Social media summary: New paper ‘Psychosocial and energetic factors on human female pubertal timing: a systematized review’ by @GlassDelaney, Joy Geerkens and Melanie Martin. With systematic review results, they reflect on bio mechanisms and socioecological mediation of pubertal timing.
Introduction
In human females, pubertal timing (referring to the onset and pacing of pubertal development) is proposed to be flexibly attuned to energetic resources and social cues in the environment (Ellison et al., Reference Ellison, Reiches, Shattuck-Faegre, Breakey, Konecna, Urlacher and Wobber2012). Across populations, median ages at menarche range from around 7 to 16 (Ellison et al., Reference Ellison, Reiches, Shattuck-Faegre, Breakey, Konecna, Urlacher and Wobber2012; Parent et al., Reference Parent, Teilmann, Juul, Skakkebaek, Toppari and Bourguignon2003; Thomas et al., Reference Thomas, Renaud, Benefice, De Meeüs and Guegan2001). Within this range of variation, optimal pubertal timing represents a life history trade-off between energetic investment in continued growth vs. reproduction, with consequences for lifetime reproductive success (Charnov & Berrigan, Reference Charnov and Berrigan1993; Chisholm, Quinlivan, et al., Reference Chisholm, Quinlivan, Petersen and Coall2005; Kramer & Ellison, Reference Kramer and Ellison2010; Reiches et al., Reference Reiches, Ellison, Lipson, Sharrock, Gardiner and Duncan2009; Stearns, Reference Stearns1992). Life history trade-offs are particularly pronounced in females, as higher oestrogen levels promote ovarian maturation and increased body fat promotes earlier closure of epiphyseal plates, leading to reduced growth in length/height (Rogol et al., Reference Rogol, Roemmich and Clark2002). Another trade-off is that earlier puberty can lead to earlier first reproduction and higher fertility, but at increased risk of maternal and offspring morbidity and mortality (Weibel et al., Reference Weibel, Tung, Alberts and Archie2020). Conversely, continued body growth that delays reproductive maturity may increase maternal and offspring fitness, but decrease maternal lifetime fertility (Fraser et al., Reference Fraser, Brockert and Ward1995; Stearns, Reference Stearns1992; Weibel et al., Reference Weibel, Tung, Alberts and Archie2020; G. C. Williams, Reference Williams1966).
Flexible pubertal timing within humans has been proposed to relate to energetic availability or sufficiency in childhood, wherein greater energetic resources favour earlier pubertal timing, and chronically low resources favour delayed or slower pubertal progression (Ellison, Reference Ellison1990, Reference Ellison1996; Wasser & Barash, Reference Wasser and Barash1983). However, research has also posited that greater childhood stress can accelerate pubertal timing, which may be evidenced by assessing differences in demographic patterning. Across species, demographic strategies exist along a continuum, occurring either relatively faster or slower (Charnov & Berrigan, Reference Charnov and Berrigan1993; Stearns, Reference Stearns1992). Faster strategies are described as rapid juvenile growth, earlier puberty, and smaller adult size, whereas slower strategies are characterized by slower juvenile growth, later puberty, and larger adult size (Charnov & Berrigan, Reference Charnov and Berrigan1993; Stearns, Reference Stearns1992). Humans and other animals who have long lifespans are particularly sensitive to harsh conditions earlier in life, which may shorten life expectancies (Douhard et al., Reference Douhard, Plard, Gaillard, Capron, Delorme, Klein and Bonenfant2014; Stearns & Rodrigues, Reference Stearns and Rodrigues2020). Recent formal mathematical models contest earlier verbal models suggesting that extrinsic adult mortality selects for shorter lifespans and earlier senescence (Moorad et al., Reference Moorad, Promislow and Silvertown2019). However, early life adversity has been shown to reduce life expectancies in humans and other primates (Weibel et al., Reference Weibel, Tung, Alberts and Archie2020). Accelerated reproduction may be favoured when lifespans are shortened, highly dependent on local conditions (Nettle, Reference Nettle2011; Stearns, Reference Stearns1992). Thus, it was suggested that greater psychosocial stress experienced in early life (i.e. perceived threats or insults to survival or wellbeing) may favour a relatively faster life history strategy (Bateson et al., Reference Bateson, Gluckman and Hanson2014; Belsky, Reference Belsky1991; Chisholm, Burbank, et al., Reference Chisholm, Quinlivan, Petersen and Coall2005; Chisholm, Quinlivan, et al., Reference Chisholm, Quinlivan, Petersen and Coall2005; Draper & Harpending, Reference Draper and Harpending1982; Ellis, Reference Ellis2004). The Psychosocial Acceleration Theory (Acceleration Theory hereafter) proposed by Draper and Harpending (Reference Draper and Harpending1982) and expanded on by Belsky (Reference Belsky1991) suggests that early life adversity cues developmentally plastic responses. For example, many researchers have posited that the stress (Ellis & Garber, Reference Ellis and Garber2000; Moffitt et al., Reference Moffitt, Caspi, Belsky and Silva1992) or resource limitation resultant from father absence may promote earlier puberty (Belsky, Reference Belsky1991).
However, the relative effects of childhood psychosocial stress on pubertal timing may be confounded. First, confounding may occur when poverty is associated with both rapid growth or obesity and high psychosocial stress. For example, in high resource environments such as the USA, disadvantaged socioeconomic status (SES) is generally associated with greater risk for overweight/obesity and more rapid physical and sexual maturation (Bleil et al., Reference Bleil, Booth-LaForce and Benner2017; Braithwaite et al., Reference Braithwaite, Moore, Lustig, Epel, Ong, Rehkopf and Hiatt2009; Deardorff et al., Reference Deardorff, Ekwaru, Kushi, Ellis, Greenspan, Mirabedi and Hiatt2011; Obeidallah et al., Reference Obeidallah, Brennan, Brooks-Gunn, Kindlon and Earls2000). Socioeconomic status is a major driver of health inequalities, and obesity rates are higher among disadvantaged SES groups because of nutritional excess, processed foods, and food insecurity, as well as a more sedentary lifestyles (owing to a lack of safe places for physical activity; Fleming et al., Reference Fleming, Kane, Meneveau, Ballantyne and Levin2021; Working Committee on Social Determinants of Health and WHO, 2008; Oberle et al., Reference Oberle, Romero Willson, Gross, Kelly and Fox2019; D. R. Williams et al., Reference Williams, Priest and Anderson2019). Culturally specific or place-based social stratification may play a role in differentiating psychosocial and energetic exposures one experiences in childhood. These have the potential to impact growth and pubertal timing and we suspect that they probably exert effects in concert with each other. For example in the USA, racism has structured discriminatory access to resources, which impacts risks of living in poverty, disadvantaged SES and experiencing heightened psychosocial stress (Sant et al., Reference Sant, Milligan and Mollett2021; Semega et al., Reference Semega, Fontenot and Kollar2017; Trent et al., Reference Trent, Dooley, Dougé and Wallace2019). Thus differences in pubertal timing among racialized and historically excluded groups probably reflect effects of structural racism (Bleil et al., Reference Bleil, Booth-LaForce and Benner2017).
Conversely in other populations high psychosocial stress and energetic scarcity may co-occur. For example, rates of obesity have typically been higher among more advantaged SES groups in lower and middle-income countries (McLaren, Reference McLaren2007; Sear et al., Reference Sear, Sheppard and Coall2019; Wang, Reference Wang2001; A. S. Williams et al., Reference Williams, Ge, Petroski, Kruse, McElroy and Koopman2018). Within these countries, social inequalities have been associated with growth stunting or poor growth outcomes among more disadvantaged groups, rather than rapid growth (B. A. Bogin & MacVean, Reference Bogin and MacVean1978; Candler et al., Reference Candler, Costa, Heys, Costello and Viner2017; Monteiro et al., Reference Monteiro, Benicio, Conde, Konno, Lovadino, Barros and Victora2010). However, these patterns are shifting because obesity is increasingly prevalent among disadvantaged, middle and elite classes in low and middle income countries in both childhood and adulthood (Monteiro et al., Reference Monteiro, Moura, Conde and Popkin2004).
Beyond population differences in rates of obesity and social inequalities, environmental mismatch may help explain why the effects of childhood growth and psychosocial stress on pubertal timing are so difficult to differentiate. In ancestral environments, psychosocial stress was probably paired with energetic exertion (Lee et al., Reference Lee, Emerson and Williams2016), whereas psychosocial stressors in some high resource settings coexist with high-fat, high-carb diets (Stearns & Rodrigues, Reference Stearns and Rodrigues2020). Thirdly, there may be residual confounding wherein psychosocial stress is directly impacting child growth and therefore pubertal timing. Thus, while ample research has demonstrated associations between energetic factors and psychosocial stressors on pubertal development, it is unclear the extent to which they have addressed potential confounding by assessing these factors simultaneously.
This systematic literature review seeks to determine if and to what extent existing research on pubertal timing has simultaneously examined the influence of energetic and psychosocial factors. First, we summarize the energetic and psychosocial frameworks applied to human female pubertal development, including the mechanisms proposed by which energetic availability and psychosocial stress may promote more rapid pubertal development, evidence for these associations, and critiques of observational research in these areas. We then present results from an unregistered systematic review conducted with the following aims: (a) to identify original studies that have assessed both energetic and psychosocial influences on pubertal timing; and (b) where possible, to compare the relative effects of energetic vs. psychosocial influences on earlier pubertal development.
Background
Biology of puberty
Puberty is the short-term shift (over months to years) from a childhood state of negative feedback in gonadal hormones on the hypothalamic–pituitary–gonadal (HPG) axis to positive feedback loops; adolescence is the more than 6 year transition from a pre-reproductive to an adult reproductive status (B. Bogin, Reference Bogin2015). Throughout puberty, body composition, stature and secondary sexual characteristics dynamically transform. This process begins when gonadotropin-releasing hormone resumes a pulsatile pattern after being dormant in childhood (Reiches, Reference Reiches2019). The HPG axis is responsible for ‘switching on’ gonadotropin-releasing hormone and it regulates the production and release of gonadal hormones such as oestrogen and testosterone, in addition to growth hormone (Reiches & Ellison, Reference Reiches and Ellison2022). These hormones help coordinate increases in linear growth, peripheral fat, lean muscle mass, body hair and reproductive organ development, as well as menarche (Ellis, Reference Ellis2004; Nepomnaschy et al., Reference Nepomnaschy, Rowlands, Prescivalli Costa and Salvante2020; Reiches, Reference Reiches2019). Alongside these changes, the hypothalamic–pituitary–adrenal axis (HPA) is subtly changing because areas of the brain related to HPA responsivity and reactivity (e.g. the amygdala, prefrontal cortex, and hippocampus) substantially mature during puberty (Romeo, Reference Romeo2010). This results in potentially heightened biological stress responses governed by the HPA (Romeo, Reference Romeo2010). The pubertal transition requires allocation of environmental and somatic resources (e.g. food, body fat) to increase growth and reproductive development, coordinated by a complex interplay of energy balance and multiple physiological, metabolic and neuroendocrine systems (Ellis, Reference Ellis2004; Ellison, Reference Ellison2017; Reiches, Reference Reiches2019; Reiches & Ellison, Reference Reiches and Ellison2022).
Energetics hypotheses
Evolutionary theorists suggest that pubertal timing is facultative at the species level (MacDonald, Reference MacDonald1999; Stearns, Reference Stearns1992; Surbey, Reference Surbey, Ziegler and Bercovitch1990; Wasser & Barash, Reference Wasser and Barash1983). There is robust evidence that pubertal onset (from relatively early to more delayed on a continuum) is highly heritable, with heritability commonly estimated greater than 0.40 (Eaves et al., Reference Eaves, Silberg, Foley, Bulik, Maes, Erkanli and Worthman2004; Howard, Reference Howard2019; Morris et al., Reference Morris, Jones, Schoemaker, Ashworth and Swerdlow2011; Zhu et al., Reference Zhu, Kusa and Chan2018). However, because a single genotype can exhibit myriad phenotypic expressions (‘norm of reaction’), the probabilistic impact of genetics on pubertal timing may well depend on local socioecological conditions (Ellis, Reference Ellis2004; Mcintyre & Kacerosky, Reference Mcintyre and Kacerosky2011; Stearns & Koella, Reference Stearns and Koella1986; Worthman et al., Reference Worthman, Dockray and Marceau2019). For example, a large norm of reaction has been observed for age of first menstruation, with median ages at menarche ranging from 12.5 in post-industrial contexts to 18+ in other horticultural contexts (Mcintyre & Kacerosky, Reference Mcintyre and Kacerosky2011; Worthman et al., Reference Worthman, Dockray and Marceau2019). Thus, local energetic conditions may influence where an individual falls within the reaction norm.
Energetics theory of timing of pubertal development (henceforth Energetics Theory) proposed that natural selection favoured mechanisms coordinating pubertal timing to signals of resource sufficiency or availability (Ellison Reference Ellison2001, Ellis Reference Ellis2004). The pubertal trade-off (i.e. invest in reproductive development now or wait) is influenced by the presence of a metabolic surplus wherein metabolic energy exceeds the costs of maintenance (Ellison, Reference Ellison1990, Reference Ellison2001). As depicted in Figure 1, energetic or resource sufficiency is the latent variable driving Energetics Theory. What is observed to capture energetic sufficiency is typically wealth, food or nutritional availability or adequacy. Moreover, advantaged wealth or food availability/adequacy is predicted to positively impact (+) childhood growth, which will result in earlier pubertal timing (−) (see Figure 1).

Figure 1. Energetic model of pubertal timing
Ideally, to measure energetic status or energetic sufficiency, researchers should utilize multiple longitudinal measures of childhood growth that can capture trajectories of both prior and current conditions. Measures of longitudinal growth or size that can be observed are changes in height and weight. Variability in height in puberty is overwhelmingly attributed to variation in growth tempo (which characterizes how fast or slow someone is growing relative to others; Hermanussen, Reference Hermanussen2016). Height is also useful to characterize the pubertal linear growth spurt, such as measuring peak height velocity, incremental changes in height over a given period or growth tempo. It is therefore critical to have repeated measurements when assessing pre-pubertal and pubertal growth because prior conditions will impact current conditions, as an individual's developmental pace tends to stay consistent across child and adolescent development (Hermanussen, Reference Hermanussen2016). However, there are some limitations of using anthropometric indicators such as height to proxy resource sufficiency. While instances such as starvation do result in linear growth deficiencies, recent analyses of historical data suggest that relationships between height and nutrition may not be as causally linked as once considered (Hermanussen & Wit, Reference Hermanussen and Wit2017). Moreover, height may be highly heritable whereas weight or body mass index (BMI) may be less heritable, which suggests that weight or BMI may be more sensitive to current or changing energetic conditions (Wainschtein et al., Reference Wainschtein, Jain, Zheng, Cupples and Visscher2021; Yang et al., Reference Yang, Bakshi, Zhu, Hemani, Vinkhuyzen, Lee and Visscher2015; Yengo et al., Reference Yengo, Sidorenko, Kemper, Zheng, Wood and Weedon2018).
Both static measures of body weight and BMI may prove useful, at least at the population level, to proxy current energetic conditions. Weight and change in weight over time can signify nutritional status (Cole et al., Reference Cole, Faith, Pietrobelli and Heo2005). Body mass index strongly correlates with total body fat percentage and it is a reasonable proxy for accrued resource availability in young females (Bygdell et al., Reference Bygdell, Kindblom, Jansson and Ohlsson2021; Dietz & Bellizzi, Reference Dietz and Bellizzi1999; Goulding et al., Reference Goulding, Gold, Cannan, Taylor, Williams and Lewis-Barned1996; Hall & Cole, Reference Hall and Cole2006; Widhalm et al., Reference Widhalm, Schönegger, Huemer and Auterith2001), especially when it is used longitudinally to understand composite measures of body size changes over time (Nuttall, Reference Nuttall2015). However, associations between exact body composition (i.e. fat mass) and BMI are weak and unreliable at the tails of the BMI distribution or outside of the ‘normal’ BMI range when using it as a categorical variable (Goulding et al., Reference Goulding, Gold, Cannan, Taylor, Williams and Lewis-Barned1996; Woo, Reference Woo2009). With this in mind, it is better in the context of pubertal timing and energetic stores to use BMI as a continuous variable (raw units), not binned into categories (i.e. ‘normal’ or ‘underweight’). In doing so, relative developmental pace and prior conditions can be tracked, whereas a BMI Z-score for age may be a more suitable measure in contexts where repeated measures are not attainable (Cole et al., Reference Cole, Faith, Pietrobelli and Heo2005). Researchers are often constrained to limited sampling frequency and therefore may rely on comparing categorical BMI or height/weight for age Z-scores. While these may be appropriate for capturing current energetic conditions, longitudinal sampling necessarily allows observation of energetic status fluctuations, proxied by childhood growth.
Epidemiological and historical evidence further suggest that rapid childhood growth as well as adequate or over-nutrition are associated with earlier pubertal timing (Ahmed et al., Reference Ahmed, Ong and Dunger2009; Biro et al., Reference Biro, Khoury and Morrison2006; Cho et al., Reference Cho, Park, Shin, Hur, Kim, Kim and Kim2010; Durda-Masny et al., Reference Durda-Masny, Hanć, Czapla and Szwed2019; Hossain et al., Reference Hossain, Islam, Aik, Zaman and Lestrel2010; Ossa et al., Reference Ossa, Munoz, Amigo and Bangdiwala2010; Parent et al., Reference Parent, Teilmann, Juul, Skakkebaek, Toppari and Bourguignon2003; Pasquet et al., Reference Pasquet, Biyong, Rikong-Adie, Befidi-Mengue, Garba and Froment1999). For example, in a large Swedish population-based study, higher BMI (used as a proxy for nutritional status) measured at ages 2–8 was associated with greater increases in height attainment in the same period and earlier pubertal timing, but it was not associated with final height (He & Karlberg, Reference He and Karlberg2001). A recent analysis of cohort trends from 27 low- and middle-income countries demonstrated that age at menarche has broadly declined, probably resulting from a combination of changes in nutrition, reductions in physical activity and changes in wealth distributions (Leone & Brown, Reference Leone and Brown2020). However, the rate of decline varied by location; for example, in Egypt and Tunisia, menarcheal trends were flatter (e.g. stalling), whereas in the Philippines and Colombia rapid declines were present. Leone and Brown (Reference Leone and Brown2020) similarly found that poorer individuals had earlier menarche in earlier surveys, whereas in later surveys richer individuals had earlier menarche in Yemen and the Philippines, but a reverse trend was found in Egypt. Furthermore, evidence from Brazil, India and Pakistan also suggests that undernutrition or malnourishment (when in comparison with adequately nourished individuals) may delay pubertal timing (Barros et al., Reference Barros, Kuschnir, Bloch, Silva, Barros, Kuschnir and Silva2019; Campisi et al., Reference Campisi, Humayun, Wasan, Soofi, Islam, Hussain and Bhutta2021; Qamra et al., Reference Qamra, Mehta and Deodhar1990; Satyanarayana & Naidu, Reference Satyanarayana and Naidu1979).
Similar associations have been detected longitudinally between food insecurity and later ages at menarche in Colombia with over 15,000 participants (Jansen et al., Reference Jansen, Herrán and Villamor2015a) and in Ethiopia (Belachew et al., Reference Belachew, Hadley, Lindstrom, Getachew, Duchateau and Kolsteren2011) with 900 participants. However, in the USA, food insecurity or inadequate nutrition has been associated with earlier puberty (M. Burris et al., Reference Burris, Miller, Romero-Daza and Himmelgreen2020; M. E. Burris & Wiley, Reference Burris and Wiley2021). This counterintuitive observation may suggest that pubertal timing is delayed by more extreme or sustained nutritional deprivation than that experienced by marginalized US children who may face nutritional insecurities of excess processed foods (Ellis, Reference Ellis2004; Kirkwood et al., Reference Kirkwood, Cumming and Aherne1987). While there are certainly heterogenous populations in the USA and other Western countries, very little research (to our knowledge) has been conducted on pubertal development in subpopulations within these contexts where more sustained nutritional and psychosocial deprivation may be occurring, for example among unsheltered teens or children in immigration or punitive detention centres.
Psychosocial stress hypotheses
Energetics Theory was extended to include psychosocial stressors in Stress Suppression Theory (henceforth Suppression Theory; Ellis, Reference Ellis2004), i.e. chronically low energetic availability or psychosocial stress insults would act as a cue to delay pubertal timing until conditions improved (Cameron, Reference Cameron1997; MacDonald, Reference MacDonald1999; Miller, Reference Miller1994; see Figure 2). Mechanistically it was proposed the HPG is suppressed via co-activation of corticotropin-releasing hormone and locus coeruleus–norepinephrine, the HPA, and the autonomic nervous system until there are improvements from psychosocial stress insults or greater energetic availability (Ellis, Reference Ellis2004; McEwen & Seeman, Reference McEwen and Seeman1999; Rivier et al., Reference Rivier, Rivier and Vale1986). Evidence for the HPG being suppressed in response to stress has come from observations of HPG suppression in chronically stressed adult females (Ellison, Reference Ellison2001; Iwasa et al., Reference Iwasa, Matsuzaki, Yano, Mayila and Irahara2018; Nappi & Facchinetti, Reference Nappi and Facchinetti2003), although it is less clear if HPG suppression occurs in adolescence in response to diverse forms of psychosocial stress (Shirtcliff et al., Reference Shirtcliff, Dismukes, Marceau, Ruttle, Simmons and Han2015).

Figure 2. Psychosocial model of pubertal timing.
Many studies testing Suppression Theory in non-human animals have examined correlations between stressful situations such as antagonistic encounters and HPA activation and HPG suppression (Ellis, Reference Ellis2004; Rivier et al., Reference Rivier, Rivier and Vale1986; Viau & Sawchenko, Reference Viau and Sawchenko2002). In primates living in dominance hierarchies, it was proposed that being lower ranked would be stressful and result in higher circulating glucocorticoids as well as suppression of pubertal development (Blanchard et al., Reference Blanchard, McKittrick and Blanchard2001; Cameron, Reference Cameron1997). However, Creel (Reference Creel2001) found that basal and circulating glucocorticoids were too variable across species and across differently ranked individuals to warrant evidence for this hypothesis. Importantly, Creel (Reference Creel2001) explained that social dominance hierarchies evolved to mitigate highly costly conflicts and intergroup escalations, and there would be no expectation of higher glucocorticoid levels in less dominant primates (Creel, Reference Creel2001). There is currently no consensus about whether being lower or higher ranked in social hierarchies is inherently more stressful (and reflected in glucocorticoid variability) across species (Beehner & Bergman, Reference Beehner and Bergman2017; R. M. Sapolsky, Reference Sapolsky2005).
However among humans who do not exist in strict dominance hierarchies but rather in a web of hierarchies, SES has been robustly associated with psychosocial stress profiles and disease outcomes (R. Sapolsky, Reference Sapolsky2005; R. M. Sapolsky, Reference Sapolsky2005). For example, in a systematic review of 36 studies, Niere et al. (Reference Niere, Spannemann, Stenzel, Bogin, Hermanussen and Scheffler2020) suggests that psychosocial factors (emotional and social) may influence linear growth in childhood depending on the environment. Among the studies they reviewed, they report that advantaged socioeconomic status, social positioning, and parental education are associated with more rapid growth tempo whereas disadvantaged SES, parental education and social mobility were associated with slower growth (Niere et al., Reference Niere, Spannemann, Stenzel, Bogin, Hermanussen and Scheffler2020). Citing evidence from studies of emotional deprivation (Spencer, Reference Spencer2017), social isolation of migrant children (Özer & Scheffler, Reference Özer and Scheffler2018) or childhood institutionalization (Kroupina et al., Reference Kroupina, Toemen, Aidjanov, Georgieff, Hearst, Himes and Sharmanov2015), Niere et al. (Reference Niere, Spannemann, Stenzel, Bogin, Hermanussen and Scheffler2020) and B. Bogin (Reference Bogin2021b) suggest these types of psychosocial stressors may also suppress growth hormone (via a biological stress response) resulting in poorer growth outcomes (B. Bogin, Reference Bogin2021b; see Figure 3). However, as they note, stress responses are modulated by stability of the environment, which is notoriously difficult to capture in humans (Creel et al., Reference Creel, Dantzer, Goymann and Rubenstein2013; Knight & Mehta, Reference Knight and Mehta2017; Niere et al., Reference Niere, Spannemann, Stenzel, Bogin, Hermanussen and Scheffler2020; R. M. Sapolsky, Reference Sapolsky1992).
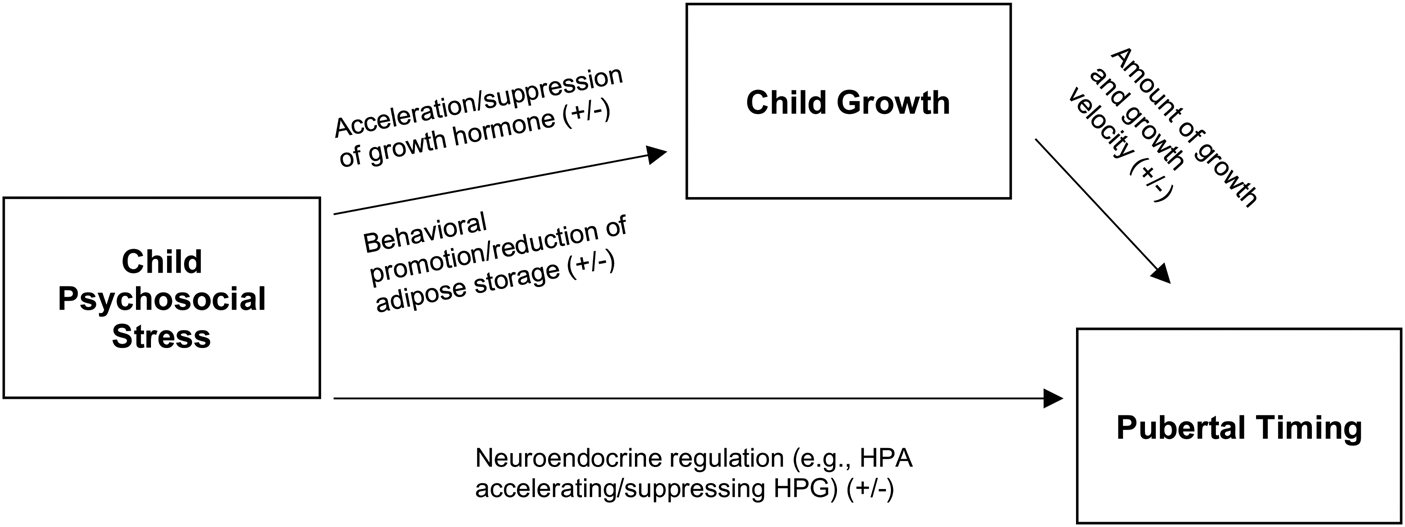
Figure 3. Proposed biological mechanisms between psychosocial stress, growth and pubertal timing.
One striking context where Suppression Theory may be invoked to explain variable pubertal timing is among individuals experiencing eating disorders such as anorexia nervosa or avoidant/restrictive intake disorder. These are characterized by very low or infrequent energy intake or caloric restriction, weight suppression (Lowe et al., Reference Lowe, Piers and Benson2018) and the co-occurrence of high psychosocial stress or psychological comorbidities like depression and anxiety (Becker et al., Reference Becker, Keshishian, Liebman, Coniglio, Wang, Franko and Thomas2019). In this situation, chronically low energy intake paired with high psychosocial stress has been shown to delay puberty, with the caveat that catch-up growth may occur with potentially negligible impacts on final adult height (Neale et al., Reference Neale, Pais, Nicholls, Chapman and Hudson2020).
Overall, however, it appears that psychosocial stress has rarely been shown to suppress pubertal development in human studies, despite observations of suppression in adulthood (Shirtcliff et al., Reference Shirtcliff, Dismukes, Marceau, Ruttle, Simmons and Han2015). The extent to which psychosocial stress impacts pubertal timing may also depend on the timing, duration and biobehavioural needs of the individual (Shirtcliff et al., Reference Shirtcliff, Dismukes, Marceau, Ruttle, Simmons and Han2015). For example, it has been proposed that psychosocial stressors may exert smaller effects later in puberty compared with pre-puberty, because (a) stress exposures pre-puberty may matter more for developmental plasticity of puberty and (b) by the time puberty is well under way, energetic and neuroendocrine reorganization (e.g. increases in gonadal and steroid hormones, physiological changes) may limit the effects of stressors (Phan et al., Reference Phan, Hulle, Shirtcliff, Schmidt and Goldsmith2021; Shirtcliff et al., Reference Shirtcliff, Dismukes, Marceau, Ruttle, Simmons and Han2015). Thus, puberty may be a particularly unique period wherein stress co-occurs with, rather than inhibits, gonadal hormone production, via synthesis of the HPG–HPA axes; this ‘hormonal coupling’ can be tested by tracking changes in the trajectories gonadal and steroid hormones across puberty (Marceau et al., Reference Marceau, Shirtcliff, Hastings, Klimes-Dougan, Zahn-Waxler, Dorn and Susman2014, Reference Marceau, Ruttle, Shirtcliff, Essex and Susman2015; Shirtcliff et al., Reference Shirtcliff, Dismukes, Marceau, Ruttle, Simmons and Han2015).
In contrast to Suppression Theory, Psychosocial Acceleration Theory (Acceleration Theory furthermore) posits that stressful early life ecological and familial conditions favour accelerated pubertal development (Draper & Harpending, Reference Draper and Harpending1982). We highlight in Figure 2 that risk or uncertainty in the environment is the latent variable driving Psychosocial Acceleration Theory and Stress Suppression Theory. Examples of observed psychosocial stressors indicative of risk or uncertainty may include father absence, disadvantaged SES, and exposures to violence. How observed psychosocial stress impacts pubertal timing, either earlier (−) or later (+) depends on the socioecological context (see Figure 2). Belsky (Reference Belsky1991) originally hypothesized that internalizing behaviour in response to perceived stress (e.g. social withdrawal, nervousness, irritability, eating more/less) in females promoted fat storage and lowered metabolism, resulting in more rapid growth, but did not elucidate mechanisms for this (Belsky, Reference Belsky1991). It is, however, plausible that heightened psychosocial stress may result in disordered eating patterns, reduce physical activity and result in more rapid weight gain and growth. Biobehavioural factors such as appetite (operating through ghrelin or leptin) and sociocultural diet expectations (Mousa et al., Reference Mousa, Al-Domi, Mashal and Jibril2010) may also have an impact on energy intake or adiposity, with downstream consequences for pubertal timing (Michels, Reference Michels2019). Chisholm, Burbank et al. (Reference Chisholm, Quinlivan, Petersen and Coall2005), Chisholm, Quinlivan, et al. (Reference Chisholm, Quinlivan, Petersen and Coall2005) and Belsky et al. (Reference Belsky, Ruttle, Boyce, Armstrong and Essex2015) later suggested that chronic activation or greater elevation of basal cortisol levels may be one mechanism through which stress accelerates sexual development, invoking the Adaptive Calibration Model (Belsky et al., Reference Belsky, Ruttle, Boyce, Armstrong and Essex2015; Chisholm, Burbank, et al., Reference Chisholm, Quinlivan, Petersen and Coall2005; Chisholm, Quinlivan, et al., Reference Chisholm, Quinlivan, Petersen and Coall2005; Del Giudice et al., Reference Del Giudice, Ellis and Shirtcliff2011; Doom & Gunnar, Reference Doom and Gunnar2013). Chisholm, Burbank et al. (Reference Chisholm, Quinlivan, Petersen and Coall2005) and Chisholm, Quinlivan, et al. (Reference Chisholm, Quinlivan, Petersen and Coall2005) further emphasized that because individuals are acclimated to different sociological environments, pubertal timing will exhibit intra- and inter-individual variation in relation to stress reactivity.
Acceleration Theory has frequently examined parental investment as a primary cue of socioecological conditions, proposing that low parental investment during childhood is perceived as a reliable cue of low future investment (Belsky et al., Reference Belsky, Steinberg and Draper1991). In such circumstances, earlier reproduction is favoured as delayed reproduction would not benefit from future accrued parental resources (Belsky, Reference Belsky1991; Ellis, Reference Ellis2004). Many studies testing Acceleration Theory have detected associations between early life adversity (e.g. family conflict, father absence, parental attributes) and earlier pubertal timing (Belsky et al., Reference Belsky, Steinberg, Houts, Friedman, DeHart, Cauffman and Susman2007, 2015; Ellis et al., Reference Ellis, Shirtcliff, Boyce, Deardorff and Essex2011; Moffitt et al., Reference Moffitt, Caspi, Belsky and Silva1992; Tremblay & Frigon, Reference Tremblay and Frigon2005). Associations between father absence and earlier pubertal timing have been robust, and it has been suggested that this may result from a higher likelihood of unstable relationships and parenting behaviour in adulthood (Belsky, Reference Belsky1991; Draper & Harpending, Reference Draper and Harpending1982). However, Sear et al. (Reference Sear, Sheppard and Coall2019) cautions that the duration of father absence, the reason for his absence (e.g. death, divorce, never having been present) and the absence of other family members may generate varied effects on pubertal timing. Furthermore, in environments characterized by low mortality, high energetic availability, normative conventions of a nuclear family and established secular trends of earlier development, associations between father absence and earlier puberty are robust (Sear et al., Reference Sear, Sheppard and Coall2019). In all other contexts where there is not a normative nuclear family structure, higher mortality and lower energetic availability, father absence has been an inconsistent predictor of pubertal timing if at all (Sear et al., Reference Sear, Sheppard and Coall2019).
Mixed cross-cultural evidence for a relationship between father absence and earlier pubertal timing may suggest that psychosocial stress distinctly correlates with resource availability across varied socioecological contexts. For example, father absence in contexts of normative nuclear family structures may threaten resource availability or social network connections, leading to psychosocial stress, whereas in other contexts, the father's impact on child wellbeing may be minimal or easily substituted (Sear et al., Reference Sear, Sheppard and Coall2019). It is also possible that across contexts, psychosocial and resource environment factors interact in complex ways. For example, Hulanicka (Reference Hulanicka1999; Hulanicka et al., Reference Hulanicka, Gronkiewicz and Koniarek2001) found that among disadvantaged SES Polish female adolescents, poverty (resource scarcity) delayed menarcheal timing overall. However among disadvantaged SES adolescents, greater chronic psychosocial stressors (parental death and divorce, familial deviance like alcoholism, prolonged illness of family member) were associated with earlier menarche (Hulanicka, Reference Hulanicka1999; Hulanicka et al., Reference Hulanicka, Gronkiewicz and Koniarek2001). The latter implies that the effect of psychosocial stress may outweigh the effect of resource scarcity in this context, which would otherwise delay menarcheal timing.
With Energetics Theory, Suppression Theory and Acceleration Theory in mind, there are conceptual, theoretical and methodological critiques that have emerged in recent years that warrant further discussion. First, one of the tenets underpinning Acceleration Theory is the idea that a fast–slow life history continuum can be applied to individuals. However, statistical associations between measures of greater psychosocial stress and earlier pubertal timing are not necessarily evidence of an evolutionary strategy favouring a fast life history (Sear, Reference Sear2020; Stearns & Rodrigues, Reference Stearns and Rodrigues2020). Variable reproductive strategies may simply represent normal variation within a reaction norm (Stearns & Rodrigues, Reference Stearns and Rodrigues2020). Moreover, Stearns and Rodrigues (Reference Stearns and Rodrigues2020) argue that the fast–slow continuum applies when comparing demographic life history patterns across species, but not among individuals. In experiments citing evidence of selection for faster reproduction within a population, adult mortality rates, not juvenile cues of a risky environment, were key selective factors (Stearns & Rodrigues, Reference Stearns and Rodrigues2020).
Furthermore, pubertal timing is assumed to be associated with timing of other reproductive outcomes such as first intercourse and pregnancy that may influence lifetime reproductive success, yet few studies have actually tested this assumption with sufficient biodemographic data, let alone in relation to psychosocial stressors (Sear, Reference Sear2020). Leveraging over 48 years of continuous longitudinal data among wild Amboseli baboons in Kenya, Weibel et al. (Reference Weibel, Tung, Alberts and Archie2020) tested Nettle and Bateson's Internal Predictive Adaptative Response model (Nettle & Bateson, Reference Nettle and Bateson2015) to probe the adaptive benefits of accelerated reproduction in light of experiencing early life adversity (Weibel et al., Reference Weibel, Tung, Alberts and Archie2020). They found that early life adversity predicted shorter lifespans, but it did not accelerate reproduction. Furthermore, accelerated reproduction was positively associated with greater lifetime reproductive success only in baboons that lived longer lives (Weibel et al., Reference Weibel, Tung, Alberts and Archie2020). In humans, it is still unclear whether earlier pubertal timing indeed reflects an adaptive strategy, and whether such an evolutionary strategy can be witnessed on an individual scale and how cues from the environment are meaningfully adopted to adjust bodily strategies. Amir et al. (Reference Amir, Jordan and Bribiescas2016) found that high subjective environmental risk and low access to economic resources predicted earlier menarche in US females similar to prior work (Šaffa et al., Reference Šaffa, Kubicka, Hromada and Kramer2019). Their findings suggest that perception of local environmental risk or local life expectancies may provide further evidence of an individual strategy, however, to date the mechanisms are unclear.
Furthermore, psychosocial ‘stress’ is often broadly and differently defined across studies (Shirtcliff et al., Reference Shirtcliff, Dismukes, Marceau, Ruttle, Simmons and Han2015). Environmental cues that can be signalled via psychosocial stress and that are proposed to influence life history strategies include adult extrinsic mortality, resource availability, and predictability, which may be at odds with the vast types and definitions of psychosocial stress utilized in studies (Shirtcliff et al., Reference Shirtcliff, Dismukes, Marceau, Ruttle, Simmons and Han2015). This is compounded by the fact that few studies have tested Acceleration Theory or Suppression Theory while also accounting for biomarkers of stress or HPA activation/suppression (Negriff et al., Reference Negriff, Saxbe and Trickett2015; Peckins et al., Reference Peckins, Susman, Negriff, Noll and Trickett2015; Saxbe et al., Reference Saxbe, Negriff, Susman and Trickett2015). Some of these semantic, methodological tensions are also evident when using SES, which has been used as both a proxy for resource availability (specifically risk of food insecurity) and psychosocial stress. While we do not argue that SES is definitively a psychosocial stress variable, we believe that it may represent both the psychosocial and energetic conditions that humans are embedded in. Psychosocial stress developing from exposure to poverty or disadvantaged social status may not have a clear-cut suppressive effect on pubertal timing. Socioeconomic status may be confounded in environments where poverty is associated with both greater psychosocial stress and food insecurity, which may promote obesity (M. Burris et al., Reference Burris, Miller, Romero-Daza and Himmelgreen2020; M. E. Burris & Wiley, Reference Burris and Wiley2021; Gundersen et al., Reference Gundersen, Mahatmya, Garasky and Lohman2011; R. Sapolsky, Reference Sapolsky2005; R. M. Sapolsky, Reference Sapolsky2005).
In other contexts, poverty may not be associated with obesity but there may be substantial differences in health and nutritional status by wealth or class such that individuals who belong to advantaged class groups or have greater wealth tend to mature earlier than individuals belonging to disadvantaged class groups (Jansen et al., Reference Jansen, Herrán and Villamor2015b; Karim et al., Reference Karim, Qaisar and Hussain2021; Montero et al., Reference Montero, Bernis, Loukid, Hilali and Baali1999; Padez, Reference Padez2003; Rimpelä & Rimpelä, Reference Rimpelä and Rimpelä1993; Shaik et al., Reference Shaik, Hashim, Alsukait, Abdulkader, AlSudairy, AlShaman and Neel2016). A meta-analysis by Zhang et al. (Reference Zhang, Zhang and Sun2019) sought to quantify the effect of early life adversity (measured via total adverse childhood events) on pubertal timing. While total adverse childhood events were not associated with earlier pubertal timing across the 43 studies they reviewed, individual events such as sexual abuse, father absence and family dysfunction were associated with earlier pubertal timing and exhibited high heterogeneity. Thus, owing to heterogeneity among existing evidence along with the rationale discussed above, there are multiple potent reasons why energetic status should be accounted for in studies of psychosocial stress and pubertal timing (Zhang et al., Reference Zhang, Zhang and Sun2019).
Methods
Systematized review aims and protocol
The primary aim of this review was to identify studies that simultaneously assessed the effect of psychosocial stress and childhood energetic status on pubertal timing, and critically evaluate the relative strength of the evidence for these respective factors across selected studies. We designed an unregistered systematic review protocol based on select PRISMA guidelines (what we term a systematized review). Following this protocol, we: (a) specified aims for the review; (b) identified data sources; (c) determined exclusion and inclusion criteria; and (d) validated concept maps and search strings before conducting the search. We did not attempt to discern risk of bias – typically operationalized as a qualitative or quantitative assessment of study design quality (Moher et al., Reference Moher, Liberati, Tetzlaff, Altman, Altman, Antes and Tugwell2009) – due to the broad inclusion criteria we defined. A metanalysis was not possible owing to methodological differences across studies, including how factors were operationalized, analysed and reported. The search was conducted in April 2019 across PubMed, Web of Science, JSTOR, ProQuest and APA PsycInfo databases.
Search strategy
We created concept maps to produce a list of search strings for each individual database. These were tailored according to the specific database search style. We identified Ellis and Essex (Reference Ellis and Essex2007) as a ‘key’ study to validate our search strings for returning relevant studies. We also used Jean et al. (Reference Jean, Wilkinson, Spitz, Prokhorov, Bondy and Forman2011) and Amir et al. (Reference Amir, Jordan and Bribiescas2016) to ensure that our search strings were returning relevant results in theme or scope, given that these were known studies on pubertal timing (Amir et al., Reference Amir, Jordan and Bribiescas2016; Jean et al., Reference Jean, Wilkinson, Spitz, Prokhorov, Bondy and Forman2011). A complete list of search strings for each database and the full protocol for this systematized review are available on Open Science Framework (https://osf.io/nckrh/). For every search string developed, we used search terms or check boxes to specify prospective or retrospective pubertal staging and psychosocial stress measures but only prospective childhood energetic measures. For example, the search string used for PubMed was (‘biosocial’ OR ‘Socioeconomic Factors’[MeSH Terms] OR ‘psychosocial’ OR ‘early life adversity’ OR ‘energetic stress’ OR ‘father absence’ OR ‘sexual abuse’) AND (‘Body Composition’[MAJR] OR ‘growth’ OR ‘height’) AND (‘Menarche’[MAJR] OR ‘Menarche/physiology’[MAJR] OR ‘pubertal timing’ OR ‘reproductive timing’) AND (longitud* OR prospective OR cohort OR ‘Multivariate Analysis’[MeSH Terms]) AND (‘Adolescent’[MeSH Terms]).
JG conducted the initial title search using final search strings across five databases. A total of 1868 titles were returned, with 1069 remaining after removing duplicates: PubMed, n = 236; Web of Science, n = 85; JSTOR, n = 392; ProQuest, n = 310; APA PsycInfo, n = 46.
Screening
From the 1069 non-duplicate titles returned, JG selected 400 titles for further review based on qualitative assessment of title relevance. If titles were vague, she quickly reviewed abstracts for more information. DG and JG independently reviewed abstracts and methods from the title selection. DG and JG selected 36 abstracts from the 400 selected titles based on inclusion and exclusion criteria (Table 1). If it was unclear whether the abstract met our criteria, the article was retained until further screening. After the abstract selection, JG and DG performed a methodology screening and selected 13 papers for review. Conflicts in study selection at all stages were resolved by MM. DG, JG, and MM then independently screened references of selected studies (n = 601), selecting seven additional papers for critical review after consensus discussion. In total we selected 20 papers for critical review (Figure 4), representing 1.25% of non-duplicate titles returned from the initial search.
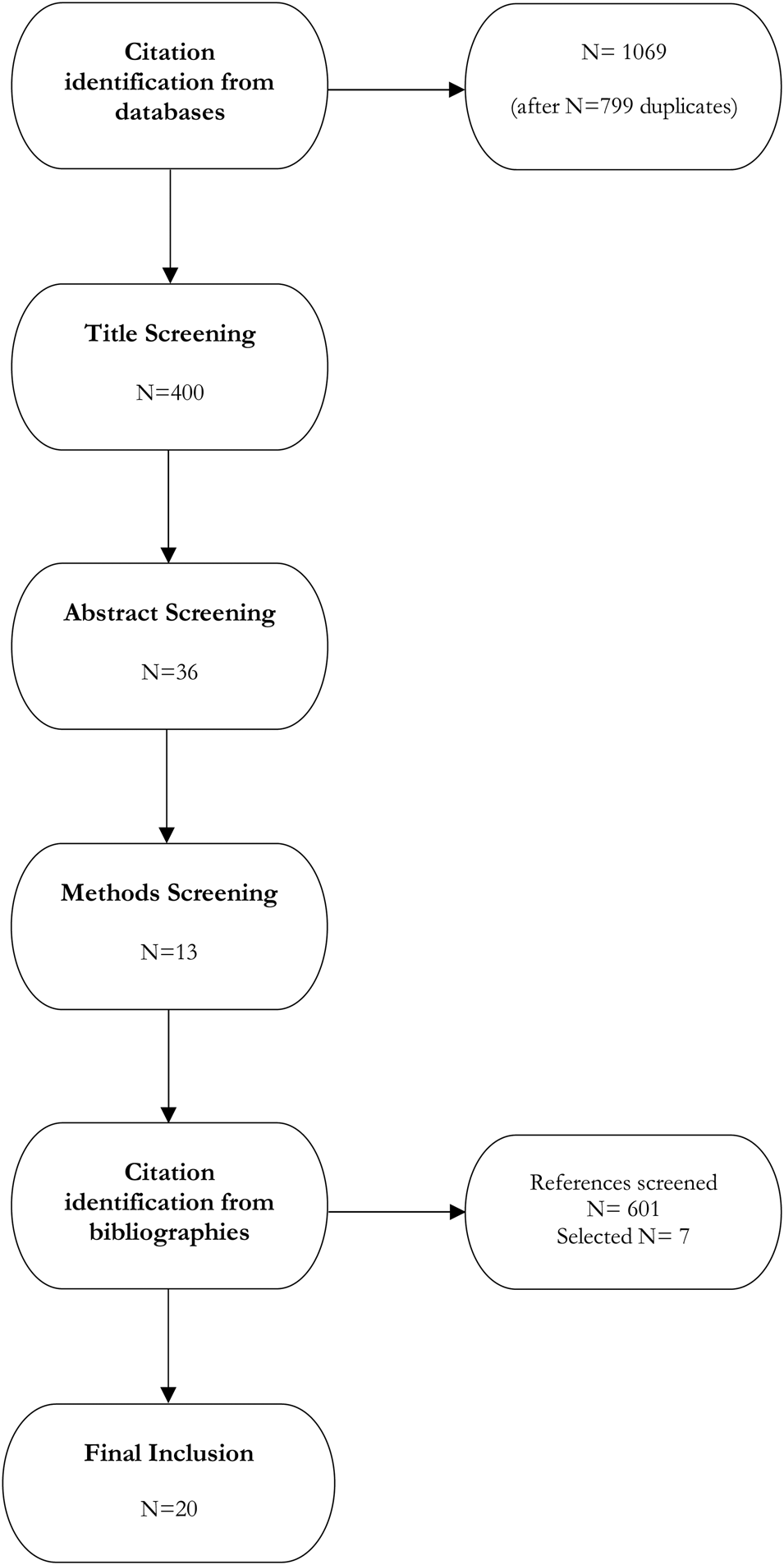
Figure 4. Flow chart of systematized review process.
Table 1. Inclusion and exclusion criteria

The primary inclusion criteria for selected studies at all stages (Table 2) were assessment of both energetic and psychosocial factors in relation to pubertal outcomes. To avoid confounding owing to weight and adipose tissue gain after mid-puberty in females, we included only studies that measured energetic factors prior to the specified pubertal outcome, or that used the status quo method with relatively sufficient numbers of individuals falling into pre- and post-outcome status categories. Acceptable energetic measures included anthropometrics such as body fat percentage, height, weight, height or weight increase, height or weight velocity, skinfold measurements or body mass index. We accepted pubertal outcomes observed prospectively, by status quo methods, or retrospectively if energetic factors had been measured prior to outcomes. Acceptable pubertal measures included age at menarche, menarcheal status, Tanner Stages, height velocity or other parameters of pubertal timing or relative pace of development. We accepted psychosocial factors measured prospectively, by status quo or retrospectively (e.g. father absence at age 5, reported at time of pubertal outcomes). Psychosocial criteria were intentionally kept broad but specific measures such as early life adversity, socioeconomic status, psychosocial (broad MESH term), father absence and sexual abuse were included in search strings. In general, any studies with factors conceived of as psychosocial variables according to the authors were acceptable for inclusion.
Table 2. Summary of author aims, location, study size, design and measurement of independent and dependent variables among the 20 selected studies

Data synthesis
The following information was extracted from selected studies: author names, study date, study location, study design type, author aims and operationalized measures for pubertal outcomes, energetic, and psychosocial factors (Table 2). We then summarized associations between psychosocial and energetic variables and pubertal outcomes observed across studies (Table 3). We separately evaluated studies in which energetic and psychosocial effects on pubertal outcomes were evaluated in the same statistical models (n = 11). For these studies, we report statistically significant and non-statistically significant parameter estimates and the type of statistical analysis used (Table 4). Search and inclusion criteria were intentionally broad, which maximized the range of studies included, but limited our ability to directly compare and synthesize findings across studies. We included them in Table 4 if a large proportion of participants were still premenarcheal or sampled at age ranges before the mean or median age of menarche reported for the sample population.
Table 3. Factors measured and their association with pubertal timing
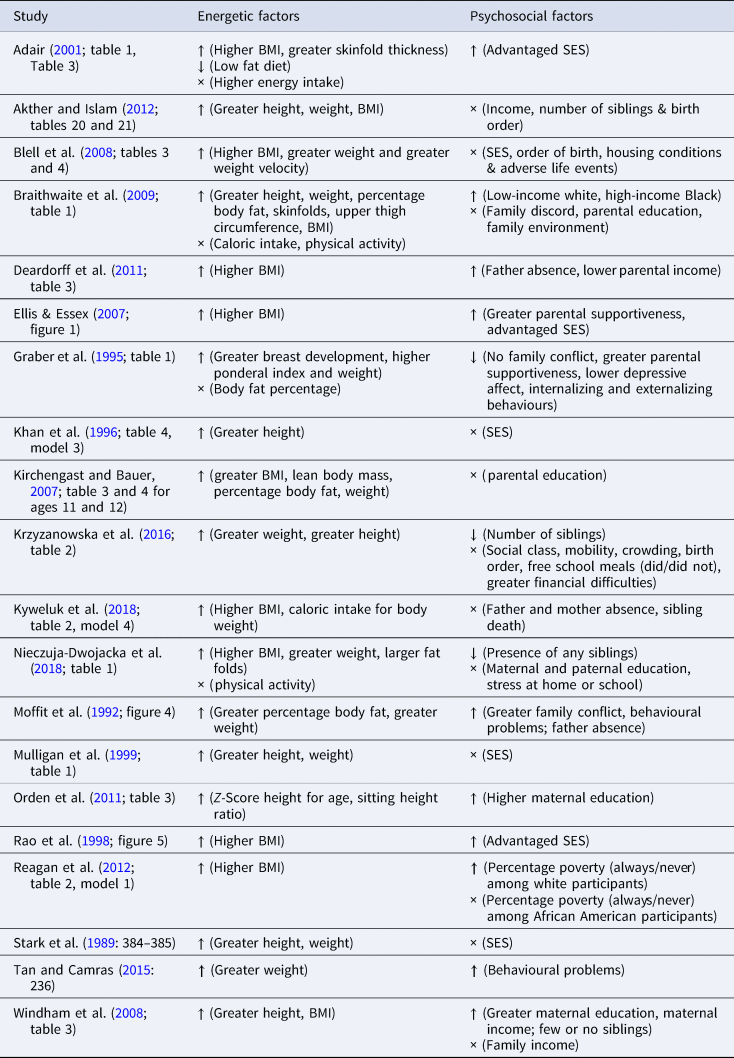
Key: ↑ = earlier development, ↓ = later development, × = no statistically significant relationship.
Table 4. Multivariate results

Symbols: (t)= trend (p < 0.10); *p < 0.05; **p < 0.01; ***p < 0.001. Coef or b, linear regression; OR, odds ratio; OLS, ordinary least squares regression; exp(b), exponentiated β.
Format: Variable – parameter estimate [confidence interval if available] or parameter estimate (standard error estimate).
Abbreviations: HAZ, height-for-age Z-score; BMI, body mass index; SES, socioeconomic status.
Results
Search results and study characteristics
We identified 20 studies (<2% of non-duplicate search results returned) that simultaneously assessed the effects of psychosocial and childhood energetic status on pubertal timing (Table 2). Seven of these studies were designed primarily to investigate energetic effects on pubertal timing (Adair, Reference Adair2001; Khan et al., Reference Khan, Schroeder, Martorell, Haas and Rivera1996; Krzyzanowska et al., Reference Krzyzanowska, Mascie-Taylor and Thalabard2016; Mulligan et al., Reference Mulligan, Bailey, Voss and Betts1999; Nieczuja-Dwojacka et al., Reference Nieczuja-Dwojacka, Siniarska, Koziel, Marchewka and Zablocka2018; Rao et al., Reference Rao, Joshi and Kanade1998; Stark et al., Reference Stark, Peckham and Moynihan1989), but analyses adjusted for or separately analysed psychosocial influences as well. Conversely, seven studies explicitly assessed psychosocial effects but adjusted for or separately analysed energetic factors (Braithwaite et al., Reference Braithwaite, Moore, Lustig, Epel, Ong, Rehkopf and Hiatt2009; Ellis & Essex, Reference Ellis and Essex2007; Graber et al., Reference Graber, Brooks-Gunn and Warren1995; Moffitt et al., Reference Moffitt, Caspi, Belsky and Silva1992; Reagan et al., Reference Reagan, Salsberry, Fang, Gardner and Pajer2012; Tan & Camras, Reference Tan and Camras2015; Windham et al., Reference Windham, Zhang, Longnecker and Klebanoff2008). Six of the reviewed studies addressed both sets of factors as primary study aims (Akther & Islam, Reference Akther and Islam2012; Blell et al., Reference Blell, Pollard and Pearce2008; Deardorff et al., Reference Deardorff, Ekwaru, Kushi, Ellis, Greenspan, Mirabedi and Hiatt2011; Kirchengast & Bauer, Reference Kirchengast and Bauer2007; Kyweluk et al., Reference Kyweluk, Georgiev, Borja, Gettler and Kuzawa2018; Orden et al., Reference Orden, Vericat and Apezteguía2011). Five of the selected studies were motivated by or explicitly tested hypotheses from a Life History perspective such as Acceleration Theory (Deardorff et al., Reference Deardorff, Ekwaru, Kushi, Ellis, Greenspan, Mirabedi and Hiatt2011; Ellis & Essex, Reference Ellis and Essex2007; Graber et al., Reference Graber, Brooks-Gunn and Warren1995; Kyweluk et al., Reference Kyweluk, Georgiev, Borja, Gettler and Kuzawa2018; Moffitt et al., Reference Moffitt, Caspi, Belsky and Silva1992). The other 15 papers utilized epidemiological approaches to understand relationships between childhood psychosocial experiences (e.g. SES or adverse life events) and pubertal timing. Specifically, many of the latter authors were interested in how biological and social factors might interface to produce variable pubertal outcomes or they were specifically interested in antecedents of earlier menarche.
Most studies (n = 18) used age at menarche as the primary indicator for pubertal timing. In addition to age at menarche, some researchers elected to measure breast Tanner Stage (Graber et al., Reference Graber, Brooks-Gunn and Warren1995), Peak Height Velocity (Mulligan et al., Reference Mulligan, Bailey, Voss and Betts1999) and a pre-pubertal development scale (Tan & Camras, Reference Tan and Camras2015). Two studies did not use age at menarche at all but instead used breast and pubic Tanner Stage (Deardorff et al., Reference Deardorff, Ekwaru, Kushi, Ellis, Greenspan, Mirabedi and Hiatt2011) or a combination of the Pubertal Development Scale, Tanner Stage and adrenal hormones at age 7 (Ellis & Essex, Reference Ellis and Essex2007). Energetic factors examined in relation to pubertal timing included height, weight, BMI, skinfold thickness, measures of body fat, thigh or arm circumference, breast development, stature, sitting height and subischial leg length (Table 2).
Psychosocial factors examined included measures of SES and environmental or familial stressors (e.g. adverse life events, familial conflict, parental stability, father absence, housing quality, sibling number and order, child behavioural problems and child psychological adjustment). At the very least, studies including SES typically measured it as a scale variable with education, income or both (Adair, Reference Adair2001; Akther & Islam, Reference Akther and Islam2012; Blell et al., Reference Blell, Pollard and Pearce2008; Deardorff et al., Reference Deardorff, Ekwaru, Kushi, Ellis, Greenspan, Mirabedi and Hiatt2011; Ellis & Essex, Reference Ellis and Essex2007; Kirchengast & Bauer, Reference Kirchengast and Bauer2007; Moffitt et al., Reference Moffitt, Caspi, Belsky and Silva1992; Mulligan et al., Reference Mulligan, Bailey, Voss and Betts1999; Nieczuja-Dwojacka et al., Reference Nieczuja-Dwojacka, Siniarska, Koziel, Marchewka and Zablocka2018; Orden et al., Reference Orden, Vericat and Apezteguía2011; Rao et al., Reference Rao, Joshi and Kanade1998; Tan & Camras, Reference Tan and Camras2015; Windham et al., Reference Windham, Zhang, Longnecker and Klebanoff2008). Other psychosocial factors used instead of or in addition to income–education SES markers included birth order (Akther & Islam, Reference Akther and Islam2012; Deardorff et al., Reference Deardorff, Ekwaru, Kushi, Ellis, Greenspan, Mirabedi and Hiatt2011; Krzyzanowska et al., Reference Krzyzanowska, Mascie-Taylor and Thalabard2016), recall of time lived in poverty (Reagan et al., Reference Reagan, Salsberry, Fang, Gardner and Pajer2012), family size or number of siblings (Akther & Islam, Reference Akther and Islam2012; Krzyzanowska et al., Reference Krzyzanowska, Mascie-Taylor and Thalabard2016; Windham et al., Reference Windham, Zhang, Longnecker and Klebanoff2008), housing conditions or crowding (Blell et al., Reference Blell, Pollard and Pearce2008; Deardorff et al., Reference Deardorff, Ekwaru, Kushi, Ellis, Greenspan, Mirabedi and Hiatt2011; Khan et al., Reference Khan, Schroeder, Martorell, Haas and Rivera1996; Krzyzanowska et al., Reference Krzyzanowska, Mascie-Taylor and Thalabard2016) and social mobility (Krzyzanowska et al., Reference Krzyzanowska, Mascie-Taylor and Thalabard2016).
Independent associations of childhood energetic factors and psychosocial stress with pubertal timing
In all studies critically reviewed, at least one measure indicative of greater pre-pubertal energetic status (e.g. larger relative body size) was significantly associated with accelerated pubertal timing. However, the specific energetic factors associated with pubertal timing varied across studies (Table 3). For example, Braithwaite et al. (Reference Braithwaite, Moore, Lustig, Epel, Ong, Rehkopf and Hiatt2009) found that earlier age at menarche was associated with greater height, weight, percentage body fat, BMI, skinfolds and upper thigh circumference (see Table 3), but not with higher caloric intake or greater physical activity (Braithwaite et al., Reference Braithwaite, Moore, Lustig, Epel, Ong, Rehkopf and Hiatt2009). Null associations between pubertal timing and higher energy diets or physical activity were also observed in Nieczuja-Dwojacka et al. (Reference Nieczuja-Dwojacka, Siniarska, Koziel, Marchewka and Zablocka2018) and Adair (Reference Adair2001).
Measures of psychosocial stress and their associations with pubertal timing were also varied. While some studies only included one measure such as SES, others included many measures (see Table 2 and 2). Eight studies (Adair, Reference Adair2001; Deardorff et al., Reference Deardorff, Ekwaru, Kushi, Ellis, Greenspan, Mirabedi and Hiatt2011; Ellis & Essex, Reference Ellis and Essex2007; Graber et al., Reference Graber, Brooks-Gunn and Warren1995; Moffitt et al., Reference Moffitt, Caspi, Belsky and Silva1992; Orden et al., Reference Orden, Vericat and Apezteguía2011; Rao et al., Reference Rao, Joshi and Kanade1998; Tan & Camras, Reference Tan and Camras2015) found associations between earlier pubertal timing and all of their measured psychosocial variables, including advantaged SES, parental education, father absence, greater family conflict and low parental income. Graber et al. (Reference Graber, Brooks-Gunn and Warren1995) similarly found that all psychosocial variables (family conflict, less parental supportiveness, more depressive affect, and more internalizing and externalizing behaviours) were associated with earlier pubertal timing (Table 3).
Five studies (Braithwaite et al., Reference Braithwaite, Moore, Lustig, Epel, Ong, Rehkopf and Hiatt2009; Krzyzanowska et al., Reference Krzyzanowska, Mascie-Taylor and Thalabard2016; Nieczuja-Dwojacka et al., Reference Nieczuja-Dwojacka, Siniarska, Koziel, Marchewka and Zablocka2018; Reagan et al., Reference Reagan, Salsberry, Fang, Gardner and Pajer2012; Windham et al., Reference Windham, Zhang, Longnecker and Klebanoff2008) found mixed associations between psychosocial exposures and pubertal timing. For example, in Windham et al. (Reference Windham, Zhang, Longnecker and Klebanoff2008), earlier age at menarche was associated with greater maternal education and income and having few or no siblings but not with family income at age 7 (Table 3). In contrast, in Nieczuja-Dwojacka et al. (Reference Nieczuja-Dwojacka, Siniarska, Koziel, Marchewka and Zablocka2018) menarche was delayed with the presence of any siblings and there was no association between pubertal timing and maternal and paternal education or stress at home or school.
Reagan et al. (Reference Reagan, Salsberry, Fang, Gardner and Pajer2012) found that white participants who had always lived in poverty experienced earlier menarche as compared with those who had never lived in poverty, but there was no association between poverty and menarche among Black participants. Conversely, Braithwaite et al. (Reference Braithwaite, Moore, Lustig, Epel, Ong, Rehkopf and Hiatt2009) observed earlier age at menarche among lower income white and higher income Black participants (compared with higher income white and lower income Black participants), while age at menarche was not associated with family discord/environment or parental education across all participants. Kryzanowska et al. (Reference Krzyzanowska, Mascie-Taylor and Thalabard2016) found that qualifying for free school meals and greater financial difficulties were associated with slower pubertal timing but found no association between pubertal timing and social mobility, social class or birth order. In both Reagan et al. (Reference Reagan, Salsberry, Fang, Gardner and Pajer2012) and Kryzanowska et al. (Reference Krzyzanowska, Mascie-Taylor and Thalabard2016) slower pubertal timing was associated with poverty, financial difficulties and qualifying for free school lunches. In seven studies (Akther & Islam, Reference Akther and Islam2012; Blell et al., Reference Blell, Pollard and Pearce2008; Khan et al., Reference Khan, Schroeder, Martorell, Haas and Rivera1996; Kirchengast & Bauer, Reference Kirchengast and Bauer2007; Kyweluk et al., Reference Kyweluk, Georgiev, Borja, Gettler and Kuzawa2018; Mulligan et al., Reference Mulligan, Bailey, Voss and Betts1999; Stark et al., Reference Stark, Peckham and Moynihan1989) none of the measured psychosocial variables (SES, birth order, adverse life events, parental income, number of siblings, sibling death and housing conditions) were associated with pubertal timing.
What are the independent effects of psychosocial stress on pubertal timing when adjusting for energetic status?
All studies minimally included univariate tests or other independent assessments of both energetic and psychosocial factors on pubertal timing. However, only 11/20 studies evaluated energetic and psychosocial factors together in multivariate models. We summarized general findings across these studies, as methods were still too varied to allow for meta-analysis. We address only univariate vs. multivariate effects as a minimal essential step to consider confounding. Moreover, univariate and multivariate models have distinct explanatory power. Univariate models, when conducted as a first modelling step, help to identify dependent and independent relationships between psychosocial stressors and energetic factors on pubertal timing. However, one consequence of only presenting univariate results is heightened risk of unmeasured confounding, lack of interaction testing, and lack of precision. Thus, when psychosocial stress and energetic factors are measured together in a multivariate fashion, covariances can be decomposed more fully to unravel the biological meaning and plausibility of these relationships. Multivariate modelling conducted by authors is a first step towards necessary causal modelling, although causal inference models were rarely used in the studies reviewed.
In 9/11 multivariate models (Adair, Reference Adair2001; Akther & Islam, Reference Akther and Islam2012; Khan et al., Reference Khan, Schroeder, Martorell, Haas and Rivera1996; Krzyzanowska et al., Reference Krzyzanowska, Mascie-Taylor and Thalabard2016; Kyweluk et al., Reference Kyweluk, Georgiev, Borja, Gettler and Kuzawa2018; Orden et al., Reference Orden, Vericat and Apezteguía2011; Reagan et al., Reference Reagan, Salsberry, Fang, Gardner and Pajer2012; Windham et al., Reference Windham, Zhang, Longnecker and Klebanoff2008) energetic factors were significantly associated with pubertal timing when also controlling for psychosocial variables. Two studies used mediation models to test whether psychosocial stress was indirectly associated with pubertal timing via influence on childhood growth or body composition – reasoning, for example, that child behavioural problems are associated with negative psychological affect, which may promote increased body fat through altered eating, metabolism or physical activity (Ellis & Essex, Reference Ellis and Essex2007; Moffitt et al., Reference Moffitt, Caspi, Belsky and Silva1992). Moffit et al. (Reference Moffitt, Caspi, Belsky and Silva1992) first tested for main effects of father absence, child behavioural problems at age 7, family conflict and weight at age 9 and found that all but child behavioural problems were significantly associated with earlier age at menarche. In additive models, menarche was predicted to be earlier among individuals with two or three of these risk factors and much earlier in individuals with all four risk factors. However, father absence appeared to be the main driver of these associations across all cumulative risk combinations. In mediation models, behavioural problems and family stress (i.e. father absence/family conflict) were not indirectly associated with menarche via body weight at age 9. Ellis and Essex (Reference Ellis and Essex2007) similarly hypothesized that BMI may mediate the relationship between marital conflict/depression and pubertal development in the fifth grade, after finding that BMI, maternal age at menarche, maternal parental supportiveness and SES each independently associated with pubertal timing. They also did not find evidence of mediation by BMI. In contrast, they did find a large direct effect of BMI on pubertal development in the fifth grade and a slightly smaller indirect effect of marital conflict/depression on pubertal development (Table 4).
In 8/11 multivariate models (Adair, Reference Adair2001; Akther & Islam, Reference Akther and Islam2012; Ellis & Essex, Reference Ellis and Essex2007; Krzyzanowska et al., Reference Krzyzanowska, Mascie-Taylor and Thalabard2016; Moffitt et al., Reference Moffitt, Caspi, Belsky and Silva1992; Orden et al., Reference Orden, Vericat and Apezteguía2011; Tan & Camras, Reference Tan and Camras2015; Windham et al., Reference Windham, Zhang, Longnecker and Klebanoff2008), at least one psychosocial factor was significantly associated with pubertal timing when controlling for measured energetic factors. In the remaining three (Khan et al., Reference Khan, Schroeder, Martorell, Haas and Rivera1996; Kyweluk et al., Reference Kyweluk, Georgiev, Borja, Gettler and Kuzawa2018; Reagan et al., Reference Reagan, Salsberry, Fang, Gardner and Pajer2012), none of the psychosocial variables measured were significantly associated with pubertal timing when controlling for energetic factors. For example, in a multivariate model in Kyweluk et al. (Reference Kyweluk, Georgiev, Borja, Gettler and Kuzawa2018), age at menarche was inversely related to BMI and caloric intake for body weight but was not associated with sibling death or maternal or paternal absence.
Discussion
In our selection process, we identified only 20 studies that examined the influence of both childhood energetic factors and psychosocial stress on pubertal timing, and only 11 of these accounted for both in multivariate models to address potential confounding (Table 4). Across all selected studies, energetic factors were more consistently associated with pubertal timing than were psychosocial factors, with measures indicative of rapid childhood growth generally predicting earlier pubertal timing. Across the 20 studies, eight found associations between pubertal timing and all measured psychosocial variables, five found mixed results and seven found no associations. In multivariate models in which both energetic and psychosocial factors were significantly associated with pubertal timing, we observed no consistent patterns of stronger effects for energetic vs. psychosocial variables.
It is important to note that both insults from psychosocial stressors and energetic resources vary at higher-order population levels and more local, granular levels. In this review, most studies (n = 13) were conducted in environments such as the USA or the UK where greater psychosocial stress and obesity rates may be higher among impoverished or disadvantaged groups owing to structural inequalities and highly sedentary lifestyles (El-Sayed et al., Reference El-Sayed, Scarborough and Galea2012; Min et al., Reference Min, Xue and Wang2018). However, in other contexts reviewed here, such as Guatemala, impacts on pubertal growth and timing may play out differently in the context of dual burdens of rising overweight and obesity (overnutrition), and undernutrition (Doak et al., Reference Doak, Campos Ponce, Vossenaar and Solomons2016). In this way, it is reasonable that there may be some ascertainment bias in our selected sample of studies.
To highlight the complexity and importance of considering population contexts, we show how psychosocial and energetic factors may result in diverse outcomes based on varying environmental contexts in Table 5. In environments characterized by high psychosocial stress and low resources it is expected that puberty will be delayed from an energetic perspective. Psychosocial Acceleration Theory might suggest pubertal timing is accelerated in a high stress context, whereas Suppression Theory may suggest it is delayed. In a Low Stress/Low Resource context, we expect energetic factors and psychosocial stress to delay puberty, although in the modern context, we are not sure Low Stress/Low Resource environments exist (thus marked by a question mark). In High Stress/High Resource contexts, both energetic and psychosocial factors are expected to accelerate pubertal timing, especially when poverty is associated with both higher BMI and high psychosocial stress at the population level. Lastly, in a Low Stress/High Resource setting, we might expect that advantaged wealth may relate to higher BMI but may also be protective towards psychosocial stress and thus we expect opposite effects when considering energetic or psychosocial impacts on pubertal timing. Moreover, we underscore that those latent variables such as energetic sufficiency or obesity are not often modelled and when they are modelled – for example as SES – impacts on pubertal timing may be variable (see Table 5). Future research can more definitively assess the influence of psychosocial stress on pubertal timing by incorporating cross-group comparisons drawn from populations in which obesity is and is not correlated with greater wealth and socioeconomic status. Opportunities for such cross-population comparisons may become increasingly limited as obesity risks are trending upwards among marginalized populations in low- and middle-income countries as well (Masood & Reidpath, Reference Masood and Reidpath2017).
Table 5. Summary of potential energetic and psychosocial impacts on pubertal timing under varying resource and stress environments

−, Earlier puberty; +, delayed puberty; low/high resource, relative energetic scarcity or abundance.
Our review demonstrates the need for greater theoretical and mechanistic clarity as to how and why childhood psychosocial stress may affect the plasticity of pubertal timing (see Figures 1–3). Only nine studies we reviewed provided at least a partial explanation or discussion of why psychosocial factors were expected to influence pubertal timing (Braithwaite et al., Reference Braithwaite, Moore, Lustig, Epel, Ong, Rehkopf and Hiatt2009; Deardorff et al., Reference Deardorff, Ekwaru, Kushi, Ellis, Greenspan, Mirabedi and Hiatt2011; Ellis & Essex, Reference Ellis and Essex2007; Graber et al., Reference Graber, Brooks-Gunn and Warren1995; Khan et al., Reference Khan, Schroeder, Martorell, Haas and Rivera1996; Kirchengast & Bauer, Reference Kirchengast and Bauer2007; Kyweluk et al., Reference Kyweluk, Georgiev, Borja, Gettler and Kuzawa2018; Moffitt et al., Reference Moffitt, Caspi, Belsky and Silva1992; Stark et al., Reference Stark, Peckham and Moynihan1989). Of these, five referred specifically to Acceleration Theory or the potential for evolutionary life history strategies to calibrate pubertal timing based on psychosocial stress experienced in childhood (Deardorff et al., Reference Deardorff, Ekwaru, Kushi, Ellis, Greenspan, Mirabedi and Hiatt2011; Ellis & Essex, Reference Ellis and Essex2007; Graber et al., Reference Graber, Brooks-Gunn and Warren1995; Kyweluk et al., Reference Kyweluk, Georgiev, Borja, Gettler and Kuzawa2018; Moffitt et al., Reference Moffitt, Caspi, Belsky and Silva1992). While we do not view SES as a psychosocial variable per se, some authors perceived it as such. For example, 4/9 studies provided discussion about the ways SES or psychosocial stress may be felt through differential social, nutritional or economic status (Braithwaite et al., Reference Braithwaite, Moore, Lustig, Epel, Ong, Rehkopf and Hiatt2009; Khan et al., Reference Khan, Schroeder, Martorell, Haas and Rivera1996; Kirchengast & Bauer, Reference Kirchengast and Bauer2007; Stark et al., Reference Stark, Peckham and Moynihan1989). However, explicit pathways were not explored to plausibly connect psychosocial stress signals to pubertal outcomes. As we highlight in Table 5, we might expect contradictory pubertal outcomes depending on population context and the extent to which SES or poverty are considered.
Moreover, if SES continues to be used as an indicator of stress, it would be worthwhile to disentangle stressful experiences related to social inequalities from direct effects of resource availability. One such way to do this would be to integrate both objective and subjective measures of SES. For example, García et al. (Reference García, Gurven and Blackwell2017) highlight how direct (e.g. resource availability) and indirect (e.g. perceived unequal distribution of resources) correlates of SES may differentially impact HPA activation (García et al., Reference García, Gurven and Blackwell2017). Taking it a step further, testing objective and subjective SES in relation to HPA reactivity and HPG hormones prospectively up until puberty may help distinguish which dimensions of SES (e.g. perceived distribution of resources, social status stress, or crude resource availability) are signals of psychosocial stress and how they may influence pubertal timing via acceleration or suppression of the HPG.
While about half of our selected studies explicitly outlined mechanisms or at least acknowledged their predictive potential, there should be more attention paid to further explain biologically meaningful differences among proxy measures of psychosocial stress such as adverse childhood events or familial conflict. For example, B. Bogin (Reference Bogin2021a, Reference Boginb) proposes two specific pathways by which adverse experiences can impact growth, such as upregulation of glucocorticoids and calcitonin via chronic stress and downregulation of growth hormone/IGF-1 with downstream neuroendocrine effects (B. Bogin, Reference Bogin2021a, Reference Boginb). Thus, it would be particularly beneficial given the heterogeneity of stressors examined here, to think through which factors are more reliably or more likely to promote these biological processes. As an example, the extent to which studied factors such as household crowding, birth order or parental report of marital problems consistently signal psychosocial stress is unclear in our view. The biological plausibility that these factors reliably impact childhood growth or promote acute or chronic biological stress responses is not well tested to our knowledge.
Likewise, for many stress indicators there is the issue of construct validity. For example, psychosocial stress variables such as lifetime stress, adverse stress scales or binary variables related to school or home stress are unlikely to quantify individual perception or appraisal of psychosocial stress or continuity of stress effects. Still, regardless of whether observed factors signal psychosocial stress, latency between childhood exposures and pubertal outcomes introduces potential ‘noise’, especially when underlying biological mechanisms or upstream programming (e.g. epigenetic programming) go unmeasured. At the same time, some psychosocial variables studied here, such as sibling loss or adverse family experiences do attempt to capture environmental stressors which may have an impact on demographic patterning. Future researchers may consider implementing multiple types of study instruments to achieve more comprehensive measurement, innovate methods to study psychosocial stress signals and their subjective and biological impact, and use more complex statistical approaches such as causal inference and structural equation modelling to capture latent vs. observed psychosocial impacts on pubertal timing. Even more important, is that future research will be bolstered by considering socioecological contexts (see Table 5) and integrating these into study designs and theoretical predictions to merge the apparent gap between evolutionary theory and biological plausibility.
Belsky, Chisholm and others have long acknowledged the importance of mechanisms linking psychosocial stress and earlier menarcheal timing, such as HPA axis ‘dysregulation’ (Belsky et al., Reference Belsky, Steinberg, Houts, Friedman, DeHart, Cauffman and Susman2007; Chisholm, Burbank, et al., Reference Chisholm, Burbank, Coall, Gemmiti, Ellis and Bjorklund2005). Of note, we excluded Belsky et al. (Reference Belsky, Steinberg, Houts, Friedman, DeHart, Cauffman and Susman2007) during our methods screening. This paper examined family factors associated with earlier puberty but did not mention energetic factors as variables of interest in the methods nor include any anthropometric measures as covariates in models. However preliminary analyses reported in the results did note that both height and weight in fourth grade were moderately inversely correlated with pubertal onset. Only two studies in our review attempted (Ellis & Essex, Reference Ellis and Essex2007; Moffitt et al., Reference Moffitt, Caspi, Belsky and Silva1992) to account for confounding between psychosocial variables and relative adiposity through mediation analysis.
In addition, we did not formally assess risk of bias. However, we qualitatively assessed whether primary aims of the selected studies may have influenced their results. Of seven studies designed to primarily test the influence of energetic factors on pubertal timing, all of them found evidence for those predicted relationships. Four of those studies also found associations between psychosocial variables and pubertal timing, the other three did not. Similarly, all seven studies designed to explicitly test the influence of psychosocial factors on pubertal timing found evidence for at least one the predicted relationships, and all of them found associations with energetic factors. For the remaining six studies explicitly designed to test both psychosocial and energetic factors, all found an association between energetic factors and pubertal timing, but four of the six found no association with psychosocial factors.
Overall, our review highlights that in the pubertal context, few studies simultaneously account for factors indicative of childhood energetic status and psychosocial stress on pubertal timing. We have highlighted some key future research directions and we have helped synthesize and update prior critiques of evolutionary hypotheses about variable pubertal timing. Critically, we also advance ideas to bring together evolutionary hypotheses explaining variable pubertal timing and knowledge about human biological variation in adolescence. Taken together, we hope to propel fruitful research directions regarding psychosocial stress exposures and energetic status. A strength of this review is that it includes only studies that measured energetic factors prior to menarche – either prospectively or using status quo methods with a sufficiently large proportion of premenarcheal participants – which limits confounding in results owing to continued post-menarcheal increases in stature and body fat. However, we included studies that measured pubertal outcomes and psychosocial factors retrospectively, which may be biased by recall error. Lastly, the studies presented here focused on human female pubertal timing, and thus do not represent all potential variability among sexes (e.g. intersex, male).
Acknowledgements
We would like to acknowledge Lynly Beard and Anne Davis, research librarians at the University of Washington who gave useful consultation and instruction on the necessary systematic review protocols. We would also like to acknowledge Dr Barry Bogin and Dr Cara Ocobock for their detailed and useful feedback on this manuscript at early stages.
Author contributions
MM conceived of the original review aims and DG conceived of the additional review aims and contributions. JG created search strings and conducted original title search. JG and DG reviewed and selected studies at abstract, methods and critical review stages. DG and JG created tables and figures and MM revised. DG wrote the full article and JG and MM contributed to revisions and idea development. All authors contributed to and approved the final manuscript.
Financial support
This research received no specific grant from any funding agency, commercial or not-for-profit sectors.
Conflicts of interest
DG, JG and MM declare none.
Research transparency and reproducibility
Our systematized review protocol and search strings can be found on Open Science Framework: https://osf.io/nckrh/











