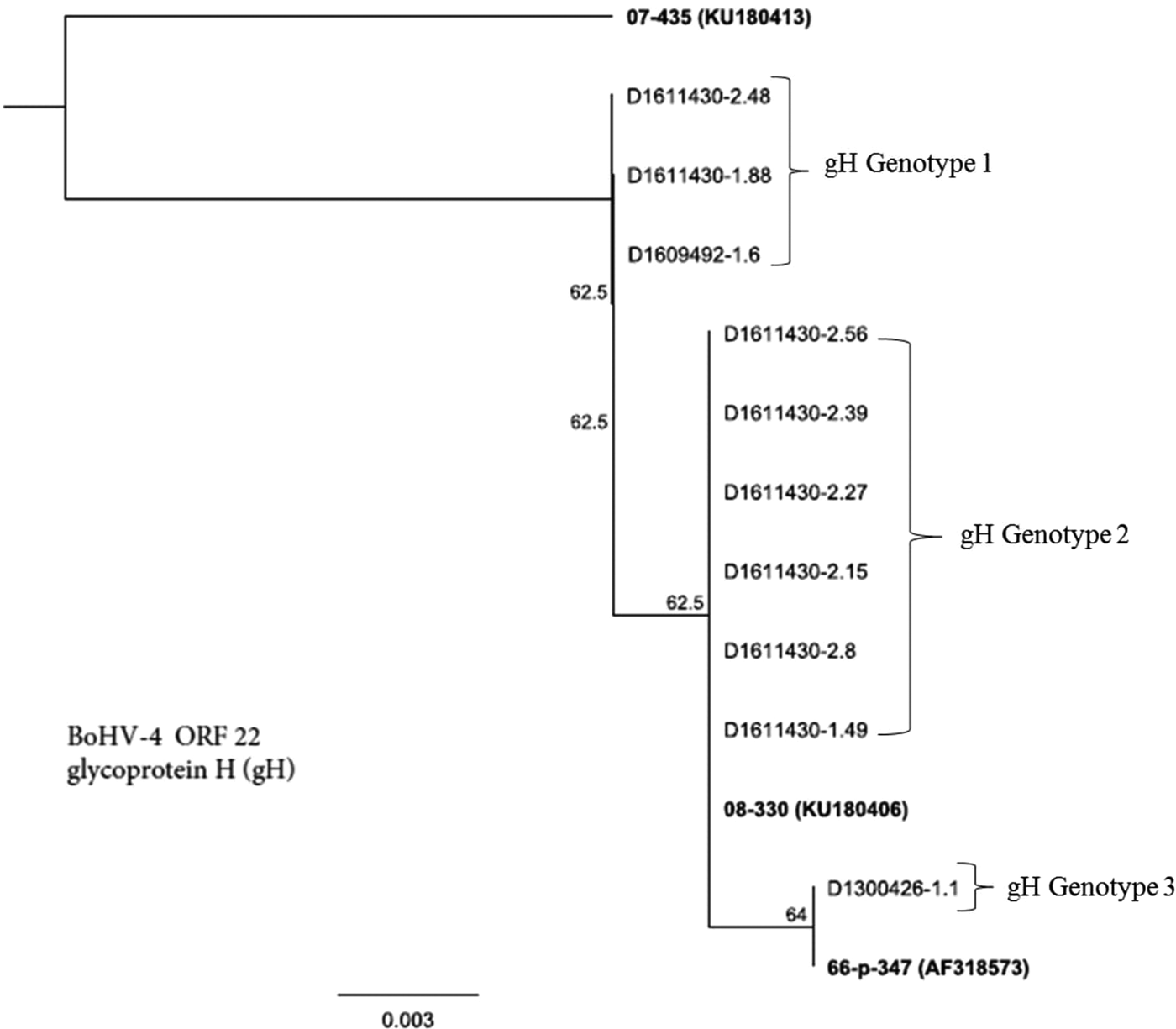
Fig. 3. Genetic relatedness of BoHV-4 isolates (n = 10) obtained from uterine/vaginal discharge samples of California dairy cows with post-partum metritis. Phylogenetic tree was constructed using Neighbour-Joining method from sequencing of ORF-22, a gene that encodes for glycoprotein H.
Discussion
This is the first comprehensive study documenting the prevalence and associated risk factors of BoHV-4 infection in California dairy herds and the dominant genotypes of the virus circulating in the area. We documented BoHV-4 prevalence of 22.3% (33/148) among studied dairy cows. Our study results confirmed a significant positive association of BoHV-4 with clinical metritis, after adjusting for other recorded variables. Early stage of lactation (<120 days) and having multiple lactations were risk factors for BoHV-4 infection. However, breed, geographic location and seasons when the study were not associated with BoHV-4 infection. Furthermore, phylogenetic analysis of 10 BoHV-4 viruses isolated from uterine/vaginal samples revealed the association of distinct and novel genotypes of BoHV-4 with clinical post-partum metritis in dairy cows. On the other hand, failure to isolate virus from PCR-positive samples was observed and this can be attributed to a number of factors including poor quality of incoming samples. It is likely that samples collected from metritis-negative cows were of poor quality since comparing rate of viral isolation between the two groups (metritis vs. no metritis) was not the primary objective of the study, and that less attention was given to the quality of samples to be collected from metritis-negative cows. Additional possible explanation could be the inherent difficulty of isolating BoHV-4 from samples. Despite its tropism for many cell types, it is relatively difficult to isolate BoHV-4 even in commonly used cell culture types such as MDBK cells due to its slow replication cycle [Reference Wellenberg22].
The prevalence of BoHV-4 varies widely across the world depending on dairy husbandry system, geographic localities and more importantly diagnostic test used. Therefore, comparability of prevalence records from different geographic places and dairy practices should take in account the diagnostic tools used to detect the virus. The PCR-based 22.3% prevalence of BoHV-4 recorded in our study is comparable with 21.0% reported from aborting dairy cows in Serbian farms that employed a similar diagnostic test [Reference Cvetojevic18]. Serological tests detected as high as 84.37% of BoHV-4 antibodies from cows with post-partum metritis [Reference Nikolin23]. In contrast, a study conducted in Southern California to determine the causes of abortion in dairy herds (based on combination of necropsy, isolation, immunology, serology and toxicology), reported no isolation of BoHV species, including type 4, among the list of viral causes [Reference Anderson24]. From the same region yet in a different time, Frazier et al. [Reference Frazier25] reported a 36% (107/296) seroprevalence of BoHV-4 from post-partum cows with ulcerative endometritis. This indicates substantial variability in the prevalence estimates of BoHV-4 across geographic regions and dairy husbandry practice based on diagnostic tests employed to detect the infection.
Our finding of strong association of BoHV-4 with post-partum metritis (Table 2) is consistent with the findings from previous studies [Reference Nikolin23, Reference Frazier25, Reference Donofrio26]. Viruses isolated and genotyped in our study (percent isolation = 76.9 (10/13)) were all obtained from clinical metritis cows vs. no virus isolation from non-metritis cows, which may suggest BoHV-4 as a potential causative agent of post-partum metritis. However, since our study was cross-sectional aimed to establish disease–risk factor association, it would be speculative inference to discuss about causal relationship. However, an experimental study conducted by Donofrio et al. [Reference Donofrio27] demonstrated BoHV-4 as pathogenic agent of uterine disease based on cellular and molecular mechanisms. Persistent survival of BoHV-4 in latent form in immune cells such as monocyte-macrophage lineage [Reference Donofrio and van Santen28] may lead to cell lysis and subsequent immunosuppression in host and increased severity of pathogenesis due to flare up of bacterial infections. Association of multiparity with increased risk of BoHV-4 infection (Table 2) suggests the existence of age and stress-related predisposing factor for exposure to latent infection with BoHV-4.
Our study also demonstrated a significant association of BoHV-4 with early stage of lactation (Table 2) (<120 days), and this is consistent with the findings of Frazier et al.’s study [Reference Frazier25], which demonstrated the occurrence of BoHV-4-associated endometritis during the 3–28 days post-partum period. The concern of whether the high infection prevalence observed in the early stage of post-partum period was due to carryover from gestation period or due to exposure post-partum needs to be investigated further. Higher prevalence of BoHV-4 infection in metritis cows in the early stage of lactation might be explained by its concurrent occurrence with bacterial-induced uterine infection. Several studies have documented the association of BoHV-4 with bacterial-induced metritis [Reference Frazier6, Reference Czaplicki and Thiry13, Reference Frazier25]. Pathogenic bacteria of various species are implicated as major causes of uterine infection in the early stage of lactation [Reference Sheldon and Dobson7, Reference Sheldon17]. This is due to the suppression of uterine immune system by progesterone, which is secreted from the first formed corpus luteum following parturition [Reference Lewis29, Reference Lewis30].
The uterus of post-partum cows is usually contaminated with several species of pathogenic bacteria that might overwhelm the immune system of the uterine and cause frequent uterine infections that cause decreased reproductive performance [Reference Lewis30, Reference Borsberry and Dobson31]. It is documented that the bacterial infection of uterus following parturition induces secretion of prostaglandin E2 (PGE2), a key mediator of inflammatory responses, which activates BoHV-4 lytic replication in macrophages persistently infected with BoHV-4 [Reference Donofrio32–Reference Zhu34]. Additionally, newly produced BoHV-4 particles (as a result of PGE2-induced replication) might infect the uterine membranes, which further increases the secretion of PGE2 [Reference Donofrio32]. Therefore, such positive-feedback mechanism between PGE2 production and viral replication could explain a role for BoHV-4 in causing metritis [Reference Donofrio32]. Stress as well as other calving-related problems such as dystocia, stillbirth and twins [Reference Cheong35, Reference Potter36] can be an important predisposing factor for increased risk of infection with BoHV-4.
To the authors’ knowledge, BoHV-4 coinfections with PPV (five cases) and BoHV-1 (one case) in post-partum metritis cows are the first cases reported in California dairy farms. Generally, PPV as a primary or even secondary cause of post-partum metritis is rare. Likewise, concurrent involvement of BVDV and BoHV-4 in post-partum metritis was detected in one uterine sample. Similar studies have documented BVDV as a co-infecting virus in BoHV-4-associated abortion in Serbian dairy herds [Reference Cvetojevic18] and endometritis in post-partum cattle from North America [Reference Frazier6]. Another study from North America has documented coinfection of foetus with BoHV-4 and BVDV [Reference Reed, Langpap and Bergeland37]. BoHV-1 was detected in one uterine sample collected from metritis-infected cow with no BoHV-4. At this time, we are not certain if coinfection with these viruses (PPV and BVDV) has exacerbating effects on pathogenesis and pathology of post-partum metritis. Our study did not reveal the presence of BoHV-2 in any tested uterine samples.
Grouping of gB genotype 3 along with the reference strain BoHV-4-FMV-09 (Fig. 2) indicates that the genotype could be involved in causing respiratory infection in addition to uterine infection. The reference strain BoHV-4-FMV-09 has recently been found to be associated with respiratory disease from Quebec, Canada [Reference Gagnon38]. Furthermore, gB genotype 1 (Fig. 2) may have been the dominant genotype associated with post-partum metritis in California dairy cows since it is a distinct novel clade that comprised the majority (60%) of the isolates. The importance of gB to BoHV-4 has been documented recently by Franceschi et al. [Reference Franceschi8], who indicated that the gene is essential for BoHV-4 replication and its deletion inhibits productive replication of the virus, thus suggesting possibility for targeting the gene for treatment.
Phylogenetic analysis of thymidine kinase of BoHV-4 isolates revealed two distinct groups: TK genotype 1 and TK genotype 2 (Fig. 1). One possible explanation for genetic resemblance of the members of TK genotype 1 with European strain (Movar) (Fig. 1) is the possibility of disease transmission from Europe into North American through importation of animals or animal products. The novelty of TK genotype 2 and its composition of the majority of isolates (60%) (Fig. 1) suggest dominance of this genotype in post-partum metritis in California. However, with such relatively small number of isolates and sequence analysis of TK gene may not be sufficient to arrive at such conclusion. We therefore, suggest that such an assertion should be further tested by an in-depth phylogenetic analysis of a large number of isolates using multiple gene sequences including glycoprotein and TK. In order to generate accurate information about phylogenetic picture of BoHV-4, TK gene sequencing needs to be combined with other markers or several strains [Reference Verna21].
Like gB, gene sequence analysis of BoHV-4 revealed distinct and novel genotypes of BoHV-4 isolates (Fig. 3). Three distinct patterns of clades were observed with gH gene sequencing of BoHV-4 isolates from our study, gH genotype being a novel genotype, while gH genotype 2 and gH genotype 3 closely resemble previously described strain of 08-330 and 66-p-347, respectively. This suggests that phylogenetic analysis of the gene can be used as an additional biomarker for grouping of strains of BoHV-4. However, information regarding genetic variability of BoHV-4 isolates based on sequencing of gH gene is scarce, thus making it difficult to perform comparative analysis.
Our findings of high genetic diversity of BoHV-4 as evidenced by the observation of distinct genetic patterns, most resemble reference strains and two reported as being novel genotypes: TK genotype 2 (Fig. 1) and gH genotype 1 (Fig. 3) point to an ongoing natural selection possibly induced by environmental, host or pathogen factors. However, to what extent the prevailing dairy husbandry system or possibility of co-infection with multiple bacterial species, which are known to be primary agents of post-partum metritis [Reference Sheldon and Dobson7, Reference Sheldon17], impacted genetic diversity of BoHV-4 warrants further investigation. Furthermore, isolation of various BoHV-4 genotypes from post-partum metritis in California dairy cows highlights the importance of further research since this will eventually be helpful for vaccine development in the future. It is possible that genetic diversity of strains for a pathogenic virus can be a factor for potential difference in the pathogenicity of strains [Reference Gagnon38].
A limitation of our study was the relative smaller sample size (n = 148) that could have limited statistical test from providing accurate point estimates (odds ratio and standard errors). Consideration of fewer specific geographical areas in our study may limit external validity of our results. Additionally, convenience sampling of farms may have introduced a selection bias.
Conclusions
Our study demonstrated a strong association of BoHV-4 with post-partum metritis, multiparity as well as early stage of lactation signifying the need for implementing proper management (isolating infected animals, minimizing stress, early treatment of bacterial infections and proper dietary supplies) to minimise exposure to the virus from uninfected cows as well as from the environment following reactivation and shedding. The finding of high genetic variability of BoHV-4 indicates the possibility of co-infection with multiple genotypes. Further investigation is needed to demonstrate the specific role of BoHV-4 genotypes reported in our study in the pathogenesis of BoHV-4 infections.
Acknowledgements
The authors thank dairy farms owners and extension veterinarians for their assistance in data and sample collection. The authors also thank laboratory personnel of the California Animal Health and Food Safety (CAHFS) laboratories for technical support in molecular sequencing works. They are also grateful to Dr Heejung Bang from the Department of Public Health Sciences, University of California for advice with statistical analysis.
Financial support
This project was conducted by fund received from the California Animal Health and Food Safety Laboratory system (CAHFS) – Davis Laboratory-protocol #17985.
Declaration of Interests
None.






