Background
Epidemiological studies of environmental and genetic risk factors indicate that elevated triglycerides (TGs), remnant cholesterol or TG-rich lipoproteins may be causal risk factors for cardiovascular disease (CVD) [Reference Austin, Hokanson and Edwards1–Reference Do3]. Anti-retroviral therapy (ART) is associated with dyslipidaemia, including increased levels of circulating TGs in populations of European decent [Reference Souza4–Reference Manfredi and Chiodo6]. As such, long-term ART may be associated with increased risk of CVD. Indeed, observational evidence suggests that certain ART regimens may be associated with increased risk of CVD in European populations [Reference Friis-Moller7].
However, in sub-Saharan Africa (SSA), a region with the highest burden of HIV and where access to ART has substantially increased over the last decade, the association between ART and TGs has not been clarified. Importantly, the relationship between ART and risk factors for CVD in populations from SSA may be more complex because of differences in cardiometabolic risk profiles, HIV strains, efficacy of ART and environmental factors [8–Reference Li19]. It is therefore crucial to assess the association between ART and cardiovascular risk factors in SSA populations – to inform strategies to control the rising burden of CVD in the region.
We previously conducted a systematic review to provide a preliminary assessment of the relationship between HIV and ART with a set of cardiometabolic risk factors [Reference Dillon20]. In this paper, we extend this work to synthesize existing evidence using individual participant data (IPD) pooled analyses to more reliably assess the magnitude and direction of association between ART and raised TGs and other risk factors for cardiometabolic disease in SSA.
Methods
Data sources and inclusion criteria
We invited 57 investigators to contribute IPD for pooled analysis: 52 investigators who had collaborated in a previous systematic review of the association between HIV, ART and cardiometabolic risk factors [Reference Dillon20], and five investigators identified through personal communication with other collaborators. Briefly, we included studies conducted in SSA that had collected data on HIV/ART status and the relevant cardiometabolic risk factors among black Africans aged 13 years or older. Studies were excluded if they lacked a comparison group, or had too few (<10) or no participants with a risk factor based on a defined cut-off, or had a very small sample size (<10 participants).
Data collation
We requested data on cardiometabolic risk factors [blood pressure (BP), lipids, glucose and glycated haemoglobin (HbA1c)], HIV infection and ART status. We also collected additional variables for adjustment (Table 1). Data were checked for plausibility and consistency and, when necessary, collaborators were contacted for clarification before analysis. We converted all variables measured on other scales to the SI scale (mmol/L, %, mm Hg or Kg/m2).
Table 1. Data requested for estimating the magnitude and direction of association between anti-retroviral therapy (ART) and selected cardiometabolic risk factors in sub-Saharan Africa
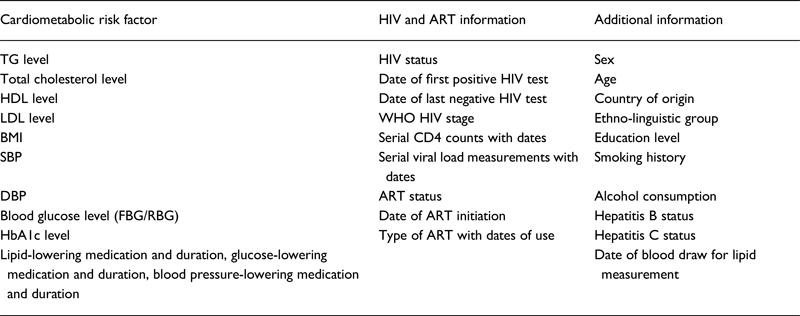
TG, triglycerides; HDL, high-density lipoprotein cholesterol; LDL, low-density lipoprotein cholesterol; BMI, body mass index; SBP, systolic blood pressure; DBP, diastolic blood pressure; FBG, fasting blood glucose; RBG, random blood glucose; HbA1c, glycated haemoglobin; ART, antiretroviral therapy; WHO, World Health Organization.
Definition of outcomes and exposure
The outcomes for this study were binary cardiometabolic risk factors defined according to predefined clinical cut-offs [Reference Ware21–26]. We analysed the following risk factors: raised TGs (>2.3 mmol/L), raised low-density lipoprotein (LDL ⩾3.3 mmol/L), raised total cholesterol (TC >5.2 mmol/L) and low high-density lipoprotein (HDL <1.3 mmol/L for women and HDL <1.0 mmol/L for men). We also analysed raised BP (>140/90 mm Hg), raised blood glucose (fasting blood glucose ⩾7.0 mmol/L or random blood glucose ⩾11.1 mmol/L) and raised HbA1c (⩾6.5%). The exposures of interest were HIV infection status (positive or negative) and ART use. HIV infection status was defined as presented in each study. An individual was considered to have untreated HIV infection if they were HIV-positive and had never received ART medication. In addition, individuals recorded as receiving ART were considered to be HIV-infected. We defined ART use as a receipt of ART medication at the time of cardiometabolic risk factor measurement.
Statistical analysis
We conducted a two-step IPD pooled analysis, analysing each dataset separately to obtain study-level estimates, before combining them using random-effects models of meta-analysis. We fitted Poisson regression models with robust sandwich estimators of variance for each outcome to obtain study-specific risk ratios (RRs) and prevalence ratios (PRs) for ART use and untreated HIV infection [Reference Zocchetti, Consonni and Bertazzi27, Reference Zou28]. RRs and PRs are collectively referred to as RR hereafter. We used multilevel mixed models to adjust for clustering of individuals within households in two cross-sectional studies and to account for correlation in repeated measurements in one longitudinal study included in the analyses.
In our primary analysis, we assessed the association between ART and each cardiometabolic risk factor by comparing individuals receiving ART (ART+) with individuals not receiving ART. Individuals not receiving ART were either untreated HIV-positive individuals (in studies of HIV-positive individuals only) or a combination of untreated HIV-positive individuals and HIV-negative (HIV-) individuals (in studies including both groups). In sensitivity analyses, we also compared associations between individuals receiving ART and HIV-negative individuals, and between individuals receiving ART and untreated HIV-positive individuals. We also compared untreated HIV-positive individuals with HIV-negative individuals to assess the impact of HIV infection on cardiometabolic risk independent of ART use.
All models were adjusted for body mass index (BMI), age and sex. In a subgroup of studies where data were available, we also adjusted for alcohol consumption, current smoking status, education level, fruit and vegetable consumption, physical activity and socio-economic position. Additionally, where data were available for lipid and glucose outcomes, we adjusted for lipid- and glucose-lowering medication, respectively. For BP as an outcome, we adjusted for BP-lowering medication where data were available. Further, for each cardiometabolic risk factor studied as an outcome variable, the other cardiometabolic risk factors were additionally adjusted for.
To pool the adjusted RRs, we estimated a weighted average of study-specific log (adjusted RR) incorporating between-study heterogeneity according to the method of DerSimonian and Laird [Reference DerSimonian and Laird29]. The I 2 statistic was used to assess the heterogeneity between study-specific estimates [Reference Higgins and Thompson30].
A predetermined set of study-level characteristics were assessed as potential sources of heterogeneity between studies using meta-regression: study type (population-based, clinic-based), study size, year the study was conducted, location of study (West, East, Central or Southern Africa), sex distribution (proportion of men), mean participant BMI and mean participant age. Lastly, we conducted sensitivity analyses to assess whether a single study could have influenced pooled RR results excessively by excluding each study from the pooled analysis in turn, and comparing results with and without the study in question.
All analyses were performed using STATA 13.1 (Stata, College Station, TX, USA).
Ethics
This study received ethical approval from the Human Biology Research Ethics Committee at the University of Cambridge, UK (Application No: HBREC.2015.05), and each primary study obtained informed consent from participants.
Results
We received data for 20 studies and included 14 studies conducted between 2003 and 2014 in the current analysis (Fig. 1). Overall, the pooled data comprised 21 023 participants aged 13–107 years, with generally fewer men than women but with varying proportions for each cardiometabolic risk factor studied (Table 2). The number of individuals and proportion of men and women included in the analyses varied by cardiometabolic risk factors because not all studies had data on all risk factors, and because of missing data within studies. Apart from one all-women study, the proportion of men across all studies ranged between 22% and 49%. In the primary analyses (comparing individuals on ART to individuals not receiving ART), the number of participants included ranged from 6364 with data on glucose to 10 620 with data on TC (Table 2).
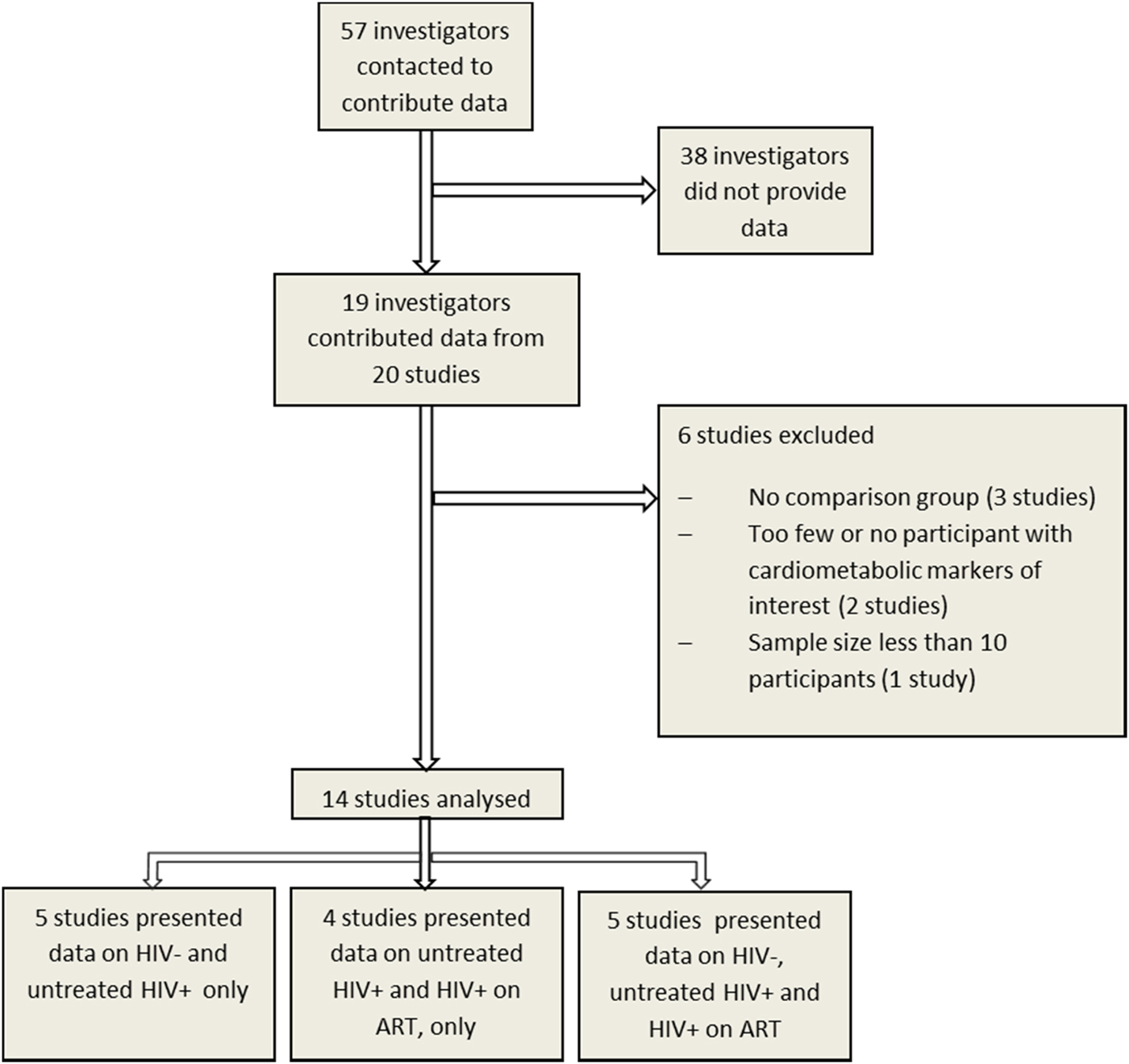
Fig. 1. Study selection for individual participant data pooled analysis to assess the association of HIV and anti-retroviral therapy with cardiometabolic risk in sub-Saharan Africa.
Table 2. Characteristics of 14 studies included in the pooled analyses to assess the association between HIV/anti-retroviral therapy (ART) and selected cardiometabolic risk factors in sub-Saharan Africa
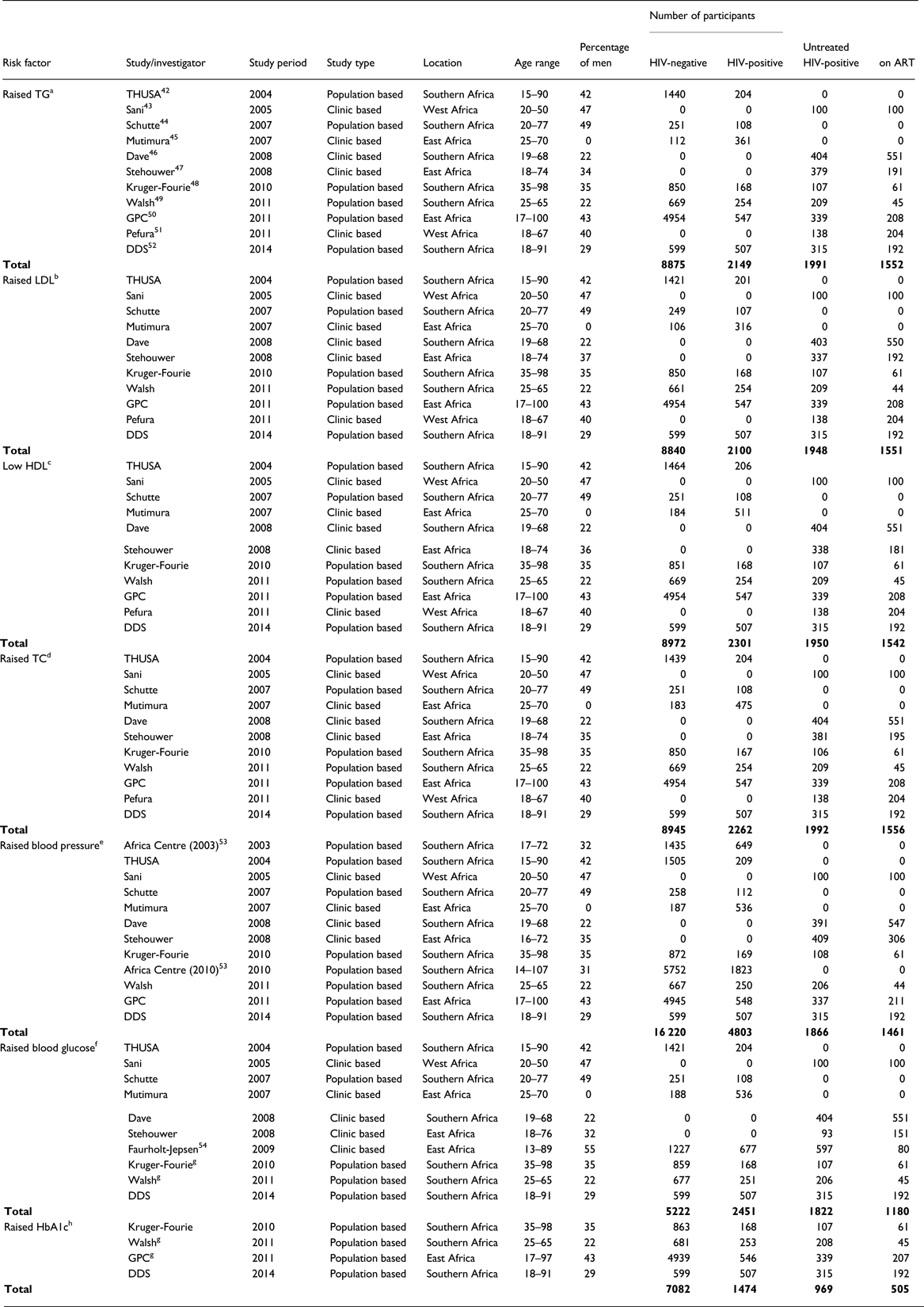
TG, triglycerides; LDL, low-density lipoprotein; HDL, high-density lipoprotein; TC, total cholesterol; HbA1c, glycated haemoglobin.
a Raised TG defined as TG >2.3 mmol/L.
b Raised LDL defined as LDL ⩾3.3 mmol/L.
c Low HDL defined as HDL <1.3 mmol/L (women) and HDL <1.0 mmol/L (men).
d Raised TC defined as TC >5.2 mmol/L.
e Raised BP defined as systolic blood pressure ⩾140 mm Hg or diastolic blood pressure ⩾90 mm Hg.
f Raised glucose defined as glucose ⩾7.0 mmol/L (fasting) or glucose ⩾11.1 mmol/L (non-fasting).
g Estimates for ART not available because of too few cases of raised glucose; GPC, General Population Cohort; THUSA, Transition and Health during Urbanization in South Africa; DDS, Durban Diabetes Study.
h Raised HbA1c defined as HbA1c ⩾6.5%.
From the above, 10 615 [36% men; age range 17–100 years; mean age 41.4 years (SD 14.0)] individuals from eight studies provided data for analyses of the association between ART and raised TG. Of these, 1552 were on ART, 1413 (91%) of whom provided data on ART regimen (Table 3). Among those with data on ART regimen, 80% were on two nucleoside reverse transcriptase inhibitors (NRTIs) [mainly zidovudine (AZT) and lamivudine, 80%] and one non-nucleoside reverse transcriptase inhibitor (NNRTI) [mainly efavirenz (EFV) or nevirapine (NVP), 87%] (two NRTIs + one NNRTI); while 13% received two NRTIs and one protease inhibitor (PI) (two NRTIs + one PI) (Table 3). In all, 87% of the individuals with TG data, who were on ART, and provided ART regimen data, were on a non-PI combination (predominantly, two NRTIs + one NNRTI), while 13% were on a combination including a PI. The prevalence of raised TG was 10.5% (95% CI 7.5–13.9), overall; 13.2% (95% CI 8.1–19.2) among individuals on ART and 8.4% (95% CI 4.9–12.6) among individuals not on ART.
Table 3. Number of individuals (with data on triglycerides) receiving specific antiretroviral therapy drug class combination and the most common regimen in pooled analyses of the association between ant-retroviral therapy and cardiometabolic risk in sub-Saharan Africa
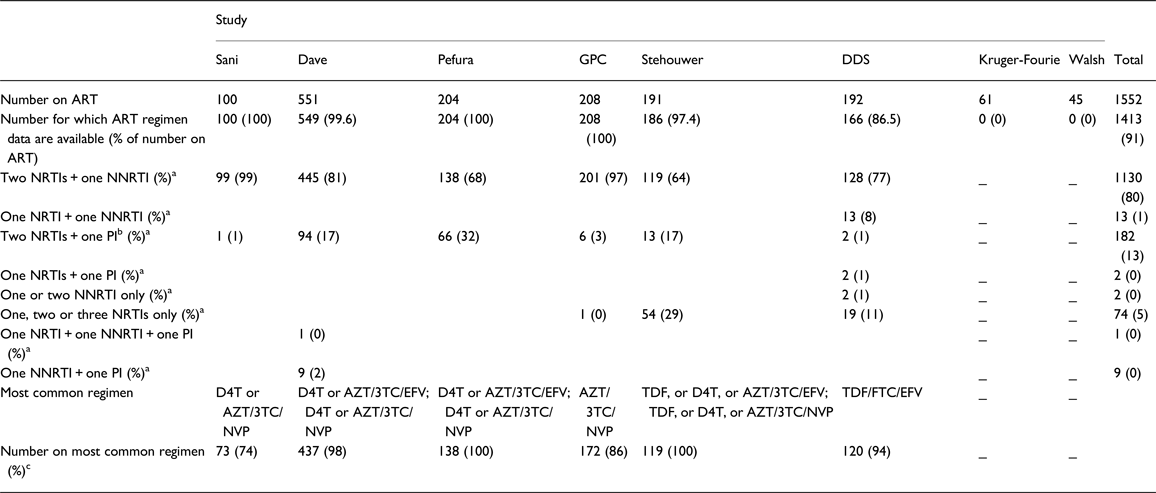
ART, antiretroviral therapy; NRTI, nucleoside reverse transcriptase inhibitors; NNRTI, non-nucleoside reverse transcriptase inhibitors; PI, protease inhibitors; GPC, General Population Cohort; DDS, Durban Diabetes Study; D4T, stavudine; AZT, zidovudine; 3TC, lamivudine; NVP, niverapine; EFV, efavirenz; TDF, tenofovir; FTC, emtricitabine.
a Percentage of number for which ART regimen data are available.
b The PI was lopinavir/ritonavir, 80% of the time.
c Percentage of number receiving 2NRTIs + 1NNRTI.
_ Regimen data not provided.
Some non-zero proportions are recorded as 0% and some percentages do not add up to 100%, because of rounding errors.
Association between ART and raised TG
Compared with individuals not receiving ART (i.e. untreated HIV-positive individuals only, or untreated HIV-positive individuals and HIV negative individuals combined), individuals receiving ART were two times more likely to have raised TG (RR 2.05, 95% CI 1.51–2.77) (Fig. 2a). This association did not vary substantially across studies (I 2 45.2%) and was consistent when ART users were compared with HIV-negative individuals only and with untreated HIV-positive individuals only (Fig. 2b, c). Additional analyses comparing untreated HIV-positive individuals with HIV-negative individuals found no association between untreated HIV infection and TG (Fig. 2d) suggesting that the association between ART and raised TG is independent of HIV infection.
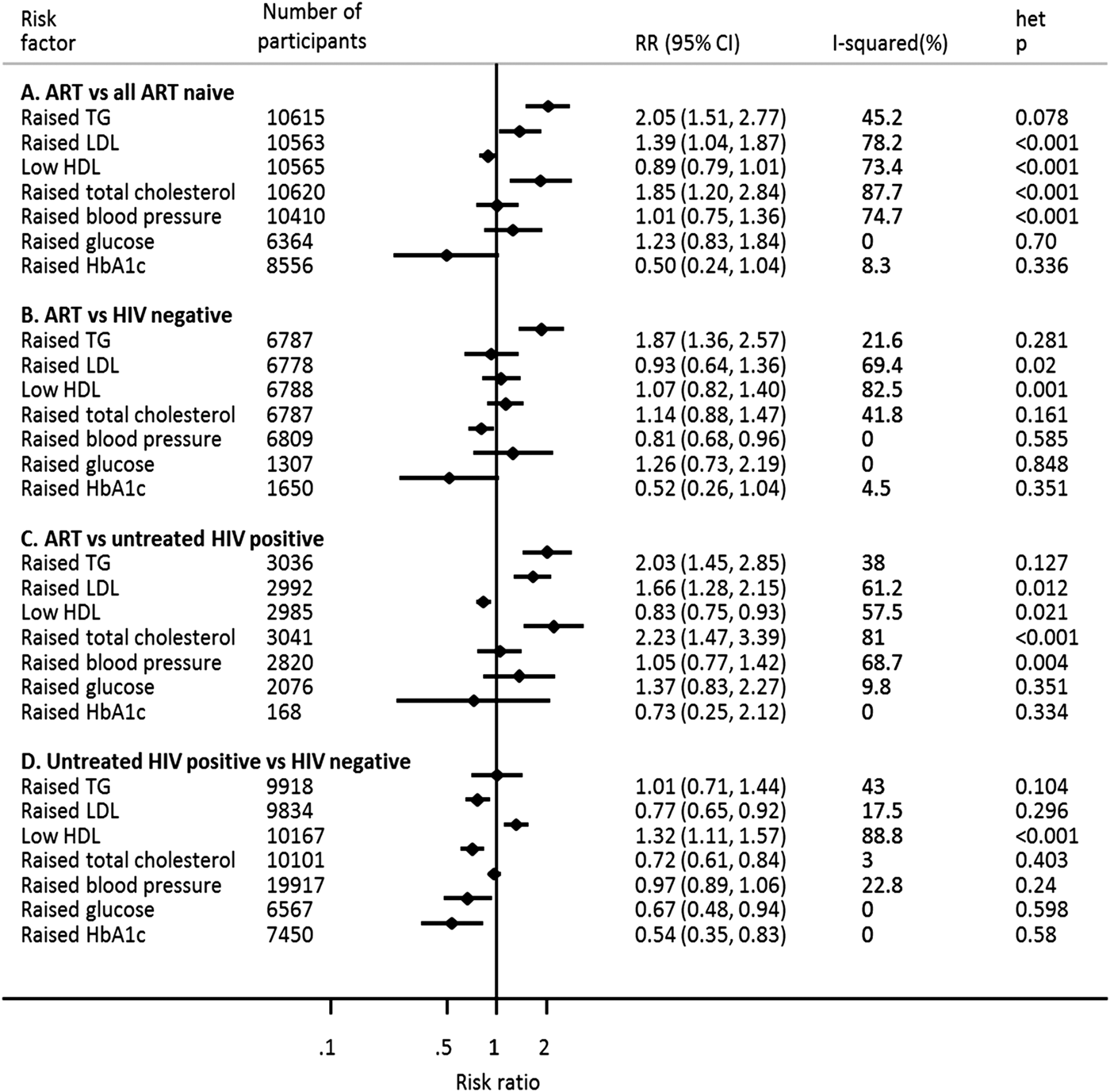
Fig. 2. Association of anti-retroviral therapy and untreated HIV infection with selected cardiometabolic risk factors in sub-Saharan Africa.
Association between ART drug class and raised TG
Additionally, we performed sensitivity analyses to assess whether there was a difference in probability of raised TG between individuals receiving an ART combination that included a PI and individuals receiving a combination not including a PI. In one of three studies with adequate numbers of individuals on PIs to allow analyses, we found some indication that individuals receiving a PI combination are more likely to have raised TG compared with individuals on a non-PI combination (Dave study: RR 2.10, 95% CI 1.06–4.02). However, this association was confounded by duration of ART. Individuals on ART drug combinations that included a PI had been on ART, on average, twice as long as individuals on non-PI combinations. Thus, when duration of ART was adjusted for, the association became non-significant (RR 1.5, 95% CI 0.72–3.11). There was no difference in the significance of the effect of PIs relative to non-PIs with and without adjustment for duration of ART in the other studies. It was, however, not possible to establish the time spent on a particular class of ART as the duration reported was simply time spent on all ART. Considering PIs are currently used as second-line drugs, individuals on PIs are likely to have spent some time on first-line non-PI combinations. Thus, in these data, there was no evidence that PIs were more strongly associated with raised TG than NNRTIs after adjusting for treatment duration. Additionally, the probability of raised TG was not significantly different between individuals whose regimen included AZT compared with stavudine and EFV compared with NVP.
Association between ART and other cardiometabolic risk factors
The estimates of association of ART (in the primary analyses) with raised LDL (RR 1.39, 95% CI 1.04–1.87) and raised TC (RR 1.85, 95% CI 1.20–2.84), though significant, were markedly heterogeneous (I 2 78.2% and 87.7%, respectively) across studies (Fig. 2a). There was no evidence of an association between ART and raised BP, glucose, HbA1c or low HDL (Fig. 2a).
When ART users were compared separately with HIV-negative individuals and with untreated HIV-positive individuals, the association of ART with raised LDL, low HDL and raised TC was inconsistent across studies (Fig. 2b,c). There was no association between ART and raised glucose or HbA1c when comparing individuals on ART with HIV-negative individuals or untreated HIV-positive individuals (Fig. 2b,c). Additionally, ART was associated with a lower risk of raised BP when individuals on ART were compared with HIV-negative individuals (Fig. 2b). However, there was no evidence of association between ART and raised high BP in comparison with untreated HIV-positive individuals (Fig. 2c).
Sources of heterogeneity and individual study influence on pooled association between ART and cardiometabolic risk factors
In primary analyses comparing individuals receiving ART to all other individuals, the magnitude of association between ART and raised TG ranged from 0.81 to 6.17, with a moderate level of heterogeneity (I 2 = 45.2%) (Supplementary Fig. S1). We found no statistically significant study-level determinants of between-study heterogeneity in the pooled association between ART and TG (Supplementary Table S1). Additionally, no single study substantially influenced the pooled estimate of the association between ART and raised TG (Supplementary Table S2). In comparisons of individuals receiving ART with HIV-negative individuals, we found only minimal between-study heterogeneity in the association between ART and raised TG (I 2 = 21.6%) (Supplementary Fig. S2). This heterogeneity was not explained by any of the study-level variables assessed (Supplementary Table S3), and the pooled association was not substantially influenced by any particular study (Supplementary Table S4). Similarly, in comparison of ART users with untreated HIV-positive individuals, between-study heterogeneity in the association between ART and raised TG was low (I 2 = 38.0%) (Supplementary Fig. S3) and not associated with any of the study-level characteristics (Supplementary Table S5). The pooled RR was also not influenced by any one study (Supplementary Table S6). In comparisons of untreated HIV-positive individuals with HIV-negative individuals, as discussed above, we found no association between untreated HIV and raised TG. This lack of association was consistent across studies (I 2 = 43.0%) (Supplementary Fig. S4) and was not significantly influenced by any of the study characteristics assessed (Supplementary Table S7). Lastly, the pooled magnitude of association was not influenced by a single study (Supplementary Table S8).
As indicated above, there was significant between-study heterogeneity in the association between ART and all the other cardiometabolic risk factors in the primary analysis, except for raised blood glucose and HbA1c. The observed heterogeneity was not associated with any of the study-level factors assessed, nor was the pooled measure of association influenced by one study alone, except for raised BP where the magnitude of association with ART tended to be higher in population studies compared with clinic-based studies (Supplementary Tables S1 and S2).
Discussion
In these pooled analyses of 10 615 individuals, ART was independently associated with a twofold higher probability of raised TG. This could have important implications for the burden of CVD in SSA as access to, and duration on, ART increases in this population, highlighting the need to better understand the effect of long-term ART on TG and its impact on the burden of CVD in SSA.
Our findings are broadly consistent with earlier reports, including a study among black African women in rural South Africa and a meta-analysis of clinical trials in European-descent populations [Reference Clark31, Reference Hill, Sawyer and Gazzard32]. These studies suggested that first-line ART is associated with raised cholesterol and TG [Reference Clark31, Reference Hill, Sawyer and Gazzard32]. In addition, a study of metabolic complications in a European-descent population found that the use of combined NNRTI and PI was associated with a fivefold higher prevalence of hypertriglyceridaemia [Reference Friis-Moller33]. In our study, 87% of the participants on ART were either on two NRTIs and one NNRTI (the standard first-line anti-HIV drugs recommended across SSA) or a non-standard combination of NRTIs and NNRTIs; while only 13% received drug combinations including a PI [Reference Wester34]. PIs, currently used as second-line ART drugs, have been the most cited in studies measuring CVD among HIV-infected people receiving ART in other parts of the world [Reference Friis-Moller7, Reference Friis-Moller33]. In this study, we found no evidence that ART combinations including PIs were more strongly associated with raised TG than non-PI combinations.
Many mechanisms by which ART may lead to raised TG levels have been proposed. It is thought that ART may reduce the clearance of TG from circulation through impairment of lipoprotein lipase activity in experimental studies [Reference den Boer35]. Additionally, ART may cause accumulation of the sterol-sensing transcription factor SREBP, the chief regulator of lipid homeostasis, which contributes to an increase in hepatic intracellular lipids [Reference Zhou36]. Further, ART may increase the level of circulating TG by altering mitochondrial proliferation, morphology and mitochondrial DNA content, or inhibiting the degradation of and increasing hepatic secretion of ApoB, the main lipoprotein for transportation of lipids [Reference Maagaard and Kvale37–Reference Liang41].
Evidence of the effect of prolonged ART use on CVD risk is currently limited to a few studies in western populations. One study found a relative rate of myocardial infarction of between 0.98 and 1.13 per year of NNRTI exposure and 1.10 and 1.23 per year of PI exposure [Reference Friis-Moller7]. Similar to this study, our findings suggest that longer duration of ART may confer greater risk of raised TG. However, the potential impact of ART on CVD risk mediated specifically through raised TG may be inferred from studies of the effect of TG on CVD. For example, in a recent meta-analysis, the odds of coronary heart disease (CHD) was nearly doubled in individuals with TG values in the top third of the population compared with those in the bottom third [Reference Sarwar2]. Additionally, in another study, an increase of 1 mmol/L in TG was associated with increases of between 14% and 37% in CVD risk after adjustment for HDL [Reference Austin, Hokanson and Edwards1]. Extrapolating the results of the studies above to our study, with TG higher in ART users by 1.11 mmol/L than the rest of the population on average, ART may be associated with 16–41% increase in CVD risk.
We note, however, that there is a paucity of published population data on the prevalence of dyslipidaemia, including raised TG, in SSA. Estimates of raised TG prevalence ranging from 5% to 20% have been reported in rural East Africa and urban West Africa, respectively [Reference Asiki42–Reference Sumner44]. This variation likely reflects differences in study design as well as potential real differences between populations. Further, available evidence consistently shows higher rates of hyperglyceridaemia among HIV-infected individuals receiving ART compared with untreated HIV-positive individuals and HIV-negative individuals [Reference Mbunkah43, Reference Julius45, Reference Tesfaye46]. Estimates of the prevalence of hypertriglyceridemia of between 14% and 42% have been reported among individuals receiving ART [Reference Mbunkah43, Reference Julius45, Reference Tesfaye46]. The heterogeneity is perhaps explained by underlying differences between populations in addition to differences in the duration of ART use – for example, comorbidities, socio-economic factors and healthcare systems.
The strength of this study is that it is the largest to date to assess the association between ART and cardiometabolic risk in SSA using IPD. In addition, we defined cardiometabolic risk factors according to clinically relevant cut-offs. Our findings may therefore be relevant for the clinical care of patients. Further, use of IPD enabled a more comprehensive adjustment for potential confounders including BMI and socio-demographic factors, as well as behavioural risk factors at the individual level. Importantly, the study assessed data on the class of ART thereby shedding more light on the impact of ART drug class on lipids, which is relevant to HIV patient treatment and care.
However, the study has some limitations. First, the majority of the studies included in the pooled analysis were cross-sectional. This precludes an analysis of the temporal relationship between ART use and the cardiometabolic risk factors studied. Second, our results may have been confounded by fasting status, as some of the studies included in the pooled analyses provided non-fasted lipids and glucose measurements. However, studies of fasting participants and studies of unfasted participants have reported only minor differences in the strength of associations between TG and CHD [Reference Sarwar2]. This, and the fact that non-fasted lipids and glucose were presented by only two studies, suggests that the impact of potential confounding due to differences in fasting status on the validity of our results is likely to be minimal.
In summary, this study provides evidence of association between ART and raised TG in SSA. Given the increasing use of ART and a potentially causal association between raised TG and CVD outcomes, our findings support the need to transition to new ARV drugs, such as dolutegravir, that have shown less adverse effects on lipids [Reference Quercia47]. Importantly, the findings highlight the need for prospective studies to clarify the impact of long-term ART and its interplay with other risk factors on CVD risk in SSA. In the interim, it might be beneficial to strengthen the monitoring of lipid levels in individuals receiving ART.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/gheg.2018.7
Acknowledgements
The authors are grateful to the study participants and the research teams of the studies included in these analyses for their effort in generating these data. The authors are also grateful to the colleagues at the Global Health and Population Sciences Group, University of Cambridge, for their useful comments.
MS, KE, EY and PK are part-funded by the African Partnership for Chronic Disease Research (Medical Research Council UK partnership grant number MR/K013491/1). KE is supported by an Islamic Development Bank Cambridge International Scholarship. MS is supported by the National Institute for Health Research Cambridge Biomedical Research Centre (UK).
Declaration of interest
None.
Author contributions
Literature search and study design: KE, DGD, EHY, MSS. Data collection and collation: KE, DGD, MSS, NS, DF, NC, MN, KA, CC, JAD, CMF, HF, LMK, CTL, DM, EM, CEN, GP, EWPY, NR, MUS, MS, AES, EHV, CW, DG, FM, AK, JS, DP, AAM, PK. Data analysis: KE, MSS. Drafted the manuscript: KE, EHY, MSS.
All authors contributed to writing the paper and reviewed the manuscript.






