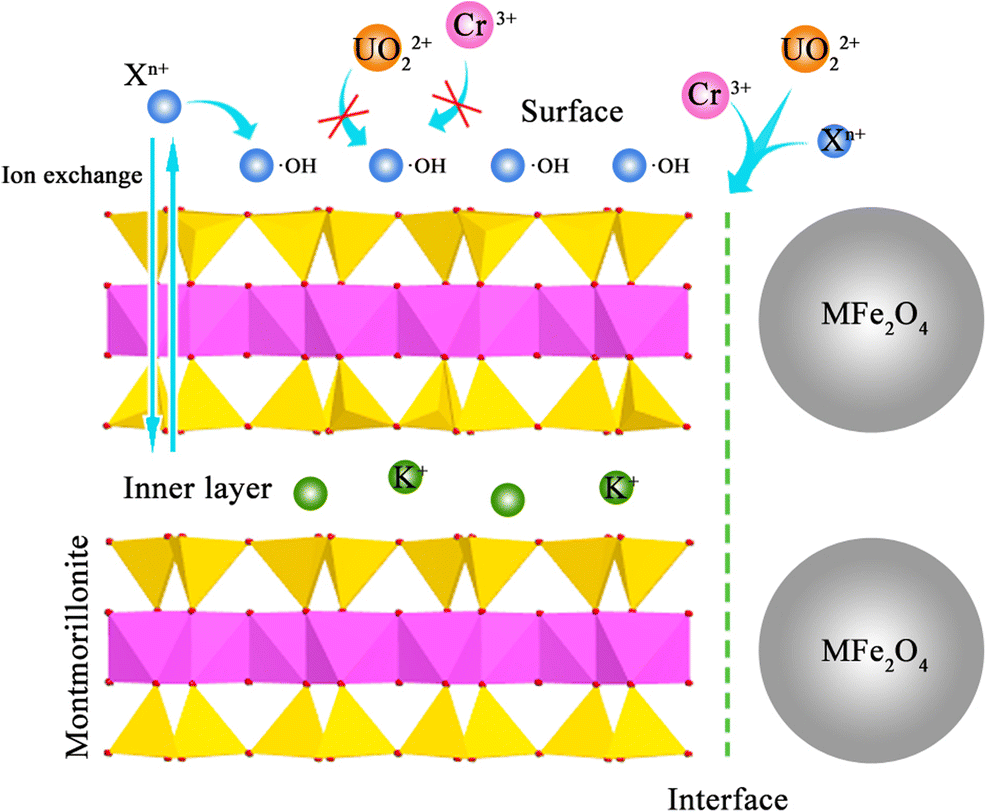
Adsorption of uranyl (UO22+) ions to mineral surfaces is a potentially effective method for removing this hazardous metal from water, but other toxic trace metal ions (Xn+: Rb+, Sr2+, Cr3+, Mn2+, Ni2+, Zn2+, Cd2+) in uraniferous wastewaters compete with UO22+ for adsorption sites and thus may diminish the capacity of adsorbents to sequester UO22+. A better understanding of competitive adsorption among these metal ions and the development of better adsorbents are, therefore, of critical importance. The purpose of the present study was to synthesize and characterize magnetic adsorbents, consisting of MFe2O4 (M = Mn, Fe, Zn, Co, or Ni) nanoparticles synthesized on montmorillonite (Mnt) edge sites, and to investigate their use as adsorbents for UO22+, including competitive adsorption with trace metal ions. Selective adsorption was studied using Langmuir, Freundlich, and Dubinin-Radushkevich isotherms, and the results showed that Xn+ ions were adsorbed primarily on MFe2O4-montmorillonite surfaces, and the UO22+ ions were adsorbed on the interfaces between montmorillonite edge surfaces and MFe2O4 nanoparticles. Using the Freundlich model, the interface adsorption capacity of UO22+ reached 25.1 mg·g–1 in mixed solution. Further, the UO22+ and Cr3+ ions had a redox reaction on the interfaces with synergistic adsorption. Herein, the adsorption capacity of Cr3+ was 60.2 mg·g–1 using the Freundlich isotherm. The results demonstrated that the MFe2O4-montmorillonite with highly selective adsorption of UO22+ ions is applicable to UO22+ treatment in the presence of toxic trace metal ions.
Graphical abstract