Objectives
The objectives of this chapter are to:
Introduction
The term ‘triad of anaesthesia’ is used to describe the components of a balanced anaesthetic:
hypnosis;
analgesia;
muscle relaxation.
The pharmacology of drugs used commonly in emergency airway management will be considered under these three headings.
In unmodified rapid sequence induction an analgesic is omitted and the patient is given a pre-calculated dose of induction drug and neuromuscular blocker only. The rationale behind this is that, should intubation fail, the patient will recover from anaesthesia and paralysis quickly, returning to spontaneous ventilation. Opioids, particularly in high doses, may prolong the time to spontaneous ventilation. Some patients may have received analgesia before the induction of anaesthesia (e.g. for pain relief in trauma), and under some circumstances it is appropriate to consider modifying an RSI to include a carefully selected dose of opioid given before the induction drug (e.g. RSI in the presence of raised intracranial pressure: see Chapter 11.2). Opioids are also useful after intubation, when they may be used in combination with a hypnotic to maintain anaesthesia and reduce sympathetic stimulation.
Midazolam is not considered an induction drug in the UK. Hypotension, bradycardia and long duration of action limit its usefulness as an induction drug in emergency airway management. However, midazolam may occasionally be given by an experienced practitioner to sedate an agitated and uncooperative patient to facilitate the process of RSI. It is also used for procedural sedation, and is therefore included in the hypnosis section.
Any modification of a standard rapid sequence induction will alter the pharmacodynamic response to induction drugs. Therefore the risks and benefits of RSI modification should be considered for individual patients.
Hypnosis
Induction drugs
The ideal anaesthetic induction drug would induce anaesthesia smoothly and rapidly without causing pain on injection. It would cause minimal depression of the respiratory and cardiovascular systems, and protect the cerebral circulation. Recovery would be rapid and the drug would have no adverse effects. Unfortunately, such a drug does not exist, and the attributes and limitations of available drugs, as well as the condition of the patient, will determine the final choice. Familiarity with the properties of a specific drug is also very important. It is not appropriate to use an unfamiliar drug for the first time in an emergency.
All induction drugs have the potential to cause hypotension to a greater or lesser extent, particularly when the patient is physiologically compromised.
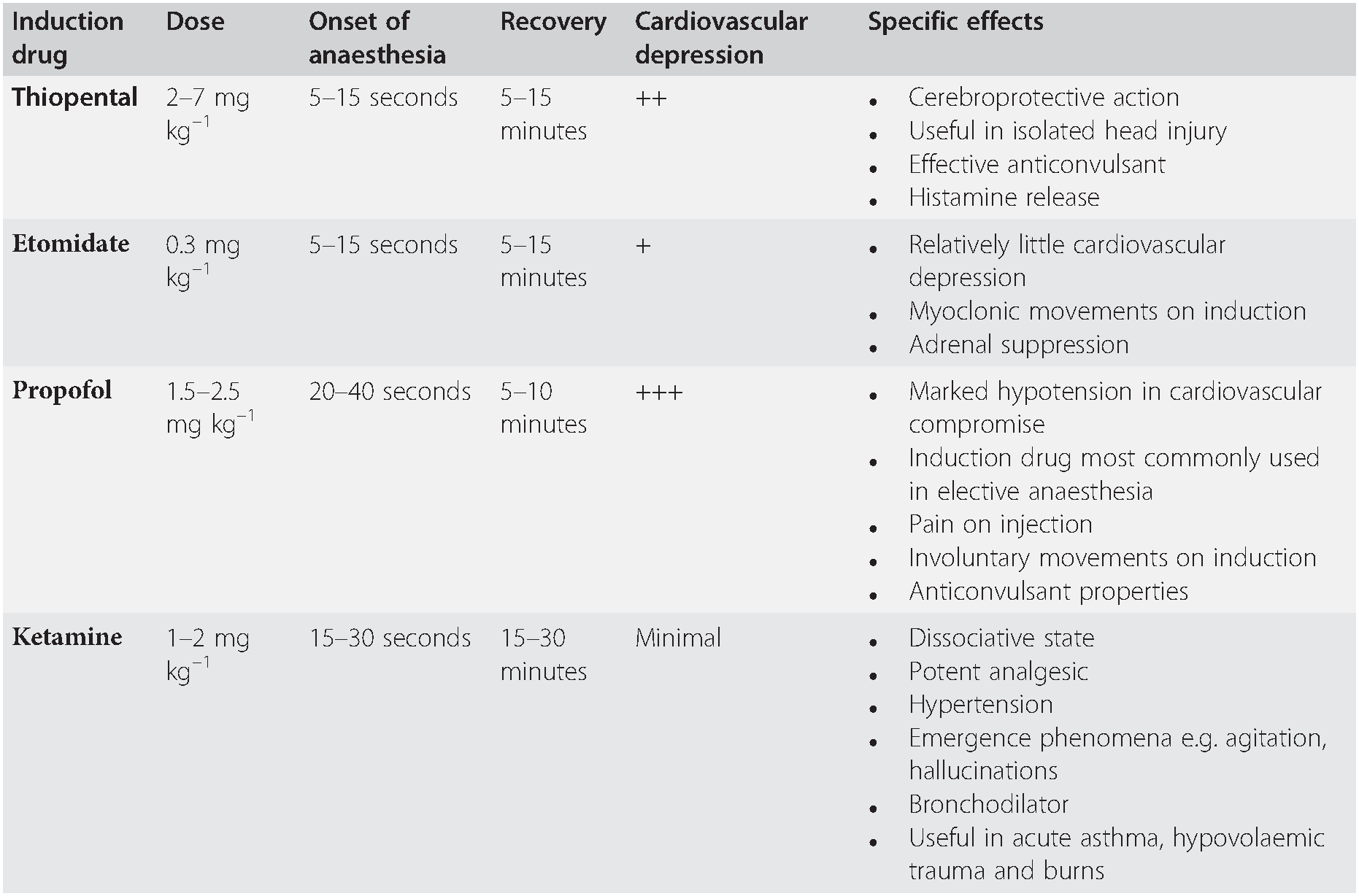
In the UK, one of four induction drugs is used in emergency RSI: these are summarized in Table 7.1, and described in more detail subsequently. However, this manual does not attempt to provide a comprehensive account of the properties of individual drugs and readers are referred to pharmacological texts for further information.
Table 7.1 Summary of the four commonly used induction drugs
| Induction drug | Dose | Onset of anaesthesia | Recovery | Cardiovascular depression | Specific effects |
|---|---|---|---|---|---|
| Thiopental | 2–7 mg kg–1 | 5–15 seconds | 5–15 minutes | ++ |
|
| Etomidate | 0.3 mg kg–1 | 5–15 seconds | 5–15 minutes | + |
|
| Propofol | 1.5–2.5 mg kg–1 | 20–40 seconds | 5–10 minutes | +++ |
|
| Ketamine | 1–2 mg kg–1 | 15–30 seconds | 15–30 minutes | Minimal |
Etomidate has fewer adverse effects on the cardiovascular system than thiopental or propofol and, particularly in the presence of hypovolaemia, will cause less hypotension than these drugs. In critically ill patients a single dose of etomidate causes adrenal suppression for up to 24 hours: the clinical significance of this is unclear, but it may be a cause of subsequent morbidity and mortality. Do not use etomidate in septic patients. Many practitioners prefer to use ketamine, thiopental or propofol, even in hypovolaemic patients, but this necessitates considerable experience of these drugs, and a carefully considered dose reduction, particularly if propofol is used. Where time allows, the placement of an arterial cannula is invaluable during RSI to provide accurate and continuous blood pressure measurement.
Ketamine is a useful induction drug in some circumstances: it is a bronchodilator, causes less hypotension and respiratory depression than the other induction drugs, and is a potent analgesic. The use of ketamine for induction is increasing, especially in critically ill patients, particularly trauma patients, and earlier concerns about adverse effects in patients with traumatic brain injury have not been demonstrated in practice. It can, however, cause hypertension. At the time of induction, the clinician must determine the clinical priorities for each patient, bearing in mind the benefits, but also the potential pitfalls, of each induction drug.
Etomidate
Indications
Induction of anaesthesia in the haemodynamically compromised patient.
Induction characteristics
5–15 seconds onset;
5–15 minutes full recovery;
myoclonic movement on injection (may be mistaken for seizures);
pain on injection.
Physiological effects
hypnotic;
relative haemodynamic stability;
attenuation of the increase in ICP that accompanies laryngoscopy;
reduced cerebral blood flow;
reduced cerebral oxygen demand;
adrenocortical suppression; must never be given by infusion;
do not use in a patient with sepsis.
Dose
0.3 mg kg–1 IV.
Propofol
Indications
most commonly used induction drug in elective anaesthesia;
can be used by infusion for maintenance of anaesthesia or sedation;
sedation in intubated patients on ICU or during transport.
Induction characteristics
slow onset (20–40 seconds) can lead to overdose;
rapid return of consciousness.
Physiological effects
hypotension is common, and may be severe in cardiovascular compromise and the elderly; it is caused mainly by vasodilatation, but also by a direct myocardial depressant effect;
apnoea after induction dose;
pain on injection with some preparations (reduced if 2 mL of 1% lidocaine is mixed with the induction dose or injected before induction);
occasional severe bradycardia;
induction often associated with involuntary movements, but anticonvulsant properties have been demonstrated on EEG studies.
Dose
1.5–2.5 mg kg–1 IV.
Thiopental sodium
Indications
haemodynamically stable patient with:
isolated head injury;
seizures.
Induction characteristics
5–15 seconds onset;
5–15 minutes to recovery.
Physiological effects
neuro-inhibition (at barbiturate receptor as part of GABA–receptor complex);
cerebroprotective, because of a dose-dependant decrease in:
cerebral metabolic oxygen consumption;
cerebral blood flow;
ICP;
maintenance of cerebral perfusion pressure;
venodilation;
myocardial depression;
central respiratory depression;
causes histamine release; can induce or exacerbate bronchospasm.
Dose
2–7 mg kg–1 IV;
dose reduced to 1.5–2 mg kg–1 IV in haemodynamically unstable patients and the elderly.
Ketamine
Indications
trauma, particularly burns;
septic shock;
cardiovascularly compromised patient;
severe bronchospasm.
Induction characteristics
15–30 seconds onset when given IV;
lack of a defined end-point makes dose calculation difficult;
excitatory phenomena.
Physiological effects
profound analgesia;
sedation;
dissociative state;
amnesia (less than benzodiazepines);
central sympathetic stimulation leading to:
increased heart rate;
increased blood pressure;
bronchial smooth muscle relaxation;
myocardial depression (in doses >1.5 mg kg–1);
respiratory depression – dose related;
enhanced laryngeal reflexes, with potential for laryngospasm;
secretions increased – pharyngeal and bronchial;
emergence phenomena:
commoner in adults;
reduced by pre-treatment with midazolam.
Dose
1–2 mg kg–1 IV;
5 mg kg–1 IM.
Midazolam
Midazolam is a water-soluble benzodiazepine that has anxiolytic, sedative and anticonvulsant properties. At physiological pH midazolam is lipid-soluble and reaches the central nervous system quickly. It has a shorter duration of action than most other benzodiazepines but causes profound, and sometimes prolonged, anterograde amnesia.
Indications
procedural sedation;
sedation of an agitated or uncooperative patient prior to RSI;
ongoing patient sedation post-intubation (often with an opioid);
reduction of side effects associated with ketamine.
Induction characteristics
onset over 2 minutes;
plasma half life is 2–6 hours, but the effects may be prolonged in elderly or debilitated patients;
substantial variations in bioavailability have been reported.
Physiological effects
sedation;
anterograde amnesia;
respiratory depression;
minimal cardiovascular depression in lower sedative doses (but may cause bradycardia as well as hypotension in higher doses);
vertigo and dizziness;
visual disturbances and nausea;
confusion in the elderly.
Dose
0.02–0.08 mg kg–1 IV usually achieves effective patient sedation.
The elderly
The elderly require special consideration. Those over 65 years form a significant proportion of patients requiring emergency RSI and tracheal intubation. They are more likely than younger patients to have comorbidities, and safe RSI, sedation or anaesthesia requires knowledge of the pharmacokinetics and pharmacodynamics in the elderly.
The normal ageing process is associated with a degeneration of structure and function of organs and tissues, but the rate at which this occurs is variable, and depends more on biological than chronological age. The elderly are more likely to be dehydrated, which increases the hypotensive effects of anaesthetic induction drugs.
A reduced stroke volume and cardiac output prolongs the arm–brain circulation time for a drug and can increase the likelihood of overdose if induction is not modified. Cardiac reserve is diminished and hypotension more likely.
Consider carefully the drug dose and speed of administration. CNS changes include a reduction in neurotransmitter synthesis and a less effective blood–brain barrier. Reductions in plasma albumin concentration with reduced drug binding contribute to the elderly patient being more sensitive to the effects of anaesthetic drugs and opioids. Impaired renal function and dehydration raise the likelihood of hyperkalaemia at presentation, and will reduce the clearance of some drugs.
Analgesia
Opioids
Although not recommended as part of the classic rapid sequence induction technique, use of opioids will attenuate the cardiovascular responses to laryngoscopy and intubation. This may be particularly valuable if intracranial pressure is raised (see Chapter 11.2), where the patient is very hypertensive or has ischaemic heart disease. Where opioids are used, the required dose of induction drug will be reduced.
Many opioids have a relatively slow onset of action. The respiratory depression caused by opioids may be troublesome if intubation fails, because the patient may remain apnoeic despite recovery from neuromuscular blockade; if necessary, reversal of the opioid with naloxone will restore spontaneous breathing.
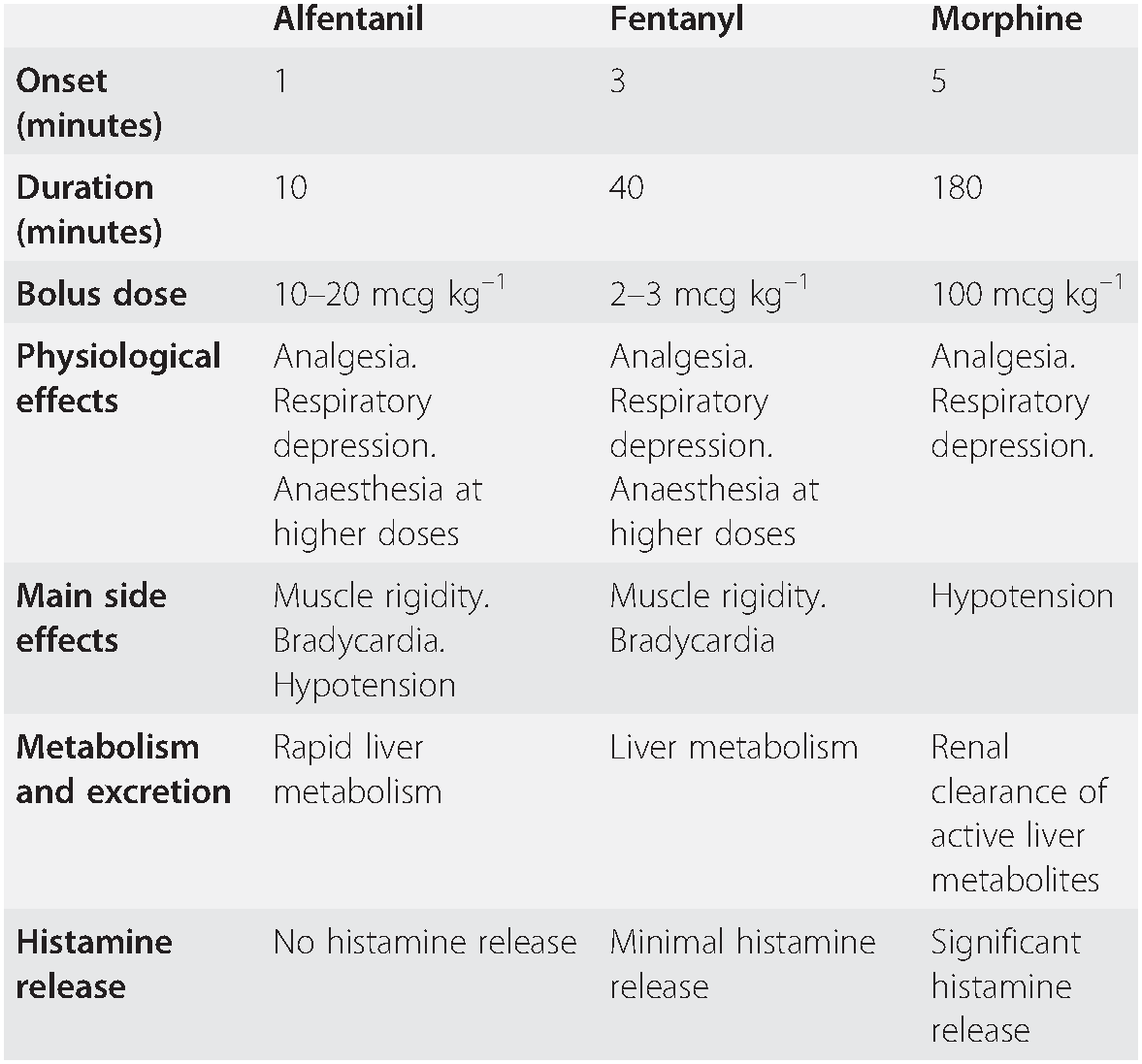
Fentanyl is a potent synthetic opioid which has a relatively fast onset (2–5 min) and short duration of action (30–60 min after a single dose). Its effects on the cardiovascular system are minimal, although large doses will cause bradycardia. Fentanyl (usual dose 2–3 mcg kg–1 IV) will reduce the hypertensive response, and offset the increase in intracranial pressure caused by laryngoscopy and intubation, providing that two to three minutes have elapsed between giving the drug and intubation.
Alfentanil (usual dose 10–20 mcg kg–1 IV) produces the same effects as fentanyl, but its peak action occurs after just 90 seconds and duration is 5–10 minutes: these characteristics make it ideal for attenuating the response to laryngoscopy and intubation.
If fentanyl or alfentanil are given, reduce the dose of induction drug (Table 7.2).
Table 7.2 Commonly used opioids
| Alfentanil | Fentanyl | Morphine | |
| Onset (minutes) | 1 | 3 | 5 |
| Duration (minutes) | 10 | 40 | 180 |
| Bolus dose | 10–20 mcg kg–1 | 2–3 mcg kg–1 | 100 mcg kg–1 |
| Physiological effects | Analgesia. Respiratory depression. Anaesthesia at higher doses | Analgesia. Respiratory depression. Anaesthesia at higher doses | Analgesia. Respiratory depression. |
| Main side effects | Muscle rigidity. Bradycardia. Hypotension | Muscle rigidity. Bradycardia | Hypotension |
| Metabolism and excretion | Rapid liver metabolism | Liver metabolism | Renal clearance of active liver metabolites |
| Histamine release | No histamine release | Minimal histamine release | Significant histamine release |
Neuromuscular blocking drugs
Suxamethonium
Suxamethonium (1.5–2 mg kg–1) produces a dense neuromuscular block of rapid onset and short duration. It remains the drug of choice for neuromuscular blockade during RSI, although use of high-dose rocuronium is increasing for this purpose. Initial depolarization at the neuromuscular junction causes muscle fasciculation within 15 seconds (although this is not always seen) and complete paralysis follows after 45–60 seconds. Spontaneous return of muscle activity follows after metabolism of the drug by pseudocholinesterase.
Suxamethonium has significant side effects (see below).
Use
remains the first line drug for muscular paralysis during rapid sequence induction.
Effects
10–15 seconds fasciculation;
45–60 seconds paralysis;
3–5 minutes first return of respiratory activity;
5–10 minutes return of effective spontaneous ventilation.
Contra-indications
ECG changes suggesting hyperkalaemia;
significant risk of hyperkalaemia (see Table 7.3).
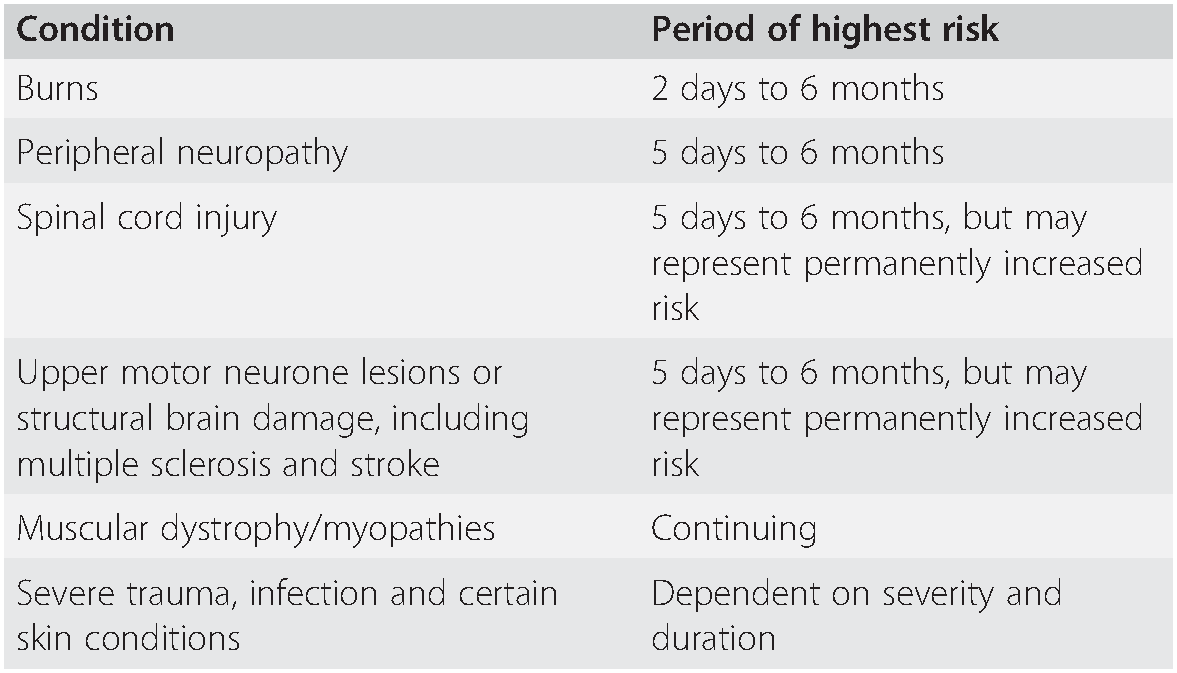
Table 7.3 Pre-existing conditions in which suxamethonium may cause significant hyperkalaemia, and the periods of highest risk
| Condition | Period of highest risk |
|---|---|
| Burns | 2 days to 6 months |
| Peripheral neuropathy | 5 days to 6 months |
| Spinal cord injury | 5 days to 6 months, but may represent permanently increased risk |
| Upper motor neurone lesions or structural brain damage, including multiple sclerosis and stroke | 5 days to 6 months, but may represent permanently increased risk |
| Muscular dystrophy/myopathies | Continuing |
| Severe trauma, infection and certain skin conditions | Dependent on severity and duration |
Side effects
hyperkalaemia;
bradycardia;
muscle fasciculation;
muscle pain;
histamine release;
anaphylaxis;
trigger drug for malignant hyperpyrexia in susceptible individuals;
trismus/masseter spasm;
prolonged neuromuscular blockade.
Dose
1.5–2 mg kg–1 IV.
Hyperkalaemia
After injecting suxamethonium, the plasma potassium concentration is increased by up to 0.5 mmol L–1, even in normal subjects. If the plasma potassium concentration is 6.0 mmol L–1 or higher the increase may precipitate arrhythmias, or even cardiac arrest. The increase in potassium concentration may be greatly exaggerated in patients with certain pathological conditions, particularly demyelinating conditions, desquamating skin conditions, major trauma, burns and several other pathologies where loss of muscle excitation secondary to denervation, immobilization or inflammation leads to up-regulation of immature acetylcholine (Ach) receptors throughout the whole muscle membrane. Depolarization of these Ach receptors by suxamethonium can cause life-threatening hyperkalaemia. Although there are recognized periods of maximum risk for patients with these conditions (Table 7.3), the exact duration of the risk period is unpredictable and an alternative to suxamethonium should be considered in each case.
Bradycardia
Suxamethonium may cause bradycardia, particularly if large or repeated doses are given: children are most at risk. This should be anticipated, and atropine must be available. Children do not need to be pre-treated with atropine routinely, but draw up the correct dose (0.02 mg kg–1) and be ready to give it whenever a child is anaesthetized.
Muscle fasciculation
The muscle fasciculation caused by suxamethonium can increase intracranial, intraocular and intragastric pressure. This effect is not significant when an adequate dose of an induction drug is given concurrently.
Muscle pain
This is most likely to occur 12–24 hours after giving suxamethonium to fit young patients and those who mobilize quickly after anaesthesia. It is seldom a clinical problem in patients undergoing emergency anaesthesia.
Histamine release
This will occur to a greater or lesser extent in all patients, and can cause significant hypotension.
Prolonged neuromuscular block
With repeated doses of suxamethonium the characteristics of the neuromuscular block change, and paralysis may be prolonged. In practice this should not be a problem as repeated doses of suxamethonium are rarely indicated. The action of suxamethonium may also be prolonged in the presence of organophosphate poisoning or cocaine use, when neuromuscular blockade may last 20–30 minutes. In patients with low or abnormal pseudocholinesterase activity, muscle paralysis after a dose of suxamethonium may last for several hours (‘sux’ apnoea). Treatment for this condition involves continued ventilation (including sedation) until normal neuromuscular activity returns.
Non-depolarizing muscle relaxants
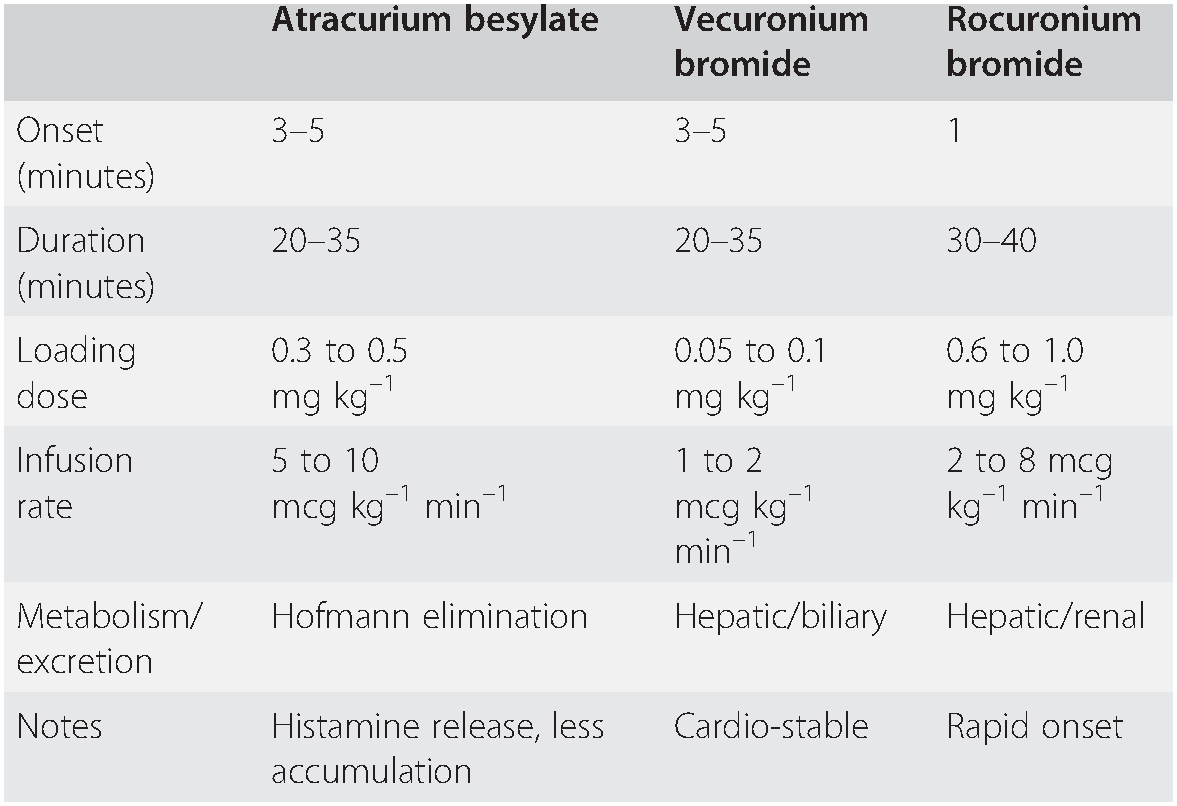
In situations when suxamethonium is contra-indicated, rocuronium 1.0–1.2 mg kg–1 can be used for modified rapid sequence induction, and will enable intubation after 60 seconds. This dose will cause paralysis for about an hour. Anaphylaxis to rocuronium occurs, but is less common than anaphylaxis to suxamethonium. Some experienced practitioners favour a modified RSI using rocuronium instead of suxamethonium, and this has been further encouraged by the introduction of sugammadex (see below). However, it should not be assumed that sugammadex will automatically rescue a practitioner from a ‘can’t intubate, can’t oxygenate’ situation, and high-dose rocuronium is therefore most suited to a patient where the option to wake up is considered non-viable, even if intubation cannot be achieved, and where difficult intubation is considered unlikely. For this reason, RSI using rocuronium is best undertaken by senior staff, and with an acceptance that timely progression to surgical airway may be required (Table 7.4).
Table 7.4 Commonly used non-depolarizing neuromuscular blockers
Sugammadex
If rocuronium has been chosen as the primary neuromuscular blocker for RSI its effect can be reversed rapidly with sugammadex given 3 minutes after rocuronium. This has potential benefits, but should be approached with caution.
Sugammadex is a cyclodextrin that encapsulates and then inactivates aminosteroid neuromuscular blocking drugs, including rocuronium. It forms a complex with the muscle relaxant in the plasma, thus reducing its ability to bind with receptors at the neuromuscular junction. Because one molecule of neuromuscular blocking drug binds to one molecule of sugammadex, the dose of sugammadex required depends on the extent of neuromuscular blockade to be reversed. For example, immediate reversal of rocuronium after RSI requires a large dose of sugammadex (i.e. 16 mg kg–1). In the obese patient, the dose should be based on actual body weight.
Most patients will have a recovery of neuromuscular function by 3 minutes. However this potential benefit should be balanced against the following considerations:
Patient oxygenation is an absolute priority.
Sugammadex is costly, and therefore it is rarely drawn up until it is known that it will definitely be required. Studies have shown it can take up to 7 minutes from a decision to give sugammadex to actual injection; this is in addition to the time spent on several intubation attempts.
Side effects include hypersensitivity, effects on haemostasis (with an increased risk of bleeding in some patient groups) and bradycardia.
The use of sugammadex for immediate reversal of rocuronium in children and adolescents has not been investigated, and so is not recommended.
Other non-depolarizing neuromuscular blockers are unlikely to be used during RSI, but may be used for maintaining muscle relaxation following recovery from suxamethonium.
Potential drug-related complications
The following complications can occur for the first time during emergency anaesthesia, but in some cases may be anticipated from the history of previous anaesthetics, allergies or family reactions to anaesthetic. This emphasizes the importance – even in emergencies – of thorough preparation, including a brief history where possible. Other information may also be found amongst the patient’s possessions, wallet or MedicAlert jewellery.
Malignant hyperthermia
Malignant hyperthermia (MH) is a life-threatening disorder of skeletal muscle calcium homeostasis. Its inheritance is autosomal dominant, with an incidence of around 1 in 30,000. The most common triggers are suxamethonium and volatile anaesthetics. Diagnosis can be difficult, and it may present insidiously over hours or as an acute life-threatening event at induction. Hyperthermia itself may be a late feature, as are the effects of rhabdomyolysis. Signs can be considered in two groups: direct muscle effects and the effects of increased metabolism. Masseter muscle spasm (MMS) may indicate MH, and can be the only sign of it, but is not pathognomonic since it also occurs in a few normal patients, especially children, after suxamethonium. After injection of suxamethonium, MMS will cause trismus at a time when relaxation would normally have been expected, but it does not usually persist long enough to hinder attempts at intubation. Treat prolonged MMS as a potential case of MH.
The most common signs of increased metabolism are:
unexplained increasing ETCO2;
concomitant tachycardia and arrhythmias;
decreasing oxygen saturation;
flushing.
Having excluded ventilatory problems and light anaesthesia, a patient displaying these signs should be treated for MH.
The Association of Anaesthetists of Great Britain and Ireland has published guidelines for the recognition and treatment of malignant hyperthermia. These guidelines should be available wherever general anaesthesia is administered, and followed if MH is suspected. Seek senior anaesthetic assistance immediately in all cases of suspected MH.
Discontinue the precipitant, hyperventilate with 100% oxygen and give dantrolene sodium as soon as the diagnosis is considered. The initial dose is 2.5 mg kg–1 IV. Repeated doses of 1 mg kg–1 are given thereafter to a maximum of 10 mg kg–1. Dantrolene has no major side effects but reconstitution for injection is time-consuming; several people may be needed to help. Further treatment comprises active cooling, treatment of hyperkalaemia, arrhythmias and disseminated intravascular coagulation, frequent monitoring of arterial blood gases, potassium, FBC and clotting, and supportive therapy as required.
Anaphylaxis
Anaphylaxis is a severe, life-threatening, generalized or systemic hypersensitivity reaction. It is characterized by rapidly developing, life-threatening problems involving: the airway (pharyngeal or laryngeal oedema), and/or breathing (bronchospasm with tachypnoea), and/or circulation (hypotension and/or tachycardia). In most cases, there are associated skin and mucosal changes. Almost any drug has the potential to cause anaphylaxis. Other causes include contact with substances such as chlorhexidine, or less commonly, latex. The clinical manifestations vary, but in the anaesthetized patient the incidences of various signs are:
cardiovascular collapse 88%;
erythema 45%;
bronchospasm 36%;
angio-oedema 24%;
other cutaneous signs (swelling, urticaria, rash).
Anaphylaxis on induction will frequently present with acute cardiovascular collapse. The presence of severe bronchospasm may make ventilation of the lungs impossible, and acute upper airway oedema makes intubation difficult. In this situation a smaller tracheal tube or even a surgical airway may be necessary as other rescue devices such as the laryngeal mask airway will be relatively ineffective. An alternative cause of hypotension, high inspiratory pressures and desaturation of arterial blood is tension pneumothorax, particularly in the trauma patient. If there is any doubt about the diagnosis, treat both conditions. Other diagnoses to consider are asthma, airway obstruction and primary myocardial pathology.
The initial treatment of anaphylaxis is to remove the precipitant and to manage the airway, breathing and circulation:
stop drug/remove precipitant;
100% oxygen;
maintain airway;
get help;
lay patient flat with legs elevated;
adrenaline IV:
50 mcg (0.5 mL of 1:10,000) increments (observing cardiac monitor) every 30 seconds until hypotension/bronchospasm improve;
100 – 500 mcg or more may be required;
1 mcg kg–1 in children = 0.1 mL kg–1 of 1:100,000;
In severe cases consider an adrenaline infusion: the usual dose is 0.05–0.1 mcg kg–1 min–1;
IV fluids – crystalloid (20 mL kg–1 in children).
Give antihistamines and corticosteroids to treat the end organ response to the mediators. A catecholamine infusion may be required as cardiovascular instability may last for hours. Check arterial blood gases regularly. Bronchodilators may be required to treat persistent bronchospasm. Before attempting extubation, ensure there is no residual oedema by deflating the tracheal tube cuff and checking for an adequate air leak.
Antihistamines: chlorphenamine 10 – 20 mg slowly IV (0.25 mg kg–1 in children);
Corticosteroids: hydrocortisone 100 – 300 mg IV (4 mg kg–1 in children).
The Association of Anaesthetists of Great Britain and Ireland has published guidelines for the recognition and treatment of anaphylaxis. These guidelines should be available wherever general anaesthesia is administered, and followed if anaphylaxis is suspected. Immediately seek senior assistance in all cases of suspected anaphylaxis.
Summary
Knowledge of the pharmacology and side effects of drugs used commonly in emergency airway management is essential.
No drug is perfect, and its advantages and disadvantages must be understood clearly.
Practitioners should choose drugs with which they are most familiar.
Practitioners must know how to treat the common complications of any drugs or techniques used.