Introduction
Paranoia is defined as a range of thoughts from mild worries that relate to social judgment to strong beliefs of reference and fear that an entity is intending to cause harm (Green et al., Reference Green, Freeman, Kuipers, Bebbington, Fowler, Dunn and Garety2011). These beliefs range from paranoid ideations to persecutory delusions. Approximately one in three people in the general population experiences paranoid thinking to some extent during their lifetime (Freeman et al., Reference Freeman, McManus, Brugha, Meltzer, Jenkins and Bebbington2011). Over 70% of people experiencing early stages of psychosis present with persecutory delusions (Coid et al., Reference Coid, Ullrich, Kallis, Keers, Barker, Cowden and Stamps2013). These delusions, held with strong conviction and resistant to contrary evidence significantly impact functioning, mood (Vorontsova, Garety, & Freeman, Reference Vorontsova, Garety and Freeman2013), and sleep (Freeman, Pugh, Vorontsova, & Southgate, Reference Freeman, Pugh, Vorontsova and Southgate2009). The severity of these beliefs, which is key to diagnosis, is determined by factors such as conviction, distress, impact on functioning, preoccupation, and resistance to change (Garety et al., Reference Garety, Freeman, Jolley, Bebbington, Kuipers, Dunn, Fowler and Dudley2005). Diagnosing paranoid ideation typically involves self-report or hetero-anamnestic evidence from interviews and questionnaires (such as the Positive and Negative Syndrome Scale (PANSS; Kay, Fiszbein, & Opler, Reference Kay, Fiszbein and Opler1987); Comprehensive Assessment of At-Risk Mental States (Yung et al., Reference Yung, Yung, Pan Yuen, Mcgorry, Phillips, Kelly, Dell’olio, Francey, Cosgrave, Killackey, Stanford, Godfrey and Buckby2005); and Green Paranoid Thoughts Scale (Green et al., Reference Green, Freeman, Kuipers, Bebbington, Fowler, Dunn and Garety2008). Interviews and questionnaires can potentially be suboptimal for diagnosing paranoia, as individuals may avoid sharing details due to mistrust or fear of judgment. Additional evidence for the diagnosis of persecutory delusions might be provided through clinical observation and other nonverbal measurements, although a synthesis of evidence for this in the literature is lacking. The aim of this article is to provide an overview of nonverbal correlates of paranoid ideation. Results can be used to further develop nonverbal components of diagnostic instruments in order to provide useful triangulation in clinical assessment.
Currently, people’s ability and willingness to verbally communicate are a vital part of diagnostic assessment. When this is hampered due to anxiety, suspiciousness, or other symptoms, the validity of the diagnostic process becomes vulnerable. Studies show that social interactions and relationships can be complicated for people experiencing paranoid delusions (Fett et al., Reference Fett, Hanssen, Eemers, Peters and Shergill2022; Freeman et al., Reference Freeman, McManus, Brugha, Meltzer, Jenkins and Bebbington2011; Gorisse et al., Reference Gorisse, Senel, Banakou, Beacco, Oliva, Freeman and Slater2021). Furthermore, language and cultural contexts can add to the challenge of the diagnostic process and interpretation of symptoms. For example, behaviors that may appear paranoid, such as heightened mistrust or hypervigilance, can stem from real threats or from experiences with racism and oppression, rather than from clinical delusional ideations (Whaley, Reference Whaley2001). When the interaction between clinician and patient is disrupted, questions that assess symptom severity can become ineffectual. In these cases, healthcare professionals have to rely on records, context, and clinical experience to assess and formulate diagnoses. Research has shown some reliability in this approach of diagnosis (Ekholm et al., Reference Ekholm, Ekholm, Adolfsson, Vares, Ösby, Sedvall and Jönsson2005). However, this diagnostic process is not standardized nor operationalized, which challenges its validity, especially in critical stages of the disorder.
Ideally, auxiliary methods in the form of observations of nonverbal correlates could provide supplemental evidence for diagnosis. Opportunities for assessing these nonverbal correlates exist on multiple facets. Anxiety and negative emotions impact the development and maintenance of persecutory delusions (Freeman & Garety, Reference Freeman and Garety2014). Negative emotional states have nonverbal correlates including posture (Oosterwijk, Rotteveel, Fischer, & Hess, Reference Oosterwijk, Rotteveel, Fischer and Hess2009), facial expressions (Ekman, Freisen, & Ancoli, Reference Ekman, Freisen and Ancoli1980), and breathing patterns (Masaoka & Homma, Reference Masaoka and Homma1997). This type of additional information could potentially aid the diagnostic process compared to reliance on verbal reports alone. Additionally, cognitive biases associated with persecutory delusions, such as jumping-to-conclusions and bias against disconfirmatory evidence (Ho-wai So, Freeman, & Garety, Reference Ho-wai So, Freeman and Garety2008; Van Dael et al., Reference Van Dael, Versmissen, Janssen, Myin-Germeys, van Os and Krabbendam2006), could be revealed through nonverbal tasks. One way to do this is through assessment in decision-making tasks when minimal evidence is presented, when decisions are made faster, that is, with less information a tendency to jump-to-conclusions could be present (Catalan et al., Reference Catalan, Tognin, Kempton, Stahl, Salazar de Pablo, Nelson, Pantelis, Riecher-Rössler, Bressan, Barrantes-Vidal, Krebs, Nordentoft, Ruhrmann, Sachs, Rutten, van Os, de Haan, van der Gaag, Valmaggia and McGuire2022). Additionally, the perception of threat can trigger physiological and neurobiological reactions (Kozlowska, Walker, McLean, & Carrive, Reference Kozlowska, Walker, McLean and Carrive2015). Finally, deficits in social cognition and emotion perception, which impair the ability to accurately interpret and respond to others’ mental states, emotions, and social cues, can be assessed nonverbally.
While studies exist that investigate nonverbal correlates, the literature is devoid of a systematic literature review of nonverbal correlates relating to paranoid ideation. To explore this topic, we conducted a systematic overview of the existing literature, with a specific focus on objectively measured correlates of a nonverbal nature. This review can help identify potential additional measures of paranoid ideations and methodological. Results may offer insights into the mechanisms of paranoid ideation and could inform the development of novel diagnostic instruments that do not depend solely on verbal communication, bridge language barriers, and potentially contributing to diagnostic accuracy.
Methods
This systematic literature review was registered with PROSPERO (ID: CRD42022288001). The research protocol follows the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) guidelines (Page et al., Reference Page, McKenzie, Bossuyt, Boutron, Hoffmann, Mulrow, Shamseer, Tetzlaff, Akl, Brennan, Chou, Glanville, Grimshaw, Hróbjartsson, Lalu, Li, Loder, Mayo-Wilson, McDonald and Moher2021). The PRISMA checklist is added in Supplementary Material A. The PICO framework (participants, interventions, comparators, outcomes) was used to determine the eligibility criteria for this study, which resulted in the inclusion and exclusion criteria below. Databases were searched in May 2022 and a search update took place in April 2024.
Inclusion and exclusion criteria
Studies were included if they met the following inclusion criteria: (1) use of standardized instruments for measurements of paranoid ideation; (2) use of standardized instrument for measurement of nonverbal correlate; (3) adult participants, aged 16 and older; (4) both clinical (delusions) and subclinical (paranoid ideations) levels of paranoia, (5) original data; and (6) published in a peer reviewed journal. Studies were excluded based on the following exclusion criteria: (1) nonverbal correlate is measured using a verbal method and (2) full text not available in English language.
No inclusion criteria were formulated around use of comparator group or study methodology, meaning articles with and without a control group were included as well as both qualitative and quantitative studies. No restrictions were placed on date of publication. No contextual restrictions for the studies were included in the review, allowing for a broad understanding of nonverbal correlates of paranoia in diverse settings.
Search strategy
Four databases were used: PsycINFO, PubMed, Web of Science, and Cienahl. Our search strategy contained terms for paranoia (e.g. paranoid ideation, persecutory delusions) and nonverbal behavior (e.g. nonverbal communication, behavioral symptoms). The search strategy for each database can be found in Supplementary Material B. As a secondary search strategy, the titles in the reference lists of included papers were screened.
Screening and data extraction
Duplicates were removed and the remaining articles were independently screened by two of the authors (RH and CG/HJ) in three phases: title, abstract, and full text (see Figure 1). During each phase, assessment of eligibility criteria was conducted with any discrepancies resolved through discussion between all review authors. Data were systematically extracted from the selected studies using a data extraction sheet including: authors, year of publication, country of study, N, gender, diagnosis, used instruments, nonverbal concept being measured, and main outcomes. Main outcome data were extracted on any quantitative measure where possible, such as correlations, odd ratios, effect sizes, relative risks, and risk ratios. Similarly, qualitative data measures were extracted, for example: thematic and axiological analyses. Data were summarized thematically and narratively.
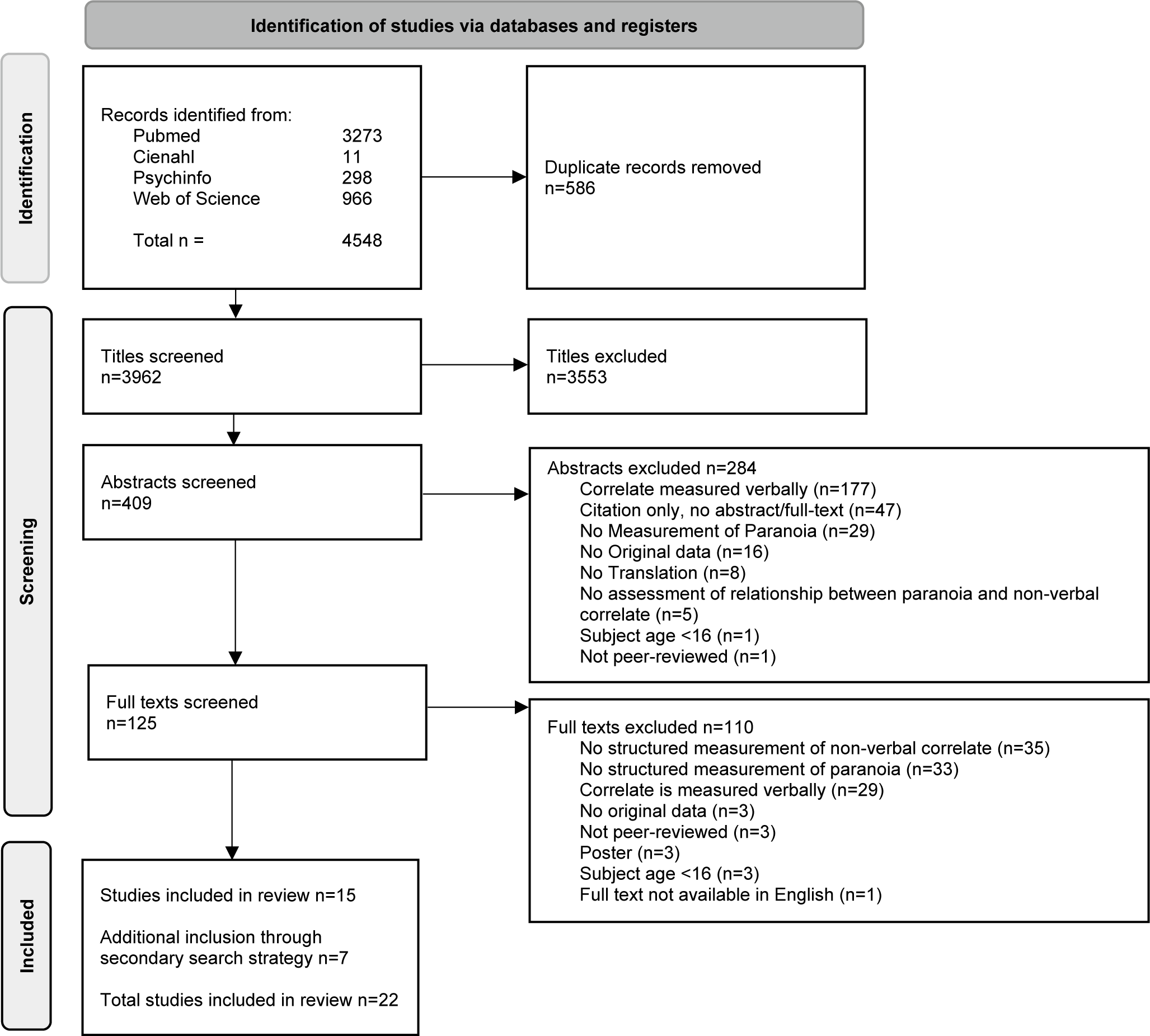
Figure 1. PRISMA flowchart.
Meta-analyses were planned in case five or more studies with the same nonverbal correlate were found. This limit was chosen as literature has shown that estimating between-study variance with fewer than five studies can be unstable and unreliable (Jackson & Turner, Reference Jackson and Turner2017). Since there were no categories with minimally five studies, meta-analyses were not conducted
Quality assessment
For the included studies, the ‘Effective Public Healthcare Panacea Project’ quality assessment (QA) tool was used (Thomas, Ciliska, Dobbins, & Micucci, Reference Thomas, Ciliska, Dobbins and Micucci2004). The Effective Public Health Practice Project (EPHPP) was selected as it allows for a standardized assessment across diverse methodologies. Given the absence of restrictions on research design in the inclusion criteria, a variety of study designs was anticipated, making the EPHPP approach particularly suitable. QA was performed by two authors independently (RH and CG/HJ). QA criteria were: selection bias, study design, confounders, blinding, data collection methods, withdrawals and dropouts, intervention integrity, and analysis. Scores ranged from 1 (strong) to 3 (weak), thus higher value indicates a lower quality. Average quality scores were calculated, full sores can be found in Supplementary Material C.
Paranoia concept formulation
Included studies show conceptual overlap concerning paranoid ideations but written terminologies have lexical variation between authors. In this review, the term paranoid ideation is used to offer uniform terminology.
Results
The final search was executed in April 2024 and resulted in a total of 3962 titles after duplicate removal. After abstract and full-text screening, 15 studies met inclusion criteria. The secondary search strategy yielded 45 potential studies of which seven met inclusion criteria, resulting in a total of 22 studies included in this review (Figure 1). Main reasons for exclusion during full-text screening (n = 125) were a non-structured measurement of either paranoid ideation or the nonverbal correlate (n = 68, 54%), and verbal measurement of the nonverbal correlate (n = 29, 23%). Of the 22 included studies, 11 (50%) included patients, and the other 11 studies were conducted with a nonclinical sample. Combined the studies included a total of 1572 participants, of which 458 (29%) were patients diagnosed with a psychotic disorder.
Table 1 provides an overview of the included studies; these are grouped in categories of type of nonverbal concept. Due to heterogeneity of data, no formal data synthesis was possible. While interpretations of effect sizes (small, medium, large) are reported where available, it is important to note that many of the included studies did not provide standardized effect sizes. Identified nonverbal concepts were spatial behavior (n = 6), brain region activity (n = 5), visual perception (n = 5), stress physiology (n = 4), information processing (n = 3), and aggression (n = 1). Two included articles studied multiple nonverbal concepts and therefore reoccur in Table 1.
Table 1. Summary of Included Studies by Nonverbal Concept: Participants, Measures, Findings, and Quality Assessment
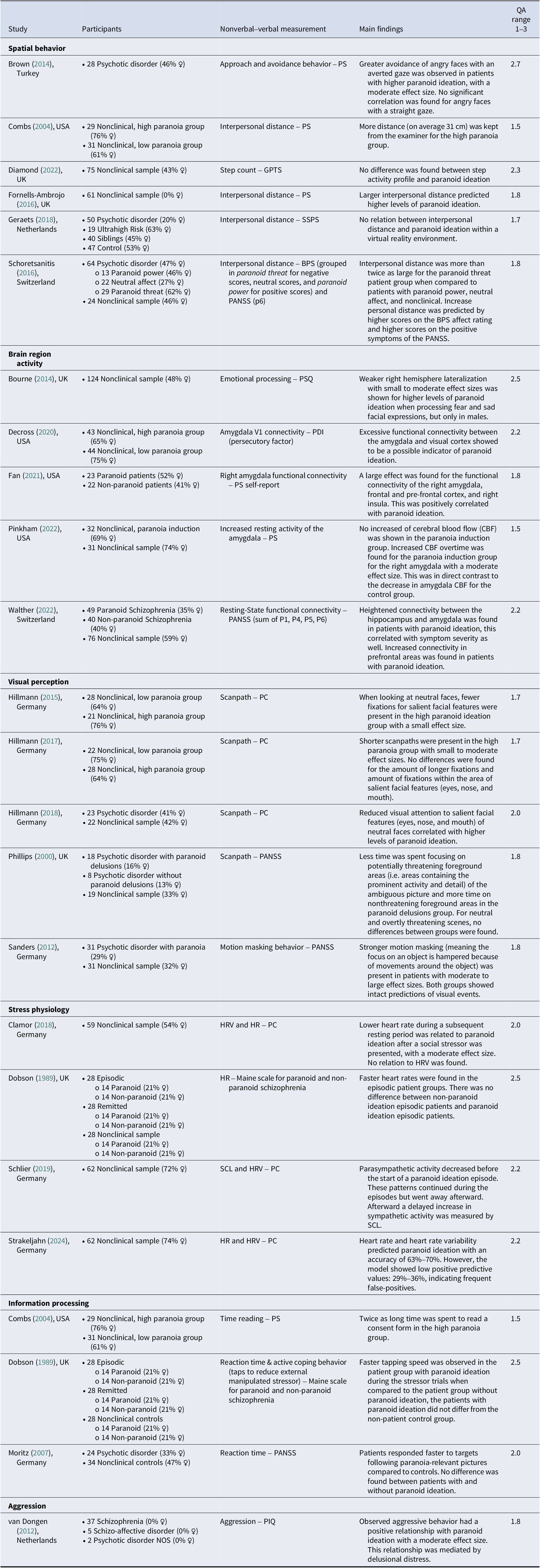
Note: BPS, Bern Psychopathology Scale; GPTS, Green Paranoid Thoughts Scale; HRV, Heart Rate Variability, HR, Heart rate; PANSS, Positive and Negative Syndrome Scale; PC, Paranoia checklist; PDI, Peters et al. Delusions Inventory; PIQ, Persecutory Ideation Questionnaire; PS, Paranoia Scale; PSQ, Paranoia/Suspiciousness Questionnaire; SCL, Tonic Skin Conductance Level; SSPS, State Social Paranoia Scale.
Spatial behavior (n = 6)
Increased paranoid ideation seemed to be associated with increased physical interpersonal distance (Combs & Penn, Reference Combs and Penn2004; Fornells-Ambrojo et al., Reference Fornells-Ambrojo, Elenbaas, Barker, Swapp, Navarro, Rovira, Sanahuja and Slater2016; Schoretsanitis et al., Reference Schoretsanitis, Kutynia, Stegmayer, Strik and Walther2016) and avoidance of potentially threatening social cues (Brown et al., Reference Brown, Tas, Kuzu, Esen-Danaci, Roelofs and Brüne2014) in both clinical and nonclinical samples. Combs and Penn (Reference Combs and Penn2004) found that the high paranoid ideation group maintained an average interpersonal distance that was 31 cm greater than those in the low paranoid ideation group. Schoretsanitis et al. (Reference Schoretsanitis, Kutynia, Stegmayer, Strik and Walther2016) found interpersonal distance increased more than twofold for patients in the paranoid threat group compared to patients in the paranoid power group (i.e. delusion of grandiosity) and controls. Brown et al. (Reference Brown, Tas, Kuzu, Esen-Danaci, Roelofs and Brüne2014) showed a moderate positive relationship between paranoid ideation and avoidance of potentially threatening social cues. Both Fornells-Ambrojo et al. (Reference Fornells-Ambrojo, Elenbaas, Barker, Swapp, Navarro, Rovira, Sanahuja and Slater2016) and Geraets et al. (Reference Geraets, van Beilen, Pot-Kolder, Counotte, van der Gaag and Veling2018) looked at personal distance in a virtual environment. Results were mixed, Fornells-Ambrojo et al. (Reference Fornells-Ambrojo, Elenbaas, Barker, Swapp, Navarro, Rovira, Sanahuja and Slater2016) found persecutory ideation predicted increased interpersonal distance, Geraets et al. (Reference Geraets, van Beilen, Pot-Kolder, Counotte, van der Gaag and Veling2018) found no relationship between persecutory ideation and interpersonal distance. Finally, a study looking at activity profiles of patients (consisting of step count, meaningful activity, and mobility issues) showed no relationship with level of persecutory ideation and activity profile group (Diamond et al., Reference Diamond, Bird, Waite, Bold, Chadwick, Collett and Freeman2022).
Brain region activity (n = 5)
The studies on neurobiological correlates of paranoid ideation showed that the amygdala is significantly involved as a key brain area in threat processing. Amygdala activity was amplified (Fan et al., Reference Fan, Klein, Bass, Springfield and Pinkham2021) and an increased connectivity was found to the visual cortex (Decross et al., Reference Decross, Farabaugh, Holmes, Ward, Boeke, Wolthusen, Coombs, Nyer, Fava, Buckner and Holt2020) and to the hippocampus (Walther et al., Reference Walther, Lefebvre, Conring, Gangl, Nadesalingam, Alexaki, Wüthrich, Rüter, Viher, Federspiel, Wiest and Stegmayer2022) in individuals with high levels of paranoid ideation. Both when comparing high and low levels of paranoid ideation in healthy samples (Decross et al., Reference Decross, Farabaugh, Holmes, Ward, Boeke, Wolthusen, Coombs, Nyer, Fava, Buckner and Holt2020) and when comparing controls with patients with paranoid ideation and schizophrenia, with Fan et al. (Reference Fan, Klein, Bass, Springfield and Pinkham2021) reporting a large effect size (Fan et al., Reference Fan, Klein, Bass, Springfield and Pinkham2021; Walther et al., Reference Walther, Lefebvre, Conring, Gangl, Nadesalingam, Alexaki, Wüthrich, Rüter, Viher, Federspiel, Wiest and Stegmayer2022). In a nonclinical sample, Bourne and McKay (Reference Bourne and McKay2014) showed weaker right hemisphere lateralization in males when processing fear and sad facial expressions when paranoid ideation was higher. Pinkham et al. (Reference Pinkham, Bass, Klein, Springfield, Kent and Aslan2022) showed an increase in connectivity in the prefrontal cortex for individuals with higher level of paranoid ideation.
Visual perception (n = 5)
Individuals with higher levels of paranoid ideation might exhibit distinct visual perception patterns. Included papers mainly looked at scanpath behavior and visual fixations. A visual scanpath refers to the amount of saccades (small eye movements) a person makes when looking at different parts of a scene or image. Meaning a reduced scanpath shows fewer eye movements (Hillmann, Ascone, Kempkensteffen, & Lincoln, Reference Hillmann, Ascone, Kempkensteffen and Lincoln2017). Visual fixation occurs when the eyes stay focused on a specific point for more than 100 m/s (Hillmann, Kempkensteffen, & Lincoln, Reference Hillmann, Kempkensteffen and Lincoln2015). Differences for visual perception were apparent in both clinical and nonclinical samples. Paranoid ideation seemed to be related to reduced scanpaths, with a small to medium effect size reported (Hillmann, Ascone, Kempkensteffen, & Lincoln, Reference Hillmann, Ascone, Kempkensteffen and Lincoln2017), reduced visual attention to salient facial features of the eyes, mouth, and nose, with a small effect size reported (Hillmann, Kempkensteffen, & Lincoln, Reference Hillmann, Kempkensteffen and Lincoln2015; Hillmann, Riehle, & Lincoln, Reference Hillmann, Riehle and Lincoln2018), and reduced visual attention of photograph areas containing activity and detail in ambiguous social scenes (Phillips, Senior, & David, Reference Phillips, Senior and David2000). Higher paranoid ideation can also affect visual processing when looking at dynamic environments. When a visual cue is moving or when it is presented in a visually complex environment, it becomes complicated to separate important details from surrounding distractions, this is called motion masking. This effect was shown to be stronger for patients with persecutory ideations, with a medium to large effect size reported (Sanders et al., Reference Sanders, Muckli, de Millas, Lautenschlager, Heinz, Kathmann and Sterzer2012). Within nonclinical samples mixed results were found for the association of paranoid ideation and visual fixations Hillmann, Kempkensteffen, & Lincoln, Reference Hillmann, Kempkensteffen and Lincoln2015; Hillmann, Ascone, Kempkensteffen, & Lincoln, Reference Hillmann, Ascone, Kempkensteffen and Lincoln2017). However, the study by Phillips, Senior, and David (Reference Phillips, Senior and David2000) with clinical sample patients with paranoid ideation showed fewer fixations and different focus of fixations compared with controls.
Stress physiology (n = 4)
No evidence of an association in clinical samples was found between stress physiology and paranoid ideation (Dobson & Neufeld, Reference Dobson and Neufeld1989). Looking at levels of paranoid ideation in nonclinical samples, the results showed low predictive value of physiological indicators, such as heart rate variability (HRV) and heart rate (HR) (Clamor & Krkovic, Reference Clamor and Krkovic2018; Strakeljahn, Lincoln, Krkovic, & Schlier, Reference Strakeljahn, Lincoln, Krkovic and Schlier2024). Schlier, Krkovic, Clamor, and Lincoln (Reference Schlier, Krkovic, Clamor and Lincoln2019) showed patients had a lower HR and a decrease in parasympathetic activity before a paranoid episode.
Information processing (n = 3)
Patients were shown on average to spend more than twice as long reading an informed consent form (65 s compared to 128 s) for participation in research, which seems to indicate slower and possible more cautious information processing in neutral or nonurgent situations in individuals with higher levels of paranoid ideation (Combs & Penn, Reference Combs and Penn2004). A study by Dobson and Neufeld (Reference Dobson and Neufeld1989) did not find differences in responses on a stressor task: subjects were instructed to control the length of a noise played through headphones by tapping the index finger of their preferred hand quickly. Moritz and Laudan (Reference Moritz and Laudan2007) did find patients responded faster to paranoia relevant pictures, but did not find differences between patients with and without paranoid ideation.
Aggression (n = 1)
The study by van Dongen, Buck, and van Marle (Reference van Dongen, Buck and van Marle2012) showed that paranoid ideation had a moderate positive relationship with observed aggressive behavior, which was mediated by delusional distress.
Quality assessment
Overall, the studies demonstrated strong performance in addressing confounders and employing robust data collection methods, while showing weaker scores in selection bias and blinding. Eleven of the included articles were of moderate to good quality (n = 50%), three of moderate quality (n=14%), and eight of moderate to weak quality (n = 36%) (see Table 1).
Discussion
This is the first systematic literature review on nonverbal correlates of paranoid ideation. This review included 22 studies that examined 5 categories of nonverbal correlates associated with paranoid ideation. First, the results for each identified category will be discussed.
Spatial behavior (n = 6)
In essence, results suggest that paranoid ideation influences physical distancing and threat avoidance, highlighting how it alters social behavior. According to the cognitive model of Freeman (Reference Freeman2016), persecutory delusions are conceptualized as threat beliefs, which appear to drive increased personal distance as a protective mechanism. In this model, safety behaviors maintain paranoid ideation as it prevents exposure to contradictory evidence, which make them a possible target for interventions (Berkhof et al., Reference Berkhof, van der Stouwe, Geraets, Pot-Kolder, van der Gaag and Veling2024). However, spatial safety behavior might be modulated by different contextual variables such as environmental familiarity or how busy an environment is. Subsequently, results from studies on spatial behavior in virtual reality show that personal distance is possibly regulated differently in a virtual environment. Not all dimensions of spatial behavior seem to be related to paranoid ideation, as seen by a lack of support for a relationship between paranoid ideation and activity profiles (Diamond et al., Reference Diamond, Bird, Waite, Bold, Chadwick, Collett and Freeman2022). Results support a threat-avoidance model, where higher paranoid ideation leads to modification of the physical environment to feel safer. However, larger, controlled studies are needed. It remains unclear to what extent spatial behavior is being modulated by factors, such as the social environment, familiarity, or specific stressors.
Brain region activity (n = 5)
Results underpin paranoid ideation’s integration of emotional and cognitive responses. Increased amygdala activation paralleled with heightened prefrontal connectivity suggests the existence of two neural pathways within paranoia: the amygdala is involved in fast threat detection while the prefrontal cortex contributes to rational decision-making and the control of thought patterns related to paranoid ideation. This seems to involve cognitive effort in managing emotional responses. The specific role of the amygdala and prefrontal cortex in paranoia appears to fit within the general framework of psychosis-related neural activity (Feola et al., Reference Feola, Beermann, Manzanarez Felix, Coleman, Bouix, Holt, Lewandowski, Öngür, Breier, Shenton, Heckers, Brady, Blackford and Ward2024) wherein the amygdala has shown to be pivotal as part of the neural mechanism in fear and anxiety, with specific associations for psychosis with the hippocampus and ventromedial prefrontal cortex.
Visual perception (n = 5)
Visual perception and specifically gazing behavior has been studied extensively in patients with a psychotic disorder. Summarizing these results in a recent scoping review Wolf, Ueda, and Hirano (Reference Wolf, Ueda and Hirano2020) concluded that longer average eye fixation, fewer total eye fixations, and shorter average scanning length are present in patients with schizophrenia. These results overlap with studies looking at paranoid ideation and gazing behavior. In terms of theoretical underpinning, the vigilance-avoidance paradigm of paranoia (Green & Phillips, Reference Green and Phillips2004) offers an explanation for the deviation gazing behavior, as mentioned by Hillmann, Riehle, and Lincoln (Reference Hillmann, Riehle and Lincoln2018).Individuals with higher paranoid ideation initially show increased bias toward potentially threatening information, which is followed by avoidant behavior in the form of reduced visual fixation on relevant areas. This avoidant behavior is shown to be present for individuals with higher paranoid ideation when presented with neutral faces. However, to which extent this explanation accounts specifically for paranoid ideation or is more general applicable to the broader spectrum of schizophrenia remains unclear. Social context seemingly plays a role in visual scanning behavior, since ambiguity of visual stimuli (either facial or environmental) influences how patients and nonclinical individuals with higher levels of paranoid ideation pay visual attention. Summarized, results suggest that paranoid ideation leads to changes in visual perception, which influences scanning behavior, visual fixation, and motion masking, these behaviors fluctuate with clinical status and social context.
Stress physiology (n = 4)
The lack of consistent physiological markers poses a challenge to simple biological models of paranoid ideation. Physiological measures such as HR and HRV, commonly used to assess autonomic nervous system activation, represent a general measure of arousal influenced by a wide range of factors – both negative, positive, and neutral. This complexity makes it difficult to isolate the direct impact of paranoid ideation. If such markers are to be useful, their application would likely require integration with other complementary measures to provide a more comprehensive understanding. Reactions could be seen as part of the body’s context-dependent response to perceived threats (Kozlowska, Walker, McLean, & Carrive, Reference Kozlowska, Walker, McLean and Carrive2015). Which would underscore the theory’s emphasis on the cascading nature of threat responses, influenced by multiple factors beyond just paranoia. As proposed by Schlier, Krkovic, Clamor, and Lincoln (Reference Schlier, Krkovic, Clamor and Lincoln2019), one hypothesis is that paranoid thinking works as a coping mechanism to deal with stress due to perceived threat. In that case, hypervigilance might cause physiological threat reactions (i.e. activation of sympathetic nervous system) but the existence of paranoid thoughts might cause parasympathetic reactions. This would explain different findings among the presented studies. Since responses vary widely, relying on physiological markers for diagnostic purposes may be limited by a lack of sufficient information at this point.
Information processing (n = 3)
Individuals diagnosed with a psychotic disorder may exhibit selective sensitivity to stimuli that align with paranoid thoughts but not a generalized change in processing speed. This might reflect the hyper vigilant state of paranoia, where one shows heightened suspicion when dealing with neutral stimuli. Heightened suspicion or threat perception influences cognitive and emotional processing, even for seemingly neutral stimuli. This aligns with previous explanation that paranoid ideation integrates emotional and cognitive responses through two distinct but interrelated neural pathways. These reflect the cognitive effort required to manage emotional responses, a dynamic central to paranoid ideation. However, as Feola et al. (Reference Feola, Beermann, Manzanarez Felix, Coleman, Bouix, Holt, Lewandowski, Öngür, Breier, Shenton, Heckers, Brady, Blackford and Ward2024) note, the roles of the amygdala and prefrontal cortex likely fall within the broader framework of psychosis-related neural activity, suggesting these mechanisms may not be exclusive to paranoid ideation but could extend to schizophrenia more generally.
Aggression (n = 1)
Although a moderate positive relationship was found for aggressive behavior and paranoid ideation, results come from one study. The particular study focused on a male sample of forensic clinical patients. Based on the specificity of this setting and low n, generalization of findings is not possible at this point in time.
Results of this systematic literature review reveal that investigations on nonverbal correlates of paranoid ideation are scarce, quite heterogeneous in approach and populations, and limited by small sample sizes. Nevertheless, measures in several domains showed statistical, clinically relevant, and theoretically plausible associations with paranoid ideations. The potential for nonverbal correlates to be used diagnostically is an interesting field, although in order to enhance diagnostic accuracy, probably the combination of both nonverbal and verbal signs is needed. Future research should focus on developing frameworks that integrate both types of indicators. Theoretically, this integration could provide a more holistic understanding of paranoid ideation, capturing both explicit verbal expressions and implicit nonverbal signals. However, given the current limitations in research, further exploration is needed to better understand how these two forms of assessment might complement each other in practice.
When comparing clinical and nonclinical populations, findings on spatial behavior and brain region activity suggest similar patterns, which could mean underlying mechanisms of paranoid ideations are shared. In terms of visual attention, results suggest that paranoid ideation is associated with changes in visual perception both in clinical and nonclinical populations. Stress physiology related to paranoid ideations has hardly been studied in clinical populations, with only one out of four studies including patients, making it difficult to determine whether stress responses differ between clinical and nonclinical groups. In terms of information processing, nonclinical subjects with high paranoid ideation took longer to read consent forms, whereas clinical subjects with high levels of paranoid ideation showed quicker responses in task-related measures compared to those with lower level of paranoid ideations. Due to the limited evidence, it remains unclear to what extent clinical and nonclinical groups differ in the nonverbal correlates of paranoia. However, the available findings suggest some overlapping patterns that require further investigation.
Finally, in some populations, nonverbal measures may be particularly useful, for example, when people cannot or will not speak, in patients with intellectual disabilities or with other communication barriers. Furthermore, investigating how contextual variables – such as social settings, environmental, or stress-inducing conditions – modulate these nonverbal correlates need to be assessed in order to ascertain the situational dynamics of paranoid ideation. Controlled virtual reality experiments may be particularly suited to test such associations. This can potentially identify predictive factors or early markers for paranoia, which can contribute to improving clinical outcomes through earlier and more accurate detection and targeted treatment strategies.
Strengths and limitations
A key strength of first systematic review into nonverbal correlates of paranoia lies in its strict inclusion criteria, excluding studies relying on verbal components. By doing so, the review reduced potential confounding factors associated with verbal elements. Subsequently, this approach narrowed the pool of eligible studies, providing a more precise exploration of the specific nonverbal correlates addressed. This helped increase precision of the synthesized evidence to stay close to the relevant review’s objectives. Nevertheless, findings should be interpreted in light of the following limitations: First, a majority of the included studies rely on small sample sizes and only half of the included papers investigated participants with clinical levels of paranoia. Therefore, results do not capture the full spectrum of paranoid ideation and subgroup variations in terms of severity or comorbidity, negatively impacting on scope and generalizability. The small sample sizes reduce reliability of findings and generalizability. It is also worth noting that the majority of included studies reported significant associations between paranoid ideation and nonverbal correlates. Although we did not conduct a formal analysis of publication bias, this uneven distribution may suggest a potential bias in favor of publishing significant findings. Second, the categories of nonverbal concepts studied are heterogeneous, as are the definitions of paranoid ideation. Within the presented categories a broad range of experimental paradigms was found. For example, experiments investigating visual fixation patterns all had slight variations in their methodological approach. This complicates synthesis of results and impacts the strength of given conclusions and its robustness. Subsequently, although a meta-analysis was planned, it was not conducted due to an insufficient number of studies per category of nonverbal correlates. Third, self-report on nonverbal correlates or informant report on nonverbal correlates were not included in the review due to reliance on a verbal instrument. Studies show that safety behavior plays an important role in paranoid ideation (Freeman, Garety, & Kuipers, Reference Freeman, Garety and Kuipers2001); however, there were no studies available that gathered data nonverbally. It is possible that similar other seemingly nonverbal correlates are excluded due to the usage of verbal measurements. Fourth, it cannot be ruled out that the nonverbal correlates identified by this review might reflect broader symptoms of psychosis or threat. The possibility that these correlates lack specificity toward paranoid ideation reduces the precision of findings. On the other hand, certain nonverbal correlates of threat responses might also coalesce with paranoia.
Conclusion
Spatial behavior and brain region activity were shown to have the most potential as possible nonverbal indicators of paranoid ideation, whereas findings on visual processing remain inconclusive due to methodological differences. Evidence for stress physiology, information processing, and aggression as potential nonverbal correlates was less robust. Further investigation of the identified categories is needed, potentially leading to new diagnostic and therapeutic possibilities that move beyond traditional verbal methods.
Supplementary material
The supplementary material for this article can be found at http://doi.org/10.1017/S0033291725001230.
Funding statement
This research received no specific grant from any funding agency, commercial, or not-for-profit sectors.
Competing interests
The authors declare none.




