Introduction
The treatment gap for people with severe mental illness (SMI) is over 90% in most low-income countries (Wang et al., Reference Wang, Angermeyer, Borges, Bruffaerts, Chiu, de Girolamo, Fayyad, Gureje, Haro, Huang, Kessler, Kovess, Levinson, Nakane, Brown, Ormel, Posada-Villa, Aguilar-Gaxiola, Alonso, Lee, Heering, Pennell, Chatterji and Ustun2007). A ‘full’ task-sharing model whereby primary healthcare (PHC) workers are equipped to deliver all aspects of first-line mental health care, with limited specialist support, is recommended to increase access to care. Full task-sharing is at the heart of the World Health Organisation (WHO)'s mental health Gap Action Programme (mhGAP) (World Health Organization, 2008). However, many studies of task-shared care for SMI have employed a model whereby a mental health specialist makes the diagnosis of SMI, initiates treatment and provides an ongoing review, combined with the involvement of non-specialists in delivering psychosocial aspects of care (Chatterjee et al., Reference Chatterjee, Naik, John, Dabholkar, Balaji, Koschorke, Varghese, Thara, Weiss, Williams, McCrone, Patel and Thornicroft2014). This ‘partial’ model of task-sharing cannot address the needs of most people with SMI in low-income countries due to the scarcity of mental health professionals. In the few examples of programmes employing a ‘full’ task-sharing approach (Ventevogel et al., Reference Ventevogel, van de Put, Faiz, van Mierlo, Siddiqi and Komproe2012; Gureje et al., Reference Gureje, Abdulmalik, Kola, Musa, Yasamy and Adebayo2015), there is only limited evidence on whether care can be delivered safely and with sufficient quality to bring about improved outcomes for people with SMD (Jordans et al., Reference Jordans, Aldridge, Luitel, Baingana and Kohrt2017; Jordans et al., Reference Jordans, Luitel, Kohrt, Rathod, Garman, De Silva, Komproe, Patel and Lund2019). This lack of evidence may be fuelling reluctance to embrace the more ambitious task-sharing approach (Hanlon et al., Reference Hanlon, Fekadu, Jordans, Kigozi, Peterson, Shidhaye, Honikman, Lund, Prince, Raja, Thornicroft, Tomlinson and Patel2016b). To address this evidence gap, we present findings from the Programme for Improving Mental health carE (PRIME) (Fekadu et al., Reference Fekadu, Hanlon, Medhin, Alem, Selamu, Giorgis, Shibre, Teferra, Tegegn, Breuer, Patel, Tomlinson, Thornicroft, Prince and Lund2016). We have shown that PRIME in Ethiopia achieved contact coverage of over 80% for people with SMI in the target population. The objective of the current study was to investigate the impact of implementing a district level mental health care plan on the 12-month clinical and social outcomes of people with SMI who engaged with primary mental health care in Ethiopia.
Methods
Study design
We conducted an intervention cohort study, with assessments conducted at the baseline of implementation of the district mental health care plan (T0), and 6 (T1) and 12 months (T2) after initial engagement of people with SMI in the new integrated service.
Setting
The PRIME study was carried out in Sodo district, in the Gurage Zone of the Southern Nations, Nationalities and Peoples' Region of southern Ethiopia from December 2014 to July 2016. Sodo has an estimated population of around 160 000 people (Hanlon et al., Reference Hanlon, Luitel, Kathree, Murhar, Shrivasta, Medhin, Ssebunnya, Fekadu, Shidhaye, Petersen, Jordans, Kigozi, Thornicroft, Patel, Tomlinson, Lund, Breuer, De Silva and Prince2014). More than 90% of inhabitants live in rural areas and rely on subsistence farming and small-scale trading. At the time of the study, health services in Sodo comprised 54 health posts, most of which were staffed by two community health extension workers with 1 year of training in general health promotion and illness prevention, and eight health centres, staffed by nurses, health officers and midwives, who provide basic curative and obstetric care. There were no doctors or mental health specialists in the district. In the nearest town of Butajira, located 30–50 km from Sodo, there was a psychiatric nurse-led out-patient clinic. The nearest in-patient mental health services were 100–120 km away in the capital city, Addis Ababa.
Sample and recruitment procedures
As described previously (Baron et al., Reference Baron, Rathod, Hanlon, Prince, Fedaku, Kigozi, Jordans, Luitel, Medhin, Murhar, Nakku, Patel, Petersen, Selohilwe, Shidhaye, Ssebunnya, Tomlinson, Lund and De Silva2018), people with possible SMI in the community were identified by community key informants and health extension workers who had received half a day of training in typical presentations of SMI (Shibre et al., Reference Shibre, Kebede, Alem, Negash, Kibreab, Fekadu, Fekadu, Jacobsson and Kullgren2002). People with possible SMI were referred to the nearest health centre and assessed by PHC workers who had been trained in mental health care for a total of 10 days: 5 days of classroom-based teaching using adapted mhGAP training materials and 5 days of practical clinical training in the Butajira psychiatric clinic. The PHC workers assessed the referrals, made a diagnosis of SMI and initiated treatment according to the evidence-based clinical guidelines in the WHO mhGAP Intervention Guide (World Health Organization, 2016). Independent diagnostic review was carried out with standardised, semi-structured clinical assessments by research psychiatric nurses using the OPerational CRITeria for research (OPCRIT) (McGuffin et al., Reference McGuffin, Farmer and Harvey1991). Diagnostic assessments were conducted for all people with PHC worker-diagnosed SMI and wherever the PHC worker was uncertain about the diagnosis. The research psychiatric nurses reviewed the initial treatment plan. Any changes made by the psychiatric nurses were communicated back to the PHC workers.
Eligibility criteria
• Confirmatory research psychiatric nurse diagnosis of a psychotic disorder (including schizophrenia, schizoaffective disorder and depression with psychotic features) or bipolar disorder.
• Providing informed consent, or caregiver permission, to participate in the study if the person with SMI lacked capacity to consent.
• Able to converse in Amharic, the official language of the region.
• Planning to reside in the district for at least 12 months.
• No cognitive or sensory impairment that interfered substantially with the clinical assessment.
• Not acutely physically unwell.
Sample size
The sample size for the PRIME SMI cohorts across countries was calculated to detect a 20% reduction in the severity of symptoms at 12 months, with 90% power, two-sided alpha of 0.05 and 20% attrition rate (Baron et al., Reference Baron, Rathod, Hanlon, Prince, Fedaku, Kigozi, Jordans, Luitel, Medhin, Murhar, Nakku, Patel, Petersen, Selohilwe, Shidhaye, Ssebunnya, Tomlinson, Lund and De Silva2018), leading to a target sample size of 150. However, in the Ethiopia district, all people who received a confirmatory diagnosis of SMI were included to establish a completely ascertained population cohort.
PRIME intervention for people with SMI
The integrated district mental health care plan for the Ethiopian setting has been described in detail previously (Fekadu et al., Reference Fekadu, Hanlon, Medhin, Alem, Selamu, Giorgis, Shibre, Teferra, Tegegn, Breuer, Patel, Tomlinson, Thornicroft, Prince and Lund2016), and involved interventions at the level of the health system, PHC facility and community.
Health system interventions
The district health office staff were involved in participatory planning using Theory of Change methodology (Hailemariam et al., Reference Hailemariam, Fekadu, Selamu, Alem, Medhin, Wolde Giorgis, DeSilva and Breuer2015). The district health office assigned a focal person for mental health co-ordination. PRIME provided ongoing technical support with medication supply management (including establishing a revolving drug fund and providing assistance with forecasting the amount of psychotropic medication required), building capacity in supervision of mental healthcare (training high-performing general health workers to supervise mental health care) and monitoring and evaluation activities (e.g. aggregating data on facility contacts and feeding back at the advisory board meetings).
PHC facility interventions
All frontline PHC workers in Sodo district (n = 128) were trained in mental healthcare. The PHC workers received monthly supervision from a psychiatric nurse trained using the mhGAP supervisor training manual. For rural health centres, supervision was conducted by telephone when weather conditions precluded travel to the facility. PHC workers could also consult the psychiatric nurse for advice. People with confirmed SMI were prescribed an antipsychotic and/or antidepressant medication and/or a benzodiazepine, as indicated. There were no mood-stabiliser medications available, so people with bipolar disorder received antipsychotic medication as per usual practice in this setting (Fekadu et al., Reference Fekadu, Hanlon, Thornicroft, Lund, Kaaya, Alem, Gureje, Cleare, Patel and Prince2015). Almost all service users had to pay for medication.
In addition to the prescription of medication, PHC workers were trained to provide psychoeducation, activate social supports, address social stressors, monitor physical health, review response to treatment and refer to specialist mental health care if needed. As prescriptions were usually for a maximum of 1 month, follow-up appointments with the PHC workers were usually scheduled monthly.
Community-level interventions
A multi-sectoral ‘community advisory board’ was established to support community awareness-raising and mobilisation, to help with trouble-shooting during the implementation phase and to review project activities and outcomes. A total of 96 community-based health extension workers were trained in case detection, outreach to re-engage people who dropped out of care, identification of medication side effects, community awareness-raising and supporting social reintegration and recovery of people with SMI. In half of the sub-districts, people with schizophrenia who had enduring symptoms or disability after 6 months received adjuvant community-based rehabilitation, delivered by trained lay workers (n = 75) or ongoing PRIME care (n = 87), as part of a nested cluster randomised trial (the RISE trial) (Asher et al., Reference Asher, De Silva, Hanlon, Weiss, Birhane, Ejigu, Medhin, Patel and Fekadu2016). Aside from the additional contacts from CBR workers in the intervention arm (weekly for 2–3 months and 2-weekly for the subsequent 5–6 months), there were no additional trial-related contacts.
Measures
Primary outcomes
• Clinical symptom severity was measured using the Brief Psychiatric Rating Scale, expanded version (BPRS-E) (Burlingame et al., Reference Burlingame, Seaman, Johnson, Whipple, Richardson, Rees, Earnshaw, Spencer, Payne and O'Neil2006). The BPRS-E is a 24-item, clinician-rated scale which has been translated into Amharic and shown to have robust psychometric properties and sensitivity to change in Ethiopia (Habtamu et al., Reference Habtamu, Alem, Medhin, Fekadu, Dewey, Prince and Hanlon2017).
• Disability was measured using the World Health Organisation Disability Assessment Schedule (WHODAS), version 2.0, 36-item version (Üstün et al., Reference Üstün, Chatterji, Kostanjsek, Rehm, Kennedy, Epping-Jordan, Saxena, von Korffe and Pullf2010). The WHODAS has been validated for use in people with SMI in Ethiopia and is sensitive to change (Habtamu et al., Reference Habtamu, Alem, Medhin, Fekadu, Dewey, Prince and Hanlon2017). The WHODAS was completed by a combination of responses from the person with SMI and the caregiver responses to the proxy-WHODAS at the post-baseline assessments (19.8% of WHODAS scores from caregivers at midline, 24.1% at endline). We used the polytomous summary score of the WHODAS scaled from 0 to 100.
Secondary outcomes
• Experience of discrimination was measured using the ‘unfair treatment’ subscale of the discrimination and stigma scale-12 (DISC-12) (Brohan et al., Reference Brohan, Clement, Rose, Sartorius and Thornicroft2013). The original DISC-12 sub-scale has 21 items. Four items lacked face validity or had a low frequency of endorsement, but the remaining 17 items loaded onto a single factor using exploratory factor analysis and were summed.
• Restraint was measured by self-report of whether the person had been ‘restrained, chained or confined’ in the preceding 12 months.
• Alcohol use disorder was measured using the lay interviewer-administered Alcohol Use Disorder Identification Test (AUDIT) (Babor et al., Reference Babor, Higgins-Biddle, Saunders and MG.2001). This 10-item scale has been adapted for local drinks in the Ethiopian setting. People scoring ⩾8 are considered to have a probable alcohol use disorder.
• Depression was measured using a locally validated version of the Patient Health Questionnaire (PHQ-9) (Kroenke and Spitzer, Reference Kroenke and Spitzer2002). In the Ethiopian setting, a cut-off of 5 or more is indicative of major depressive disorder (Hanlon et al., Reference Hanlon, Medhin, Selamu, Breuer, Worku, Hailemariam, Lund, Prince and Fekadu2015).
• Suicide attempts in the past 3 months were assessed using the Mini International Neuropsychiatric Interview (Sheehan et al., Reference Sheehan, Lecrubier, Harnett-Sheehan, Janavs, Weiller, Bonara, Keskiner, Schinka, Knapp, Sheehan and Dunbar1997).
Potential effect modifiers
Equity indicators
• Gender and residence (rural v. urban).
• Socio-economic status: a poverty index was constructed which loaded onto a unidimensional scale using exploratory factor analysis: roof material made of straw (v. corrugated iron), unimproved water source, unimproved sanitation, no electricity, no separate room for kitchen, no radio or television, no mobile phone.
• Time to access the nearest health facility, estimated in minutes, whatever the means of travel. This was dichotomised into <60 min v. 60 min or longer.
Baseline characteristic
• Diagnosis: clinician-assessed diagnosis using criteria of the Diagnostic and Statistical Manual of the American Psychiatric Association, version IV) (American Psychiatric Association, 1994) from OPCRIT (McGuffin et al., Reference McGuffin, Farmer and Harvey1991).
Process indicators
• Receipt of minimally adequate treatment used the definition proposed by Wang et al. (Wang et al., Reference Wang, Demler and Kessler2002): prescription of medication on at least one occasion combined with at least four follow-up appointments with a health worker trained in mental health. We additionally required that psychotropic medication should be prescribed at therapeutic levels (World Health Organization, 2016). Data on the number of facility contacts and prescriptions were extracted from the clinical records, cross-referenced with a facility registration book.
• Receipt of community support (measured at T1 and T2): support received with returning to work, remembering to take medication, improving self-care, meeting people and social engagement. Responses were summed and binarised: 0–2 types of community support v. 3–5 types of community support.
• Receipt of in-patient care for mental health problems, contact with specialist mental health or general health facilities and traditional or religious healers, and type of care received during primary health care contacts were measured at T1 and T2.
Descriptive baseline characteristics
• Socio-demographic characteristics (age, educational level, marital status).
• Social support was assessed using the three-item Oslo Social Support Scale (OSS-3) (Dalgard et al., Reference Dalgard, Dowrick, Lehtinen, Vazquez-Barquero, Casey, Wilkinson, Ayuso-Mateos, Page and Dunn2006), which asks about number of close supports, extent of concern from supports and amount of practical support received. The OSS-3 total score was categorised as follows: 3–8 ‘poor support’, 9–11 ‘intermediate support’ and 12–14 ‘strong support’.
• Duration of illness, type of illness onset (acute/sub-acute v. gradual), psychiatric hospitalisation in the past 12 months, presence of co-morbid medical condition and receipt of psychotropic medication at baseline were obtained from the OPCRIT (McGuffin et al., Reference McGuffin, Farmer and Harvey1991).
Data collection
The lay interviewers were individuals with an educational level of at least tenth grade who were recruited from the local area and trained for 12 days on the study questionnaires and protocols, including observed practice interviews. The trainers had master's level qualifications. Degree-level supervisors monitored data quality in the field. The clinician assessments were conducted by research psychiatric nurses who were trained for 7 days by senior Ethiopian psychiatrists. The OPCRIT diagnoses were double-checked by an Ethiopian psychiatrist by reviewing the OPCRIT responses and clinical documentation and conducting verification interviews (n = 2) where needed.
Data management and analysis
Data were double-entered using EpiData (Lauritsen and Bruus, Reference Lauritsen and Bruus2003) and analysed using Stata version 13.1 (StataCorp LP, 2016). The data were summarised descriptively, with outcome data stratified by equity indicators (gender, residence, distance from the health facility and poverty status). Comparison of the characteristics of participants remaining in the cohort with those lost to follow-up at 12 months, as well as by equity indicators, was conducted using Pearson chi-squared for categorical variables, Student's t-test for comparing means in normally distributed variables and Kruskal Wallis equality-of-populations rank test for non-normal continuous variables.
Mixed effects linear regression with random intercept was used to model the change in symptom severity, disability and depressive symptoms over time. We tested for improvement in model fit using likelihood ratio tests after adding random slopes. We also tested for any significant difference in the mean change between T0 and T1 or between T1 and T2. Mixed effects ordinal regression was used to model change in discrimination score over time. A prevalence ratio was calculated for change from T0 to T2 in probable alcohol use disorder (AUDIT ⩾ 8), suicide attempt in the preceding 3 months or restraint in the preceding 12 months.
For the primary outcomes, we calculated standardised mean difference (Borenstein et al., Reference Borenstein, Hedges, Higgins and Rothstein2009) and examined effect modification by the equity indicators, diagnosis (primary psychotic disorder v. affective disorder), process indicators (receipt of minimally adequate treatment and community support) and by the community-based rehabilitation intervention group for the RISE trial. This was done by adding an interaction term into the model and testing for improved model fit using a likelihood ratio test. Multiple linear regression analysis was conducted to examine the association between baseline duration of illness and type of illness onset with 12-month outcomes.
Ethical considerations
Ethical approval was obtained from the Institutional Review Board of the College of Health Sciences, Addis Ababa University (No. 084/11/Psy). Informed consent was obtained where possible. If the person lacked capacity and did not refuse, the accompanying caregiver was invited to give permission on the person's behalf.
Results
Of the 294 people diagnosed by PHC workers as having SMI, 279 were confirmed to have SMI, giving a positive predictive value for PHC worker diagnosis of 94.9%. A further 21 people referred by PHC workers to psychiatric nurses for diagnostic review were also found to have SMI, giving a total of 300 participants. See Fig. 1.
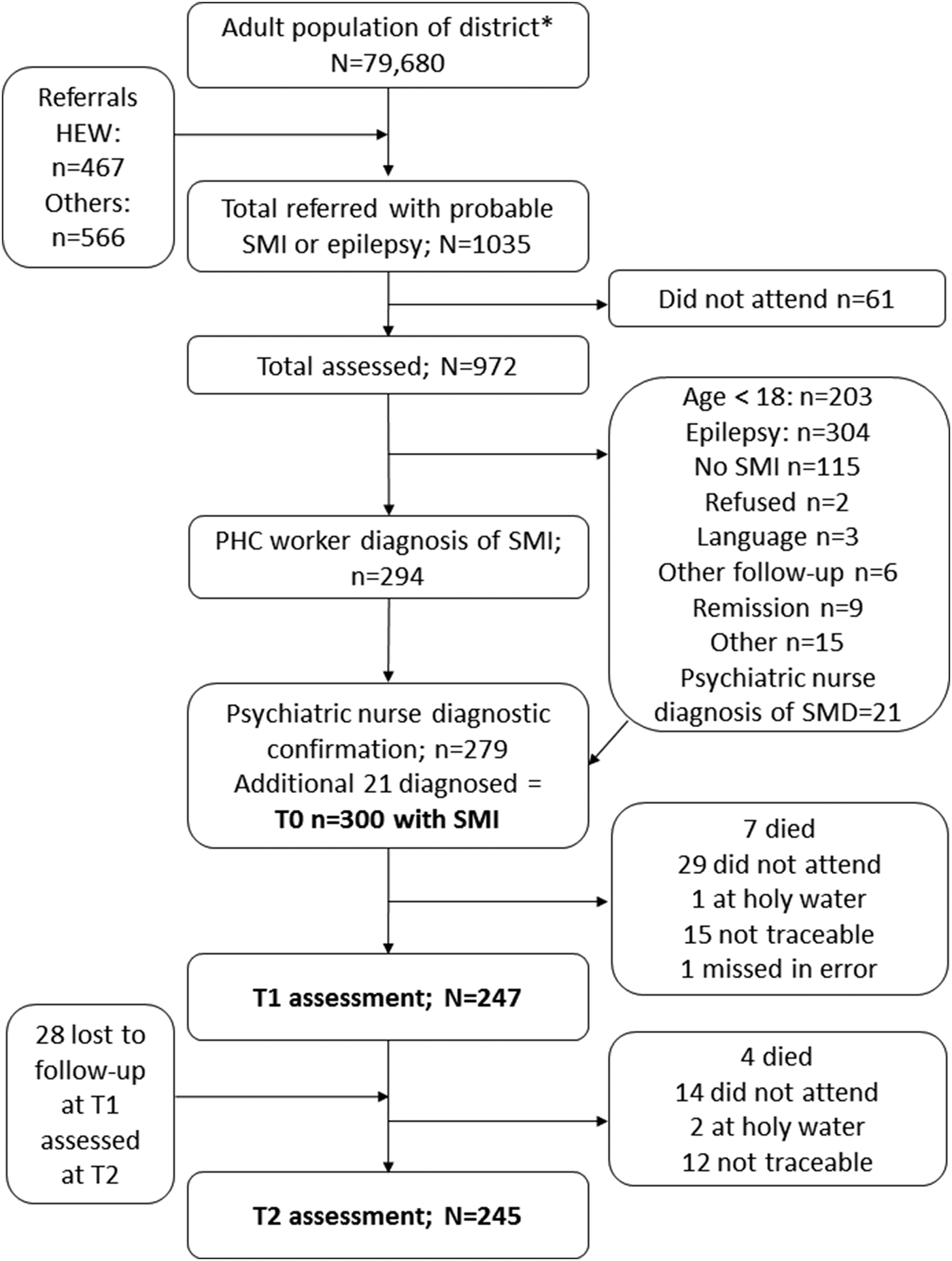
Fig. 1. Flow diagram.
Baseline characteristics
See Table 1. At baseline, there was no significant difference in symptom severity score, disability, discrimination or depressive symptoms by gender. The proportion with an alcohol use disorder (χ 2(1) 48.6750; p < 0.001) or who had been restrained (χ 2(1) 5.1154; p = 0.024) was higher in men. Baseline disability (t = 2.1012; p = 0.04) and restraint (χ 2(1) 3.9058; p = 0.048) were significantly higher in those with low socioeconomic status. Perceived negative discrimination at baseline was higher in urban residents (χ 2(1) 3.986; p = 0.049) and people of low socioeconomic status (χ 2(1) 4.195; p = 0.04).
Table 1. Baseline characteristics of the severe mental disorder cohort stratified by gender

*Poverty index = roof material made of straw (v. corrugated iron), unimproved water source, unimproved sanitation, no electricity, no separate room for kitchen, no radio or television, no mobile phone.
Cohort follow-up
A total of 247 (82.3%) people were assessed at T1 (mean 7.4 months; s.d. 1.49) and 245 (81.7%) at T2 (mean 12.3 months; s.d. 1.12) (see Fig. 1). There was no evidence of differential loss to follow-up based on baseline characteristics (online Supplementary File 1). During the follow-up period, 11 participants died.
Facility-based intervention
After review by psychiatric nurses, the medication initiated by PHC workers was unchanged or changed within the same medication class for 184 (67.7%) participants. Psychiatric nurses increased the dose or added another medication for 46 (16.9%), stopped or reduced the dose in 15 (5.9%), changed the class of medication in 15 (5.5%) and stopped a prescription of depot antipsychotic medication in 5 (1.8%).
Participants attended a median of two PHC appointments during the follow-up period (IQR 2–4; minimum 1 and maximum 12) (online Supplementary File 2). Minimally adequate treatment was received by 89 (29.8%) (online Supplementary File 3). There was no evidence of prescribing above recommended limits and only one occurrence of antipsychotic polypharmacy. Admission for in-patient care was very low (1.4% at T1 and 2.0% at T2) and <10% of participants had direct contact with a mental health specialist. A high proportion reported receiving psychosocial support and explanation about medication (online Supplementary File 4).
Community-based interventions
Most people with SMI reported receiving support with medication adherence and to improve self-care. More than half received support to get back to work, but less than a quarter were supported to get involved in social activities and <10% had support with meeting people. Almost all support was reported to come from the family. A small proportion reported contact with traditional or religious healers: 13.0% at T1 and 11.6% at T2.
Impact on clinical and social outcomes
There was a significant improvement in all clinical (symptom severity score, depressive symptoms, suicide attempts, alcohol use disorder) and social (functioning, discrimination, restraint) (Tables 2 and 3). The standardised mean difference for symptom severity was 0.18 (95% confidence interval (CI)) 0.02–0.34) between T0 and T1 and 0.28 (95% CI 0.13–0.44) from T0 to T2. For disability, the standardised mean difference was larger at both time-points: T0–T1: 0.27 (95% CI 0.13–0.41), T0–T2: 0.50 (95% CI 0.35–0.65). The test for interaction between the RISE trial intervention group and the main outcomes (symptom severity and functioning) at 12 months was non-significant.
Table 2. Mixed-effects modelling of primary outcomes stratified by equity indicators

The figures in bold are significant at p < 0.05.
Table 3. Secondary clinical and social outcomes in people with SMI stratified by gender

The figures in bold are significant at p < 0.05.
For symptom severity and functioning, the magnitude of the change did not differ between T0 and T1 compared to T1 and T2. The reduction in depressive symptoms was significantly greater between T0 and T1 than between T1 and T2 (χ 2(1) 4.26; p = 0.039); similarly for change in perceived discrimination (χ 2(1) 3.75; p = 0.053).
There was no statistically significant effect modification by the equity indicators, diagnosis or process indicators for the primary outcomes. At T1, higher receipt of community support had a borderline statistical association with a greater reduction in symptom severity (p = 0.19) and disability (p = 0.09) (online Supplementary Files 5 and 6). There was no association between duration of illness or type of illness onset and the mean improvement in the primary outcomes.
Discussion
In this community-ascertained intervention cohort of clinician-confirmed people with SMI, there was a significant improvement in clinical and social outcomes after implementation of a district level mental health care plan. PHC workers diagnosed SMI accurately, prescribed psychotropic medication safely and were reported to have delivered psychoeducation and provided support to most people with SMI. The findings from this study are generalisable to similar rural settings of low-income countries.
Although PHC workers prescribed safely (low polypharmacy and no doses above the recommended therapeutic range), the psychiatric nurses did consider that it was necessary to change the initial prescriptions of psychotropic medication in 30.1% of cases. This reinforces the need for task-shared care to be supported by input by mental health specialists, either through regular supervision or through timely review of newly diagnosed cases.
We are only aware of two previous studies, both from Nepal, where the impact of a ‘full’ task-sharing model on clinical and social outcomes of people with SMI was evaluated (Jordans et al., Reference Jordans, Aldridge, Luitel, Baingana and Kohrt2017; Jordans et al., Reference Jordans, Luitel, Kohrt, Rathod, Garman, De Silva, Komproe, Patel and Lund2019). Both studies found a significant reduction in symptom severity, disability and caregiver burden but had sample sizes under 100 and included extensive community-based psychosocial interventions (Jordans et al., Reference Jordans, Luitel, Kohrt, Rathod, Garman, De Silva, Komproe, Patel and Lund2019). Our larger study, which included a more representative population of people with SMI and a task-sharing intervention more closely based on mhGAP, provides more definitive evidence of important clinical and social benefits from the recommended WHO mhGAP approach.
In our study, the impact of the district mental health care plan care on psychotic symptoms was less marked than the reductions seen in the social outcomes, with a reduction of 4.8 on the BPRSE being less than that usually considered to be clinically significant (Hanlon et al., Reference Hanlon, Alem, Medhin, Shibre, Ejigu, Negussie, Dewey, Wissow, Prince, Susser, Lund and Fekadu2016a). In previous intervention studies for people with SMI in LMIC settings, a key factor for clinical improvement has been enhanced adherence to antipsychotic medication (Chatterjee et al., Reference Chatterjee, Naik, John, Dabholkar, Balaji, Koschorke, Varghese, Thara, Weiss, Williams, McCrone, Patel and Thornicroft2014), but only 30% of people with SMI in our study received ‘minimally adequate treatment’ over the follow-up period. Our process data indicate that engagement waxed and waned over time, rather than people dropping out of care altogether, and lends support to the acceptability of care provided as well as providing explanation for the limited impact on symptom improvement at cross-sectional assessment. In-depth interviews with study participants with SMI who had disengaged from care indicated that most had experienced symptomatic improvement, but that affordability of medication and side effects of medications was a barrier to continuous engagement. This is supported by quantitative data from the same sample, indicating high levels of poverty and out-of-pocket healthcare costs compared to the general population (Hailemichael et al., Reference Hailemichael, Hailemariam, Tirfessa, Docrat, Alem, Medhin, Lund, Chisholm, Fekadu and Hanlon2019). Mechanisms to reduce out-of-pocket healthcare costs for people with SMI are needed to achieve improved access to mental health care (Hanlon et al., Reference Hanlon, Alem, Lund, Hailemariam, Assefa, Giorgis and Chisholm2019).
Although disability is closely linked to symptom severity, our previous work in this community indicates that stigma, discrimination and poverty also make important contributions. The borderline significant effect modification indicating greater improvements in disability in people with higher receipt of community support may reflect the impact of reduced social exclusion. We observed a significant decline in perceived negative discrimination in people with SMI. The PRIME mental health care plan included a cascade model of training of community-based health extension workers to raise community awareness and reduce stigma against mental health problems (Fekadu et al., Reference Fekadu, Hanlon, Medhin, Alem, Selamu, Giorgis, Shibre, Teferra, Tegegn, Breuer, Patel, Tomlinson, Thornicroft, Prince and Lund2016); however, the extent of implementation is not known. The growing community and family awareness of the treatability of SMI, arising from the local availability of a treatment service and bearing witness to the clinical improvement of people with SMI who were well-known to the community, might have also reduced stigmatising attitudes, social exclusion and the need for people to be restrained. Furthermore, the Community Advisory Board members were selected due to their level of community influence and their endorsement of mental health care helped to reduce stigma and misconceptions about mental illness in the study site. In a recent trial in Ghana, restraint was not reduced by short-term provision of psychiatric care to people with SMI who were receiving faith healing in prayer camps (Ofori-Atta et al., Reference Ofori-Atta, Attafuah, Jack, Baning and Rosenheck2018). In our study, the follow-up period was longer, which may have allowed family members and the community to gain confidence in the beneficial effects of treatment and the PRIME intervention incorporated community and system-level interventions as well as task-shared facility-based care.
Alternative approaches to expanding access to care for people with SMI in rural populations in LMICs have been reported. One model is to utilise outreach clinics staffed by mental health specialists who diagnose, prescribe and monitor clinical progress, combined with community-level interventions by non-specialists to promote social inclusion and functional recovery (Srinivasa Murthy et al., Reference Srinivasa Murthy, Kishore Kumar, Chisholm, Thomas, Sekar and Chandrashekar2005; Chatterjee et al., Reference Chatterjee, Pillai, Jain, Cohen and Patel2009; Chatterjee et al., Reference Chatterjee, Naik, John, Dabholkar, Balaji, Koschorke, Varghese, Thara, Weiss, Williams, McCrone, Patel and Thornicroft2014). Another ‘back-referral’ model is for mental health specialists to provide initial assessment and development of a care plan which is then implemented by non-specialist health workers in the local area combined with task-shared psychosocial interventions (Xiang et al., Reference Xiang, Ran and Li1994). Augmenting the PRIME model with more intensive and systematic community-based rehabilitation delivered by non-specialists may help to address some of the gaps identified in our study by strengthening engagement with PHC and supporting livelihoods (Asher et al., Reference Asher, De Silva, Hanlon, Weiss, Birhane, Ejigu, Medhin, Patel and Fekadu2016), although the affordability and sustainability of such approaches need evaluation. There are no published reports of these task-sharing service models being successfully, safely and sustainably taken to scale and we have no evidence regarding their relative impact on effective treatment coverage for people with SMI. In the future, randomised controlled trials comparing task-shared models of care for people with SMI are needed to inform policy decisions (Hanlon et al., Reference Hanlon, Alem, Medhin, Shibre, Ejigu, Negussie, Dewey, Wissow, Prince, Susser, Lund and Fekadu2016a).
Limitations
PHC workers were informed of the research psychiatric nurse diagnosis and changes in treatment plan. For diagnosis the concordance was high and so this is unlikely to have affected the outcome, but the treatment plan reflected task-shared care with support from a mental health specialist. We only collected data on the positive and not the negative predictive value of the PHC worker diagnosis. Calculating the negative predictive value would be an important focus for future studies. Due to ethical concerns, there was no comparison group of people with SMI receiving ‘usual care’, which would have amounted to no access to evidence-based care for most people. However, outcomes in people with untreated schizophrenia (who formed the majority of our cohort) have been shown to be poor (Ran et al., Reference Ran, Xiang, Huang and Shan2001). Given the chronicity and severity of SMI at baseline, spontaneous improvement is unlikely. This is supported by the lack of an association between baseline duration of illness or type of illness onset and outcomes in our study.
Conclusions
An integrated district level mental health care plan employing a task-sharing approach safely addressed the large treatment gap for people with SMI in a rural, low-income country setting, resulting in improved clinical and social outcomes and reduced human rights abuses. Training and supporting PHC workers to provide mental health care has great potential as a sustainable and feasible approach to the care of people with SMI in resource-poor settings.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S2045796019000398.
Data
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request. The PRIME datasets will be made publicly available during 2019 (via www.prime.uct.ac.za).
Acknowledgements
We would like to express our thanks to the study participants for generously giving their time and energy to complete interviews, and to the field staff who worked tirelessly to ensure the smooth running of the project.
Financial support
This study was funded by the UK Department for International Development (DfID) [201446], as part of the Programme for Improving Mental health care (PRIME). The views expressed in this article do not necessarily reflect the UK Government's official policies. CH is supported by the National Institute of Health Research (NIHR) Global Health Research Unit on Health System Strengthening in Sub-Saharan Africa, King's College London (GHRU 16/136/54). The views expressed are those of the author and not necessarily those of the NHS, the NIHR or the Department of Health and Social Care. CH, CL, VP, AF and GM are supported by PRIME. CH additionally receives support from AMARI as part of the DELTAS Africa Initiative [DEL-15-01]. GT is supported by the National Institute for Health Research (NIHR) Collaboration for Leadership in Applied Health Research and Care South London at King's College London NHS Foundation Trust. The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR or the Department of Health. GT acknowledges financial support from the Department of Health via the National Institute for Health Research (NIHR) Biomedical Research Centre and Dementia Unit awarded to South London and Maudsley NHS Foundation Trust in partnership with King's College London and King's College Hospital NHS Foundation Trust. GT is supported by the European Union Seventh Framework Programme (FP7/2007–2013) Emerald project. GT also receives support from the National Institute of Mental Health of the National Institutes of Health under award number R01MH100470 (Cobalt study). GT is also supported by the UK Medical Research Council in relation to the Emilia (MR/S001255/1) and Indigo Partnership (MR/R023697/1) awards. LA was supported by a Wellcome Trust Fellowship in International Health [grant number 100142/Z/12/Z].
Conflict of interest
None.
Ethical standards
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008.






