Introduction
The way in which an individual understands and interacts with the social world is represented in the functions of the social brain, a set of wide-ranging neural networks involved in the perception of social cues and the regulation of socio-emotional functioning and behaviour (Cacioppo & Decety, Reference Cacioppo and Decety2011; Frith & Frith, Reference Frith and Frith2010). The social brain includes frontal, temporal and limbic regions, and parts of the basal ganglia (Adolphs, Reference Adolphs2009; Beauchamp & Anderson, Reference Beauchamp and Anderson2010). The perceptual and cognitive functions that support this system are collectively called social cognition and underpin more global social development and competence (Beauchamp & Anderson, Reference Beauchamp and Anderson2010). The development of the social brain is a dynamic process that undergoes significant and protracted evolution from childhood to early adulthood (Blakemore, Reference Blakemore2008; Burnett & Blakemore, Reference Burnett and Blakemore2009). This maturation involves a complex and dynamic set of genetically guided processes by which neural structures, particularly in frontotemporolimbic areas, interact with the environment to shape the emergence of emotional and social behaviour (Bigler et al., Reference Bigler, Yeates, Dennis, Gerhardt, Rubin, Stancin and Vannatta2013; Blakemore & Frith, Reference Blakemore and Frith2004; Jernigan, Baare, Stiles, & Madsen, Reference Jernigan, Baare, Stiles and Madsen2011; Stiles & Jernigan, Reference Stiles and Jernigan2010). As a result, the developing social brain is vulnerable to early insult, which has the potential to disrupt neural maturation, as well as the development of associated socio-cognitive skills and may result in changes to expected trajectories of social development across childhood and adolescence.
It has been suggested that the nature of brain pathology (i.e. focal vs. diffuse insult) may play a particularly important role in mediating outcome after EBI (V. Anderson, Spencer-Smith, & Wood, Reference Anderson, Spencer-Smith and Wood2011). Traditionally, it has been argued that when early damage is focal (e.g. tumor, stroke, focal dysplasia, etc.), there is a capacity for neuronal reorganisation and relatively better functional outcome (Aram & Ekelman, Reference Aram and Ekelman1986; Stiles, Reilly, Paul, & Moses, Reference Stiles, Reilly, Paul and Moses2005). In contrast, sustaining a generalised cerebral insult (e.g. traumatic brain injury (TBI), cerebral infection, etc.) is associated with slower recovery and more severe impairments (Anderson, Catroppa, Morse, Haritou, & Rosenfeld, Reference Anderson, Catroppa, Morse, Haritou and Rosenfeld2005). In keeping with this, studies on social outcomes after TBI in youth have shown that children and adolescent survivors have an elevated risk of social dysfunction, with consistent difficulties identified for social adjustment and social cognition (Anderson, Godfrey, Rosenfeld, & Catroppa, Reference Anderson, Godfrey, Rosenfeld and Catroppa2012; Rosema, Crowe, & Anderson, Reference Rosema, Crowe and Anderson2012; Yeates et al., Reference Yeates, Swift, Taylor, Wade, Drotar, Stancin and Minich2004). Amongst the sociocognitive functions affected are emotion recognition (Tonks, Williams, Frampton, Yates, & Slater, Reference Tonks, Williams, Frampton, Yates and Slater2007; Turkstra, McDonald, & DePompei, Reference Turkstra, McDonald and DePompei2001), theory of mind (Dennis et al., Reference Dennis, Simic, Gerry Taylor, Bigler, Rubin, Vannatta and Yeates2012; Snodgrass & Knott, Reference Snodgrass and Knott2006; Turkstra, Dixon, & Baker, Reference Turkstra, Dixon and Baker2004), social problem-solving (Ganesalingam, Yeates, Sanson, & Anderson, Reference Ganesalingam, Yeates, Sanson and Anderson2007) and moral reasoning (MR) (Beauchamp, Dooley, & Anderson, Reference Beauchamp, Dooley and Anderson2013; Dooley, Beauchamp, & Anderson, Reference Dooley, Beauchamp and Anderson2010). Whilst there is mounting evidence that trauma-induced neuropathology may influence social behaviour following early TBI, it is less clear how early lesions of focal aetiology impact social behaviour and social cognition. The paucity of research in this area may be attributable to several factors: (1) Data describing the impact of focal EBI on social functioning comes mainly from case studies with few group studies conducted; (2) Much of our knowledge on outcomes after focal EBI relies on aspects of general cognition (intelligence, language, motor function, etc.) and (3) As mentioned above, it is thought that focal injuries generally have better prognosis than diffuse injuries.
Existing reports of focal EBI outcome on more general aspects of cognition provide some information on the recovery of the immature brain after focal lesions. Residual language deficits following early left or right hemisphere lesions appear to be more subtle in children compared to adults (Bates et al., Reference Bates, Thal, Trauner, Fenson, Aram, Eisele and Nass1997; Chilosi et al., Reference Chilosi, Cipriani, Pecini, Brizzolara, Biagi, Montanaro and Cioni2008), whereas the development of visuospatial function following early focal brain damage corresponds more closely to findings obtained in adults with similar lesions (Stiles et al., Reference Stiles, Stern, Appelbaum, Nass, Trauner and Hesselink2008). Regarding intellectual functioning, children with focal brain lesions generally perform below expected norms, but still within the average range (Carey, Barakat, Foley, Gyato, & Phillips, Reference Carey, Barakat, Foley, Gyato and Phillips2001; Hetherington, Tuff, Anderson, Miles, & deVeber, Reference Hetherington, Tuff, Anderson, Miles and deVeber2005; Hogan, Kirkham, & Isaacs, Reference Hogan, Kirkham and Isaacs2000). Duval et al. (Reference Duval, Braun, Montour-Proulx, Daigneault, Rouleau and Begin2008) conducted a study in 240 patients with focal brain lesions and found that lower intellectual quotient (IQ) was associated with early occurring lesions rather than those sustained later in life, although scores remained in the average range. More specifically, IQ change over time was negative when the lesions occurred early in childhood, whereas adult onset cases benefited from recovery over time with age, thus highlighting the vulnerability of the developing brain to early insult.
The literature on social outcomes after focal EBI consists mostly of reports of individual cases presenting with prefrontal damage, often assessed in adulthood, or of aetiology-specific studies of global social competence after particular medical conditions. These reports suggest that early onset lesions to the prefrontal cortex could lead to severe sociobehavioural problems (Anderson, Bechara, Damasio, Tranel, & Damasio, Reference Anderson, Bechara, Damasio, Tranel and Damasio1999; Bahia et al., Reference Bahia, Takada, Caixeta, Lucato, Porto and Nitrini2013; Boes et al., Reference Boes, Grafft, Joshi, Chuang, Nopoulos and Anderson2011; Eslinger, Flaherty-Craig, & Benton, Reference Eslinger, Flaherty-Craig and Benton2004). A review of 31 studies on psychosocial adjustment in survivors of childhood brain tumours also suggests that these patients are at risk for deficits in social competence, defined as a multidimensional construct comprised of adaptive behaviour, social skills, and peer acceptance (Fuemmeler, Elkin, & Mullins, Reference Fuemmeler, Elkin and Mullins2002). Studies on paediatric stroke also document poorer social functioning following childhood ischemic damage (Anderson et al., Reference Anderson, Gomes, Greenham, Hearps, Gordon, Rinehart and Mackay2014; Galvin, Hewish, Rice, & Mackay, Reference Galvin, Hewish, Rice and Mackay2011; Lo et al., Reference Lo, Gordon, Hajek, Gomes, Greenham, Perkins and Mackay2014). In a large-scale study of 147 children with focal EBI of various aetiologies including developmental, ischemic, neuroplastic, traumatic and infective brain lesions, Greenham, Spencer-Smith, Anderson, Coleman, and Anderson (Reference Greenham, Spencer-Smith, Anderson, Coleman and Anderson2010) reported that children sustaining these types of lesions were at high risk for social impairment, according to teachers’ reports. Interestingly, lesion characteristics (location and laterality) were not predictive of social outcomes.
Despite recognition that focal EBI is associated with an elevated risk of global social dysfunction, the underlying causes are not fully understood. It seems likely that the presence of deficits in one or a number of sociocognitive functions may contribute to putative social impairments. Findings in this regard emerge from a case series of individuals with early prefrontal damage, which reports that social impairments are associated with deficits in executive functions, failure to acquire complex social knowledge and lower levels of empathy (Eslinger et al., Reference Eslinger, Flaherty-Craig and Benton2004). However, some of the patients in that study were tested during adulthood, had trauma-induced pathology, and formal testing of social cognition was limited or indirectly studied.
The recent description of heuristic models of social development have sparked interest in creating tools to assess social cognition in clinical populations with the aim of identifying the underpinnings of global sociobehavioural problems (Beauchamp & Anderson, Reference Beauchamp and Anderson2010; Yeates et al., Reference Yeates, Bigler, Dennis, Gerhardt, Rubin, Stancin and Vannatta2007). Moral reasoning (MR) is a sociocognitive function that is thought to play an important role in social competence and has been understudied in neurological populations. MR is defined as how individuals think about moral emotions and conventions that govern social interactions in their everyday lives (Haidt, Reference Haidt2001). It is now widely accepted that MR is driven by distinct neural networks that overlap with the social brain and include frontotemporolimbic regions (Greene, Nystrom, Engell, Darley, & Cohen, Reference Greene, Nystrom, Engell, Darley and Cohen2004; Sevinc & Spreng, Reference Sevinc and Spreng2014). Studies from developmental psychology suggest that MR is acquired through stages starting from an ego-oriented morality to a broader, society-oriented perspective (Gibbs, Reference Gibbs2010; Turiel, Reference Turiel1983). The development of MR is thus potentially vulnerable to disruption in cognitive growth and brain function, such as in focal EBI. In non-injured children, important links exist between MR, empathy, externalising behaviour and social adjustment which suggest that poorer MR and empathy are associated with juvenile delinquency (Jolliffe & Farrington, Reference Jolliffe and Farrington2004; Raaijmakers, Engels, & Van Hoof, Reference Raaijmakers, Engels and Van Hoof2005; Schonert-Reichl, Reference Schonert-Reichl1999; Vera-Estay, Beauchamp, & Dooley, Reference Vera-Estay, Beauchamp and Dooley2014; Vera-Estay, Seni, Champagne, & Beauchamp, Reference Vera-Estay, Seni, Champagne and Beauchamp2016). MR has also been found to be associated with several executive functions, including cognitive flexibility, inhibition and verbal fluency (Vera-Estay, Beauchamp, & Dooley, Reference Vera-Estay, Beauchamp and Dooley2014; Vera-Estay, Seni, Champagne, & Beauchamp, Reference Vera-Estay, Seni, Champagne and Beauchamp2016).
Despite these associations, studies investigating MR in focal EBI are scarce. Initial evidence of moral impairment after focal EBI emerges from three case studies of patients with prefrontal lobe damage who displayed less developmentally mature levels of MR (Anderson et al., Reference Anderson, Bechara, Damasio, Tranel and Damasio1999; Boes et al., Reference Boes, Grafft, Joshi, Chuang, Nopoulos and Anderson2011; Grattan & Eslinger, Reference Grattan and Eslinger1992). In these studies, MR was assessed during adulthood using Kohlberg's Moral Judgment Interview (Colby & Kohlberg, Reference Colby and Kohlberg1987), a semi-structured interview that presents extreme moral dilemmas. Whilst other indications of moral impairments after focal lesions are primarily anecdotal, these studies provide the groundwork for further exploration of MR in focal EBI. To our knowledge, only one study so far has examined the direct, quantitative effect of focal EBI on MR in a group of paediatric patients, reporting that 16 children with focal frontal lesions had lower levels of MR and poorer adaptive behaviour ratings than controls on a paper–pencil questionnaire (Couper, Jacobs, & Anderson, Reference Couper, Jacobs and Anderson2002).
The goals of the present study were therefore to (1) investigate social cognition, social behaviour and social adaptive skills in a group of children and adolescents with focal EBI. It was expected that these individuals would perform more poorly than their typically developing peers on measures of MR (MR maturity, moral decision-making) and empathy, display more sociobehavioural problems and have poorer social adaptive skills and (2) explore the links between social cognition (MR maturity, moral decision-making and empathy) and more general cognitive measures such as IQ and executive functioning (cognitive flexibility), as well as social behaviour in the focal EBI group. It was expected that better sociocognitive skills would be associated with fewer sociobehavioural problems and better social adaptive skills. In line with current literature in moral development (Vera-Estay et al., Reference Vera-Estay, Beauchamp and Dooley2014), it was also expected that IQ and cognitive flexibility would be positively correlated with MR maturity. We sought to meet these goals by using a novel visual MR measure that is based on an ecological approach and is adapted for children and adolescents, the Socio-Moral Reasoning Aptitude Level Task (So-Moral), and by studying a group of patients with focal frontal or temporal lesions to reflect the fact that the social brain is subsumed by both frontal and extra-frontal regions.
Methods
Participants
Thirty children and adolescents (aged 8–16 years) participated in the study. The clinical sample consisted of 15 children (10 males, 5 females) with focal frontal or temporal lobe lesions identified by magnetic resonance imaging (MRI). These children were recruited from the neurosurgery department at a tertiary urban paediatric hospital. To meet the selection criteria for the study, children were required to be fluent in English or French, be clinically stable (e.g., patients with severe or hard to control epilepsy were not included), and to have documented evidence of a focal lesion identified within frontal or temporal areas (e.g. arterioveinous malformation rupture, tumor, cerebral cyst, cavernoma, etc.). Children were excluded if they had evidence of diffuse injury. Seven patients had surgical resection and they were all seen at least 6 months post-operatively to minimise acute recovery effects. See Table 1 for the lesion characteristics of the Focal EBI group and Figure 1 for examples of focal neuropathology identified via MRI. Presence of seizure history was recorded since it is associated with poorer social outcomes (Greenham et al., Reference Greenham, Spencer-Smith, Anderson, Coleman and Anderson2010). Fifteen control participants (10 males, 5 females) were recruited from a healthy participant database. These controls were attending local elementary and high schools. They were individually matched to clinical participants on sex, age (±12 months) and parental education. Controls were excluded if they had any history of intellectual disability or developmental, psychiatric or neurological disturbance.
TABLE 1 Characteristics of the Focal EBI Patients and their Scores on the So-Moral

1. In months.
Abbreviations: AVM = arteriovenous malformation rupture; IFG = Inferior frontal gyrus; MFG = middle frontal Gyrus; SFG = superior frontal gyrus; OFG = orbital frontal gyrus; ACG = anterior cingulate gyrus; ITG = inferior temporal gyrus; MTG = middle temporal gyrus; STG = superior temporal gyrus; MR = moral reasoning.

FIGURE 1 Examples of focal lesions identified in study participants via magnetic resonance imaging. Images are in radiological perspective (subject's left on viewer's right). Sagittal and axial views are presented for Patients B, D, N and coronal and axial views for Patient I. Patient B: Intraparenchymal hemorrhagic lesion in the right ventrolateral prefrontal cortex of the inferior frontal gyrus and parts of the orbitofrontal cortex following AVM rupture. Patient D: Neuro-epithelial cystic lesion in the left anterior cingulate cortex located anterior to the left forceps minor of the genu of the corpus callosum. Patient I: Mixed neuroglial tumor in the left temporopolar cortex (parts of the inferior and middle temporal gyri) anterior to the amygdala. Patient N: Brain tumor (DNET) in the ventromedial prefrontal cortex (anterio-medial portion of the superior frontal gyrus).
Measures
Demographic and developmental variables: Demographic and developmental information was collected via an in-house questionnaire completed by parents and pertaining to their child's medical, developmental and social history.
Intellectual functioning: Participants completed the Vocabulary and Matrix Reasoning subtests of the Wechsler Abbreviated Scale of Intelligence (WASI, Wechsler, Reference Wechsler1999), to provide an estimate of general intellectual ability (Full Scale IQ, FSIQ, M = 100, SD = 15). The WASI has adequate internal consistency (α = .93), test–retest reliability (r = .87) and concurrent validity (r = .87) (Garland, Reference Garland2005).
Executive functioning: Participants completed the Trail Making Test from the Delis–Kaplan Executive Function System (D-KEFS, Delis, Kaplan, & Kramer, Reference Delis, Kaplan and Kramer2001) as a measure of cognitive flexibility. The Number–Letter sequencing versus Number–Letter switching contrast score was used in analyses (range 1–19, M = 10, SD = 3).
Moral reasoning: The Socio-Moral Reasoning Aptitude Level Task (So-Moral, Beauchamp et al., Reference Beauchamp, Dooley and Anderson2013; Dooley et al., Reference Dooley, Beauchamp and Anderson2010; Vera-Estay et al., Reference Vera-Estay, Beauchamp and Dooley2014; Reference Vera-Estay, Seni, Champagne and Beauchamp2016) is a self-paced, visual, computer-based task that presents visual moral dilemmas specifically designed for children and adolescents. The task has gender and age specific versions and includes 10 dilemmas. Each dilemma (see example in Figure 2) consists of: an introductory screen presenting the name of the dilemma (e.g. ‘wallet’); three separate screens showing first-person perspective pictures of child or adolescent actors in various social scenarios representing a moral conflict (e.g. concerns with justice, welfare/harm and rights) according to Social Domain Theory (Turiel, Reference Turiel1983); and a final screen presenting a dichotomous decision (e.g. whether or not to engage in a particular action such as stealing from a shop, cheating at a game, etc.).

FIGURE 2 Example item from the So-Moral task.
The aggregate number of morally adapted responses is compiled to obtain a moral decision making score, which ranges from 0 to 10 points. Participants are then asked to provide a justification for the choice they made. Each participant's justification is recorded verbatim and subsequently scored according to a standardised coding system (Beauchamp & Dooley, Reference Beauchamp and Dooley2012) based on the cognitive-developmental approach (Gibbs, Reference Gibbs2010; Kohlberg, Reference Kohlberg1981; Turiel, Reference Turiel1983). Developmental stages of MR have been adapted to fit the social nature of the dilemmas in the So-Moral task and consist of the following: (1) Centrations and authoritarian-based consequences; (2) Egocentric/pragmatic exchanges; (3) Interpersonal focus; (4) Societal regulation; and (5) Societal evaluation (see Table 2). Transition stages (1.5, 2.5, etc.) are used to account for answers that provide elements of two consecutive reasoning stages. When elements of non-consecutive stages are provided, the response is coded according to the highest schema detected. The MR maturity score (0 to 50 points) is obtained by summing the justification scores. The MR maturity score was translated into an overall moral maturity level as follows: Stage 0 = 0–5 points, Stage 1 = 6–15 points, Stage 2 = 16–25 points, Stage 3 = 26–35 points, Stage 4 = 36–45 points and Stage 5 = 46–50 points. All scoring was done blind to diagnosis and group membership. A second rater independently scored 20% of the So-Moral justifications. The consistency for the ratings was .92 indicating high inter-rater reliabilty. Discrepancies were resolved by discussion and consensus.
TABLE 2 Brief Description of So-Moral Coding and Examples

Empathy: Empathy was measured using the Index of Empathy for Children and Adolescents (IECA, for participants aged 12 and over) (Bryant, Reference Bryant1982) and its adapted parent version, the Griffith Empathy Measure (GEM, for participants aged 11 and under) (Dadds et al., Reference Dadds, Hunter, Hawes, Frost, Vassallo, Bunn and Masry2008). Both the IECA and GEM are general empathy questionnaires measuring an individual's understanding of other people's emotions. The IECA is a self-report questionnaire with 22 items using a yes/no format in the first-person. In the GEM, the same questions are reworded in the third person format. For example the IECA item ‘I get upset when I see an animal being hurt’ is worded as ‘My child gets upset when he/she sees an animal being hurt’ in the GEM. The original version of the GEM is a 23-item questionnaire with a nine-point Likert scale. For the purpose of this study, we removed the item that differs between the two scales and used a yes/no format to make the scaling of the two questionnaires comparable. Therefore, items on both measures were scored on a dichotomous scale (0 = non-empathic response; 1 = empathic response), with higher scores corresponding to higher levels of empathy. The IECA and the GEM have adequate validity and reliability and are well correlated (Bryant, Reference Bryant1982; Dadds et al., Reference Dadds, Hunter, Hawes, Frost, Vassallo, Bunn and Masry2008).
Social adaptive skills: Social adaptive skills were assessed using the parent-report version of the Adaptive Behaviour Assessment System-2 (Harrison & Oakland, Reference Harrison and Oakland2003). Standardised scores on the Social scale were used in the current study as a measure of children's ability to communicate and behave appropriately in social interactions (M = 100, SD = 15).
Sociobehavioural problems: Sociobehavioural problems were measured using the parent-report version of the Child Behaviour Checklist (Achenbach & Rescorla, Reference Achenbach and Rescorla2001). The CBCL assesses internalising behaviours (anxiety, depression, somatic problems, thought problems) and externalising behaviours (social problems, attention problems, aggressive behaviour, rule-breaking behaviour). It has good reliability, as well as the power to discriminate between children who are and are not referred for a clinical assessment (Achenbach, Reference Achenbach1991). Composite T-scores on the three-broadband scales (internalising, externalising and total problems) are reported (M = 50, SD = 10). Scores on the rule-breaking behaviour scale (M = 50, SD = 10) are also reported since previous studies in non-injured children have posited that failure to abide by moral guidelines is prominent in the rule-breaking behaviours that lead to criminality, violence and delinquent behaviour (Chudzik, Reference Chudzik2007; Raaijmakers et al., Reference Raaijmakers, Engels and Van Hoof2005).
Statistical Analysis
Due to the exploratory nature of the study, the probability for type 1 error was set at .05 for each measure. Prior to all statistical analyses, data were examined for violations of the assumption of normality. The means and standard deviations for the different scores were calculated separately for the Focal EBI and control group and are presented in Table 3. In accordance with the matched control study design, paired t-tests were conducted to compare the mean differences between Focal EBI patients and their matched controls on sociocognitive measures, IQ, cognitive flexibility and parent questionnaire ratings (sociobehavioural problems and social adaptive skills). Prior to this decision, we performed non-parametric sensitivity analyses (Wilcoxon signed rank test) and obtained the same results as those generated with t-tests. We also performed sensitivity analyses by excluding the 8-year old patient and the main results did not change. Therefore, we report findings on the whole sample. Within group age differences were explored using a scatterplot to graphically compare MR maturity between the two groups, taking age into account. Proportions of individuals presenting mature MR levels (stage 3 and above) in both groups were compared using Mcnemar's test. Wilcoxon signed rank tests were used to evaluate between-group differences in MR stages. Bivariate correlations were performed to investigate the relationship between social cognition measures (MR maturity, moral decision-making, empathy), general cognition measures (IQ and cognitive flexibility) and more general indicators of social functioning: externalising problems (CBCL), internalising problems (CBCL), total problems (CBCL) and social adaptive skills (ABAS).
Results
The characteristics and individual scores on the So-Moral of the Focal EBI patients are presented in Table 1. No participants were excluded based on the IQ criterion. Means, standard deviations and paired sample t-tests for the main measures are presented in Table 3. As expected after matching, no significant differences were found for age and parental education. The focal EBI group performed significantly worse on all social cognition measures (MR, moral decision and empathy), whilst they did not differ from the control group on general cognition measures (IQ and cognitive flexibility). In terms of social functioning, only the CBCL Total Problems score differed significantly between groups. No differences were found for social adaptive skills (ABAS).
TABLE 3 Means (SD) and Paired-Sample t-Tests Comparing Group Means on Main Measures

*p < .05; **p < .01. aMean of mother's and father's education in years; bTrail-Making Test Contrast Score between Number–Letter sequencing and Number–Letter switching.
Figure 3 presents a scatterplot comparing MR maturity between the focal EBI and control groups across age. This reveals a strong correlation between MR and age in both Focal EBI (r = .65, p < .01) and control groups (r = .73, p < .01). The graph further shows that whilst MR maturity score increases linearly with age in both groups, control participants almost systematically perform better than focal EBI across ages.

FIGURE 3 Regression lines and scatterplot of distribution of scores for MR maturity as assessed by the So-Moral for patients with focal EBI and controls across age. Focal EBI patients are identified by letters and their matched controls with the corresponding letter inside a square.
Mcnemar's test indicates that more Focal EBI patients perform at immature MR levels than controls (p = .016). When the distribution of patients and control participants was compared across all five MR levels (see Table 4), controls had significantly higher levels of MR than Focal EBI patients (z = −2.714, p = .007).
TABLE 4 Distribution of Participants According to MR Maturity Stage

Note: MR = moral reasoning.
As shown in Table 5, empathy was significantly correlated with MR and decision-making in the focal EBI group.
TABLE 5 Correlations Between Main Variables in the Focal EBI Group
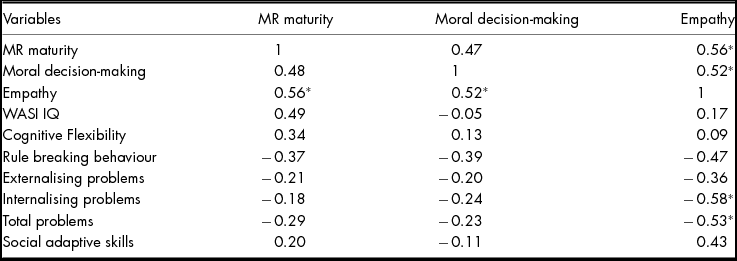
*p < .05.
Note: MR = moral reasoning.
Discussion
In the current study, social cognition and functioning were studied in a group of children and adolescents with frontotemporal lesions, with a particular interest in determining whether focal brain insults are associated with difficulties in MR during a critical period for social development. Our main results show that children with focal EBI have less mature MR in addition to displaying lower rates of moral decision-making and poorer empathy than their non-injured peers. Although these sociocognitive functions are distinct, they are interrelated and necessary foundations for adequate social functioning and they share common neural substrates (Bernhardt & Singer, Reference Bernhardt and Singer2012; Sevinc & Spreng, Reference Sevinc and Spreng2014). These results are discussed in detail below and links are provided on the complex interplay between these functions and their potential role in explaining outcomes after focal EBI.
Children with focal EBI provided significantly less mature moral justifications to realistic social dilemmas than their non-injured peers. They had a mean global stage of MR maturity that was one level (stage 2) lower than controls (stage 3). Our findings reflect both a mean group difference, as well as individual differences on MR. In fact, every patient with focal EBI had a mean score corresponding to an equivalent or lower stage of MR maturity than their matched controls. The majority (11/15, 73%) of patients with focal EBI reasoned at stage 2 or below on the So-Moral task. At these more ‘immature’ stages, individuals justify their responses to moral dilemmas based on egocentric concerns such as fear of punishment or personal gains (i.e., exchanging favours with others). They are able to recognise that different people may have different opinions about the way things are done, but their decision-making is ultimately driven from an egocentric perspective. In other words, the correct option is the one that is right for them (i.e., they are self-interested). In contrast, the majority of controls (11/15, 73%) reasoned at stage 3 or above, which marks the transition from an immature MR level (pre-conventional) to a mature MR level (conventional), according to moral development theories (Gibbs, Reference Gibbs2010; Kohlberg, Levine, & Hewer, Reference Kohlberg, Levine and Hewer1983). At stage 3, individuals justify their actions with a focus on interpersonal relationships, a sense of ‘goodness’ and feelings such as empathy and trust. At this stage, decisions are made with externally oriented motives and a prosocial perspective. Reasoning is more flexible as the individual takes into account others’ interests and/or social norms. One possible explanation for the difference in MR maturity identified between patients with focal EBI and controls is related to the notion of development of decentration; that is, the ability to shift away from a focus on the self towards attending to and interrelating of situational features or perspectives (Kaplan, Reference Kaplan, Eisenberg and Staub1989). Decentration develops during ontogenetic development in parallel with cognitive development, empathy and social opportunities (Kohlberg, Reference Kohlberg1984). It requires the integration of multiple pieces of information about causes and mental states, as well as the ability to consider conflicting emotions. Lower levels of MR after Focal EBI may thus reflect a broader cognitive impairment (i.e. executive functions, working memory), which may impede the process of decentration and reflect a failure or delay to reach a mature level of MR (stage 3 and above). Globally, our findings concord with traditional case reports (Anderson et al., Reference Anderson, Bechara, Damasio, Tranel and Damasio1999; Grattan & Eslinger, Reference Grattan and Eslinger1992) and a previous study investigating MR in children with focal frontal lesions (Couper et al., Reference Couper, Jacobs and Anderson2002), which highlighted impairment in the acquisition of mature MR as a result of focal EBI. Importantly, the current results were obtained using an ecological visual task that limits the impact of confounding cognitive factors often impaired in clinical populations such as reading, attentional and working memory skills (Dooley et al., Reference Dooley, Beauchamp and Anderson2010) and assesses reasoning during everyday moral dilemmas. Longitudinal studies would be useful to track the evolution of MR differences and verify whether these delays persist or worsen in the long-term after EBI (Beauchamp & Anderson, Reference Beauchamp, Anderson, Dulac, Di Mauro and Lassonde2013; Janusz, Kirkwood, Yeates, & Taylor, Reference Janusz, Kirkwood, Yeates and Taylor2002).
In addition to relying on less mature MR, children with focal EBI also provided significantly fewer moral responses, which were determined by the number of socially appropriate decisions taken in the dilemmas presented. In light of this, it is possible that children with focal EBI may be more inclined to engage in behaviours that do not comply with social expectations. Both cognitive and affective functions contribute to moral decision-making (Rosen, Brand, & Kalbe, Reference Rosen, Brand and Kalbe2016); the lower levels of MR and empathy found in the focal EBI group may thus provide an explanation for differences in moral decision-making. A previous study in TBI identified a distinction between knowing and understanding in the moral domain (Beauchamp et al., Reference Beauchamp, Dooley and Anderson2013). That is, children with TBI were able to identify the correct moral responses (moral decision-making), but did not have the capacity to provide mature reasoning to support their decision, indicating a lack of comprehension of the social consequences of a given decision. In the current study, we found significant differences on both moral indices (moral decision-making and MR maturity), highlighting difficulties in both the knowing and understanding facets of moral dilemmas. However, caution in drawing conclusions is advised and a larger sample of focal EBI is necessary to confirm these findings considering overall mean differences were relatively small. Studies of social behaviour in naturalistic settings could also provide further insights into the moral decision-making of these children in real-life situations.
Children with focal EBI also exhibited poorer empathy compared to control participants. This finding provides for the first time quantitative evidence of previous anecdotal accounts of lack of empathy in case studies of focal EBI (Anderson et al., Reference Anderson, Bechara, Damasio, Tranel and Damasio1999; Grattan & Eslinger, Reference Grattan and Eslinger1992). Numerous studies have suggested that the lower empathy scores of patients with frontal brain injury may be related to cognitive flexibility (Grattan, Bloomer, Archambault, & Eslinger, Reference Grattan, Bloomer, Archambault and Eslinger1994; Grattan & Eslinger, Reference Grattan and Eslinger1989; Shamay-Tsoory, Tomer, Berger, & Aharon-Peretz, Reference Shamay-Tsoory, Tomer, Berger and Aharon-Peretz2003; Shamay-Tsoory, Tomer, Goldsher, Berger, & Aharon-Peretz, Reference Shamay-Tsoory, Tomer, Goldsher, Berger and Aharon-Peretz2004). However, in the present study, no correlation was found between empathy and cognitive flexibility in the focal EBI group. The inclusion of multiple cognitive flexibility measures in future studies would be important since flexibility is hypothesised to develop in a domain-specific fashion as children gain task-specific skills and knowledge (Deak & Wiseheart, Reference Deak and Wiseheart2015; Luwel, Verschaffel, Onghena, & De Corte, Reference Luwel, Verschaffel, Onghena and De Corte2003). Empathy and morality share common neural substrates in the social brain and are both supported by a diffuse network that includes several frontotemporolimbic structures (Bernhardt & Singer, Reference Bernhardt and Singer2012; Pascual, Rodrigues, & Gallardo-Pujol, Reference Pascual, Rodrigues and Gallardo-Pujol2013; Sevinc & Spreng, Reference Sevinc and Spreng2014). The poorer empathy, MR and moral decision-making found in the Focal EBI patients may thus be attributable to disruptions of the frontotemporal circuitry underlying social cognition, as shown in studies of adults with focal lesions (see Hillis, Reference Hillis2014 for a review). Given the evidence of phases in development of both MR and emotion recognition (Tonks et al., Reference Tonks, Slater, Frampton, Wall, Yates and Williams2009), it is possible that an early brain insult to frontal or temporal regions may disrupt normal acquisition of such sociocognitive functions and potentially cause delayed effects of deficits as social demands increase.
As expected, children with focal EBI had more sociobehavioural problems than controls, although the standardised scores did not reach the clinical threshold. Patients with focal EBI had more internalising than externalising problems, in line with a previous study investigating psychopathological profiles of children with focal EBI (Duval, Braun, Daigneault, & Montour-Proulx, Reference Duval, Braun, Daigneault and Montour-Proulx2002). Elevated scores on the internalisation subscale could be attributable to physical illness in these children and emotional response to a threatening medical condition. Despite the fact that no significant differences were found on the other CBCL scales or for social adaptive skills (ABAS), children with frontotemporal lesions had consistently lower scores than their matched counterparts.
Finally, whilst the study methodology did not include a full cognitive protocol, these preliminary results suggest that social cognition may be more vulnerable to focal frontotemporal brain damage than general cognitive functions. These differential findings make intuitive sense in that functions subsumed by discrete lateralised brain regions may have greater capacity for reorganisation than those dependent on more diffuse neural networks, such as social cognition (Anderson et al., Reference Anderson, Spencer-Smith and Wood2011). They also converge with studies of TBI, which highlight impairments in emotion processing and MR despite adequate intellectual and/or executive functioning (Anderson, Barrash, Bechara, & Tranel, Reference Anderson, Barrash, Bechara and Tranel2006; Martins, Faisca, Esteves, Muresan, & Reis, Reference Martins, Faisca, Esteves, Muresan and Reis2012; Tousignant et al., Reference Tousignant, Jackson, Massicotte, Beauchamp, Achim, Vera-Estay and Sirois2016). Future work could seek to answer the question of general cognitive vs. sociocognitive deficits via more elaborate neuropsychological test batteries and a more ecological approach to the assessment of executive functions as suggested by Burgess et al. (Reference Burgess, Alderman, Forbes, Costello, Coates, Dawson and Channon2006). Also, given that the effect sizes for IQ and EF remain substantial, further studies could test the hypothesis that focal EBI results in a developmental delay that affects intellectual, executive and sociocognitive functions and leads to more sociobehavioural problems in accordance with the Socio-Cognitive Integration of Abilities Model (Beauchamp & Anderson, Reference Beauchamp and Anderson2010).
Clinical Implications
Children with focal EBI may have impaired social cognitive skills that a clinician might not suspect based on assessment of general cognitive ability and behaviour alone. The differences in social cognitive functioning that we identified are subtle and difficult to detect, yet they may potentially impact the quality of children's social life. The challenge for practitioners working with children who sustain focal EBI will be to identify those who are in need of additional intervention to target delay or deficits in social cognitive functions. In the future, the development of further ecological and standardised measures of social cognition may provide avenues for specifically assessing these functions in children with EBI.
Strengths and Limitations
Whilst the current study is the first to directly investigate different aspects of social cognition in a group of children with focal EBI, the statistical power of our analyses was limited by the small sample size. This is most obvious with some of the paired comparisons that were non-significant, but had medium effect sizes, such as IQ and EF. Still, statistically significant differences were found in some of the proposed analyses. The inclusion of patients with a history of seizure may have contributed to the findings, however, those with severe and uncontrolled epilepsy were excluded. The sample size and heterogeneity of lesions precluded examination of the individual contribution of lesion variables to outcome. Further examination of the effects of lesion size, type, location and onset with a larger group is warranted. Also, the inclusion of a non-cerebral patient control group (e.g. chronic asthma) could be insightful in controlling for the effects of hospital admission and chronic illness.
Despite these limitations, the study has several strengths. One of them is the use of an innovative MR assessment measure adapted for children and adolescents and designed to minimise methodological flaws associated with traditional MR tasks (Dooley et al., Reference Dooley, Beauchamp and Anderson2010). The combined use of both direct and indirect measures helped gain a better understanding of social functioning after focal EBI, though a direct measure of empathy was not available. This highlights the need for development of more innovative measurement approaches for directly assessing sociocognitive functions. The selection of demographically matched control participants on an individual basis represents a strength that allowed for paired comparisons. Participants were paired on age, sex, and parental education, in an attempt to eliminate potential sources of extraneous variation.
Conclusions
In summary, our results suggest that early brain insult involving focal pathology in frontotemporal regions may have an adverse effect on social cognition and behaviour. MR appears to be particularly vulnerable to such brain damage, as highlighted by lower MR maturity levels in youth with focal EBI. The variability in lesion locations highlights the diversity of frontotemporal damage likely to be associated with socio-cognitive vulnerability. Nonetheless, individual cases within this study illustrate the potential for adequate sociocognitive functioning and social behaviour despite evidence of structural damage, and suggest that multiple factors need to be taken into account in identifying children with focal EBI at risk for social dysfunction.
Acknowledgements
We gratefully acknowledge the input of Miguel Chagnon for statistical analysis.
Financial Support
This work was supported by the Natural Sciences and Engineering Council of Canada (#386527-2010).
Conflict of Interest
None.
Ethical Standards
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008.









