Highlights
-
• Prospective memory relies on processes used by bilinguals during language switching
-
• Language switching modulates the neural signals of monitoring in prospective memory
-
• No effects found in neural signals associated with retrospective processes
-
• Bilingual interactional context modulates the recall of future intentions
1. Introduction
1.1. Prospective memory (PM)
Recalling future intentions allows us to perform many activities that are essential for our daily life. For example, if you are cooking a cake, you will need to create the future intention of removing it from the oven when properly baked. Thus, if, in the meantime, you are watching a movie on TV, you will have to monitor the time and supervise the cake to take it out of the oven before it burns! The ability that allows us to recall future intentions is called Prospective Memory (PM) and it has been widely explored in the literature (Einstein & McDaniel, Reference Einstein and McDaniel2005; Kliegel et al., Reference Kliegel, Martin, McDaniel and Einstein2004; Smith, Reference Smith2003; West & Krompinger, Reference West and Krompinger2005). Usually, in a PM task, participants are asked to perform a main task, called the ongoing activity. In addition, they have to encode a prospective memory intention that should be performed only when a specific cue (termed PM cue) appears. Therefore, the PM cue is a signal that indicates the moment to perform the prospective activity and the recall of the intention. In the previous example, watching the movie could be the ongoing activity, whereas removing the cake from the oven would be the prospective intention. In this example, the golden brown on the top of the cake would constitute the PM cue, which indicates that it is the correct moment to remove the cake from the oven. In the lab, prospective memory is also studied by using lab-based ongoing task and PM-cue procedures. For example, participants may be asked to carry out a 2-back task consisting of pressing a key if the current presented letter appeared two trials before (ongoing task), and also to receive the instructions to press a different key when a given letter appears (PM task) (see Ballhausen et al., Reference Ballhausen, Schnitzspahn, Horn and Kliegel2017; West & Bowry, Reference West and Bowry2005 for similar procedures). However, note that a prospective intention can be executed within the context of different types of ongoing activities that can vary in difficulty (Meier & Zimmermann, Reference Meier and Zimmermann2015). While the example previously mentioned requires working memory processes to complete the ongoing activity, some other procedures involve ongoing tasks that are less working memory dependent (e.g., a lexical decision task). Previous research indicates that successfully responding to a PM cue while performing the ongoing task highly depends not only on the retrospective retrieval of the prospective activity but also on the working memory processes involved in monitoring the experimental context to detect the PM cue and switching to the prospective response (e.g., see the PM Multiprocess Framework by McDaniel & Einstein, Reference McDaniel and Einstein2000).
This distinction between PM processes has also been observed at the neuroanatomical level (Cona et al., Reference Cona, Scarpazza, Sartori, Moscovitch and Bisiacchi2015): prospective processes such as maintaining the intentions while simultaneously engaged in an ongoing task and the strategic monitoring for the PM cue’s presence in the environment are mediated by a dorsal frontoparietal network that includes the aPFC regions and the dorsal parietal cortex (dPC). In contrast, the retrieval of intentions is linked to a ventral frontoparietal network.
1.2. Factors impacting PM
PM can be modulated by various factors (Anderson et al., Reference Anderson, Strube and McDaniel2019): studies show that the number of PM cues to be remembered (i.e., the prospective load) impacts performance, with no significant decline for one or two cues, but performance tends to decline with three or more cues (Cohen et al., Reference Cohen, Jaudas and Gollwitzer2008). Additionally, the type of PM cue (focal vs. non-focal) affects the cognitive processes used (bottom-up vs. top-down) (Einstein et al., Reference Einstein, McDaniel, Thomas, Mayfield, Shank, Morrisette and Breneiser2005; McDaniel & Einstein, Reference McDaniel and Einstein2000). Similarly, internal factors like age and cognitive capacity also influence PM performance (Cejudo et al., Reference Cejudo, Gómez-Ariza and Bajo2019; Schnitzspahn et al., Reference Schnitzspahn, Ihle, Henry, Rendell and Kliegel2011, Reference Schnitzspahn, Stahl, Zeintl, Kaller and Kliegel2013). For instance, Brewer et al. (Reference Brewer, Knight, Marsh and Unsworth2010) found that participants with higher working memory excelled on non-focal tasks, while those with low and high working memory performed similarly on focal tasks. These findings underscore the variability in the cognitive mechanisms underlying PM.
One aspect that has not yet been investigated is the influence of bilingualism on PM. Although, at first sight, PM may seem unrelated to the language experience of the individuals, bilingual people are required to monitor the context for cues that permit them to select the most appropriate language for the situation, and this resembles PM situations where individuals need to monitor the context for cues that signal the moment to stop the ongoing task and switch to the intended prospective action. The use of monitoring in bilinguals is especially evident when the context requires frequent switches between languages (e.g., conversation with people in different languages), where they need to pay attention to specific cues in the environment to predict the incoming language. For example, being presented with a given face (Asian or Caucasian) before performing a picture naming task in Chinese or English has been shown to modulate the activation of these two languages (Liu et al., Reference Liu, Timmer, Jiao, Yuan and Wang2019a), suggesting that contextual cues may facilitate language selection. These results are consistent with the Adaptive Control Hypothesis (Green & Abutalebi, Reference Green and Abutalebi2013), which suggests that the context in which bilinguals are immersed can modulate how they control their language production. However, these interactions are not reduced to the language control attentional network. Additional studies have found that bilingual immersion experience influences domain-general cognitive control (Beatty-Martínez et al., Reference Beatty-Martínez, Navarro-Torres, Dussias, Bajo, Guzzardo Tamargo and Kroll2020; Jiao et al., Reference Jiao, Grundy, Liu and Chen2020; Timmer et al., Reference Timmer, Costa and Wodniecka2021).
1.3. Bilingualism and PM
Recent research has shown a relation between different bilingual experiences and modulations in PM at behavioral and neural levels (López-Rojas et al., Reference López-Rojas, Rossi, Marful and Bajo2022). Specifically, Lopez-Rojas et al. (Reference López-Rojas, Rossi, Marful and Bajo2022) found that bilinguals who (1) were immersed in bilingual contexts with frequent between-language switches and (2) acquired their second language (L2) during childhood, showed larger differences between the ongoing activity and the prospective intention for event-related potential (ERP) components related to PM performance (N300 and P3b) compared to monolinguals or to non-immersed bilinguals who acquired the L2 in the adolescent/adulthood. These differences were found in the more attention-demanding PM conditions, indicating that this type of bilingual was able to adapt their prospective processes to the demands of the PM task.
The concept that bilingualism modulates prospective processing raises interesting questions related to thow bilingualism affects the underlying processes associated with PM. Thus, López-Rojas et al. (Reference López-Rojas, Rossi, Marful and Bajo2022) suggested that being immersed in a bilingual linguistic context adapted individuals’ monitoring and switching strategies to the conditions of the PM task. Specifically, it was suggested that bilinguals who were used to switching between languages were better able to adapt their monitoring processes to the PM task demands than monolinguals and bilinguals immersed in a non-switching language context. In the present study, we adopted a novel approach to measure language-switching experience by experimentally providing participants with language-switching practice to investigate how that practice could impact the cognitive processes engaged in PM. In addition, we aimed to identify the specific PM processes affected by the language-switching experience.
In single-language contexts, bilinguals typically use their two languages in distinct settings (e.g., one at home and the other at work). This pattern of language use primarily relies on global control mechanisms like goal maintenance and conflict monitoring. In contrast, in dual-language contexts, which are most similar to the typical cue-based laboratory measurements of language switching, both languages are used within the same environment but with different interlocutors (e.g., both languages are spoken at work, but one with person A and the other with person B). This type of context requires constant monitoring of the situation to select the appropriate language, thus demanding a higher level of language control (see Green & Abutalebi, Reference Green and Abutalebi2013, for a detailed conceptualization of both contexts). Therefore, our assumption was that the mechanisms that naturally emerge in dual-language contexts where bilinguals frequently switch between languages are similar to the processes elicited by a language-switching task in which bilinguals from single-language contexts are forced to change between languages (Timmer et al., Reference Timmer, Calabria and Costa2019), and therefore, we expected that these mechanisms would be modulated by language-switching experience.
1.4. Language-switching practice
In the field of bilingualism, several studies have explored the impact of language-switching training on other tasks that require cognitive control. For example, Liu et al. (Reference Liu, Yang, Jiao, Schwieter, Sun and Wang2019b) explored whether language-switching training facilitated performance in two tasks that required monitoring (mixing-cost) and inhibitory control (anti-saccade). Their hypotheses were that, given that during language switching the bilingual needs to monitor the conflict between languages and inhibit cross-language representations, switching training should facilitate performance in the mixing-cost and anti-saccade tasks where these processes were also involved. Their results indicated that language-switching training improved performance in both components. Similarly, Timmer et al. (Reference Timmer, Calabria and Costa2019) compared two groups of bilinguals (language-switching group vs. language-control group) that completed a nonlinguistic task in a pre-/post-training session. In the post-training session, they found that the switching training group (but not the control group) improved their performance when measuring the switching cost (i.e., comparison between switch vs. non-switch trials) in the nonlinguistic task, concluding that at least some mechanisms of control are shared across different domains. Importantly, previous experiments have indicated that the effects of language-switching practice on cognitive control tasks could be immediate (Liu et al., Reference Liu, Fan, Rossi, Yao and Chen2016).
These conclusions have also been supported by studies with different neuroimaging techniques. For example, Chen et al. (Reference Chen, Ma, Zhang, Li, Zhang, Yuan and Guo2021) explored the neural adaptations induced by language switching using functional magnetic resonance imaging (fMRI). Their findings indicated a reduction in the connectivity from the right thalamus to the dorsal anterior cingulate cortex/pre-supplementary motor area (dACC/pre-SMA) after language-switching training. The connections between these regions were stronger when executing more demanding cognitive control processes. Therefore, these results suggest that after a language-switching training, less neural connectivity is demanded to complete the same cognitively demanding task. In addition, Zhang et al. (Reference Zhang, Kang, Wu, Ma and Guo2015) observed a modulation in the AX-Continuous Performance Task (AX-CPT) cognitive control task after switching practice. This task relies on proactive control to maintain task-relevant information over time and reactive control to solve interference when conflicts arise (Braver et al., Reference Braver, Paxton, Locke and Barch2009, Reference Braver2012). In their study, Zhang et al. (Reference Zhang, Kang, Wu, Ma and Guo2015) observed an enhancement in the use of proactive control strategies after a 10-day language-switching training compared to a pre-training condition. In fact, language-switching training produced an increase in the BSI (Behavioral Shift Index)Footnote 1, of the AX-CPT that indicates higher proactivity and a greater N2 component triggered by the cue that has been related to cognitive control. Altogether, behavioral and neural results suggest that bilinguals who completed a short-term language-switching training tend to change their strategies when completing a cognitive control task.
1.5. The present study
Therefore, in this study, we aimed to investigate whether language-switching practice modulates bilinguals’ performance in a PM task, and if so, to specify the nature of this change by measuring their brain activity and observing the ERP associated with PM. Specifically, our study evaluated Spanish–English bilinguals from Spain, immersed in a single-language context, that is, a context in which one language is used and the other language is employed in a second distinct environment (Green & Abutalebi, Reference Green and Abutalebi2013). Studying training effects in bilinguals in single-language contexts is important, since previous studies have demonstrated that these bilinguals use different modes of cognitive control compared to bilinguals immersed in dual-language contexts (Jiao et al., Reference Jiao, Grundy, Liu and Chen2020; Timmer et al., Reference Timmer, Costa and Wodniecka2021). For example, Hartanto and Yang (Reference Hartanto and Yang2016) found that bilinguals immersed in a dual-language context outperformed bilinguals immersed in a single-language context in cognitive control tasks. Interestingly, Beatty-Martinez et al. (2020) showed that bilinguals in separated contexts (e.g., South Spain) depended on reactive processes to a greater extent than bilinguals in contexts where both languages are indistinctly and more cooperatively used. Similarly, Hofweber et al. (Reference Hofweber, Marinis and Treffers-Daller2020) found that bilinguals engaged in code-switching patterns that kept languages more separate showed benefits in tasks inducing reactive control, whereas bilinguals engaged in dense code-switching situations relied more on proactive strategies. Thus, given that our bilinguals were immersed in a single-language context where both languages were used in a separated way, we expected to observe an immediate effect of the switching-between-languages practice in the PM task.
To test this hypothesis, the total sample was divided into two groups: (1) the language-switching practice group (hereinafter “switching group”) where participants carried out a picture naming L1/L2 language-switching task at the beginning of the experiment and before performing a PM task; (2) the language-control group where participants did not perform the picture naming task prior to the PM. Hence, we compared two groups of bilinguals immersed in identical single-language contexts, but only one of them was exposed to language-switching practice before performing the experimental PM task. The PM task consisted of a 2-back task (ongoing task) where colored letters were presented and participants were asked to recall if a given stimulus (a given letter or a color) appeared two trials before. Additionally, participants completed a block in which a PM intention was implemented during the ongoing activity. Hence, participants had to press a different key when a previously encoded PM cue appeared (i.e., certain stimulus colors or some specific letters). Given the nature of this practice, we expected participants in the experimental group to detect PM cues and switch between the ongoing activity and PM intention more efficiently, primarily resulting in faster response times. Additionally, if they were more skilled at monitoring the environment, this could also be reflected in improved recall of the PM’s intention. To assess possible changes in the specific processes involved in PM, we recorded the brain activity during the PM and ongoing tasks and analyzed the ERP components associated with different PM processes.
A wide body of literature has explored the ERP components associated with PM (for a review, see West, Reference West2011). Thus, a number of so-called “prospective components” have been associated with the monitoring processes required to detect the PM cue in the course of the ongoing activity, whereas other “retrospective components” have been related to the recall and updating of the intention from long-term memory. Specifically, the N300 and frontal positivity have been described as prospective components related to the detection of the PM cue in the environment. Thus, the N300 is characterized by a negative deflection in the PM trials compared to the ongoing trials around 200 ms, which could be extended until 300–500 ms. Interestingly, López-Rojas et al. (Reference López-Rojas, Rossi, Marful and Bajo2022) found that bilinguals immersed in an interactional context where both languages work in a cooperative way showed larger N300 in the more challenging conditions (when compared to monolinguals and bilinguals from a single-language context). This finding suggested that frequent language switching enhances cue detection. In another study, this N300 component did not emerge, however, when single-context bilinguals carried out the PM task in L2 (López-Rojas et al., Reference López-Rojas, Csilinkó, Bajo and Marful2023a), likely due to the higher L2 demands that impaired cue detection. Although the studies on the linguistic factors that modulate these prospective ERP components are scarce, studies with monolinguals have observed that this N300 component is more noticeable when more perceptual (compared to more semantic) cues are provided (Cousens et al., Reference Cousens, Cutmore, Wang, Wilson, Chan and Shum2015). Frequently, the N300 is accompanied by a frontal positivity, a positive deflection between 300 and 500 ms after stimulus onset that differentiates PM trials from ongoing trials. It also seems to be related to switching processes between ongoing and PM activities (Bisiacchi et al., Reference Bisiacchi, Schiff, Ciccola and Kliegel2009). Frontal positivity, however, was not examined by Lopez-Rojas et al. (Reference López-Rojas, Csilinkó, Bajo and Marful2023a, Reference López-Rojas, Marful, Pérez and Bajo2023b), but it has been demonstrated to be independent of the linguistic nature of the PM cue, occurring with both perceptual and semantic cues (Cousens et al., Reference Cousens, Cutmore, Wang, Wilson, Chan and Shum2015).
On the other hand, the P3b and frontal slow waves have been associated with retrospective processes such as retrieval from long-term memory or the realization of delayed intentions (Cona et al., Reference Cona, Bisiacchi and Moscovitch2014). Critically, the P3b has been characterized by a positive amplitude between 300–400 ms and 600–800 ms elicited by the PM trials compared to the ongoing trials. This component reflects the activity of processes related to working memory and context updating (Polich, Reference Polich2007; West et al., Reference West, Wymbs, Jakubek and Herndon2003). This component has been shown to be larger in bilinguals immersed in a language interactional context with high task demands when compared with monolinguals and single-language context bilinguals (López-Rojas et al., Reference López-Rojas, Rossi, Marful and Bajo2022). Similarly, the frontal slow waves, a component defined by a positive amplitude over the frontal region that begins around 500 ms after stimulus onset (Cona et al., Reference Cona, Bisiacchi and Moscovitch2014), is considered to reflect post-retrieval monitoring processes when a PM cue is detected (West et al., Reference West, Wymbs, Jakubek and Herndon2003). Both the P3b and the frontal slow waves are influenced by memory load and the type of information to be retrieved, with studies showing reduced P3b amplitudes under high memory loads (West & Bowry, Reference West and Bowry2005; West et al., Reference West, Bowry and Krompinger2006) and greater slow waves in PM tasks requiring effortful retrospective retrieval (Cona et al., Reference Cona, Bisiacchi and Moscovitch2014; Rösler et al., Reference Rösler, Heil and Glowalla1993; West et al., Reference West, Wymbs, Jakubek and Herndon2003).
Since the language-switching practice in the present experiment was assumed to engage monitoring and switching processes, we expected that the N300 and frontal positivity components – associated with prospective PM processes – would be modulated by this switching practice, that would result in greater ongoing-PM differences in amplitudes for the practice group than the control group. Given the prospective nature of this practice in language switching, we expected that the retrospective components (P3b and frontal slow waves) that are associated with updating and retrieval of the intention from long-term memory, would be less affected by our language-switching manipulation.Footnote 2
2. Method
2.1. Participants
This study has been approved by the Research Ethics Committee of the University of Granada (registration number 2262/CEIH/2021). A sample size of 54 was required to obtain 80% power to detect a Cohen’s effect size of f = .40. This value is considered a large effect size in Cohen (Reference Cohen1969), and it corresponds to η2= .14 based on the G*power analysis program (Faul et al., Reference Faul, Erdfelder, Lang and Buchner2007) of a 2 (IV: between-subject) × 2 (IV: within-subject) repeated measures ANOVA. A large effect size was inferred by previous results in studies on prospective memory processing in bilinguals (López-Rojas et al., Reference López-Rojas, Csilinkó, Bajo and Marful2023a; López-Rojas et al., Reference López-Rojas, Marful, Pérez and Bajo2023b).
We evaluated a total of 56 Spanish–English students from the University of Granada (11 men; mean age = 20.9, SD = 2.9). Participants were randomly assigned to the switching group (n = 28), where they completed a language-switching task before the PM task, or to the control group (n= 28), where they did not complete the language-switching task. The MELICET and LEAP-Q (Marian et al., Reference Marian, Blumenfeld and Kaushanskaya2007) tests were administered to control for language experience. Both measures were used to obtain linguistic background information from the participants (see Table 1). The MELICET assesses grammar using 50 cloze questions, each with three answer choices, while the LEAP-Q is a validated questionnaire designed to collect self-reported information on linguistic experience in both L1 and L2. In addition, to ensure that there were no differences in memory abilities between the two groups, a working memory task (digit span) was administered after the experimental task, since working memory has been related to PM (Rose et al., Reference Rose, Rendell, McDaniel, Aberle and Kliegel2010). The two groups were matched in their working memory scores (p > .05) (see Table 1).
Table 1. Mean scores and standard deviations on the LEAP-Q, MELICET and the working memory task for the control and switching groups.
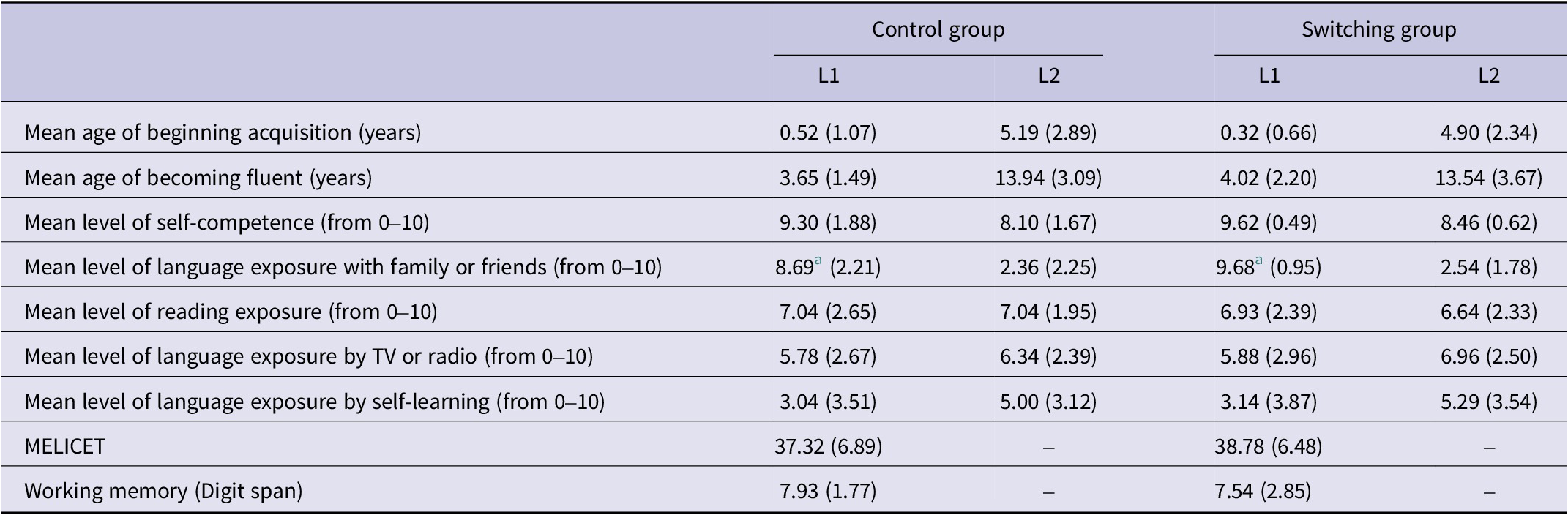
a Measures marked with an asterisk indicate significant differences between groups (p < .05).
Psychology students received course credit, while the remaining participants received €18 for their participation. All participants provided written informed consent.
2.2. Procedure
The experiment consisted of a two-hour session with two phases. Initially, participants completed the LEAP-Q on paper. In the first phase, the participants in the switching group engaged in language-switching practice for approximately 20 minutes. Importantly, prior research has demonstrated that goal-directed tasks can potentially enhance abilities related to cognitive control for subsequent cognitive processes (Gratton et al., Reference Gratton, Coles and Donchin1992; Ullsperger et al., Reference Ullsperger, Bylsma and Botvinick2005; Wu & Thierry, Reference Wu and Thierry2013). In the second phase, both groups of participants performed the PM task while their brain activity was recorded via electroencephalogram (EEG) was recorded. Note that, following established methodologies in the field (Chen et al., Reference Chen, Ma, Zhang, Li, Zhang, Yuan and Guo2021; Liu et al., Reference Liu, Yang, Jiao, Schwieter, Sun and Wang2019b; Zhang et al., Reference Zhang, Kang, Wu, Ma and Guo2015), the control group directly engaged in the PM task without completing the first phase involving language switching. Finally, all participants completed the MELICET and the digit span at the end of the session. All tasks were conducted in well-lit, individual rooms isolated from external noise.
2.3. Tasks
2.3.1. Language-switching task
A cued picture-naming task was used to practice forced language switching. The entire task lasted approximately 20 minutes. The participants in the experimental group named the pictures either in their L1 (Spanish) or L2 (English), according to the frame color of the pictures. Line drawings were selected from the database of Snodgrass and Vanderwart (Reference Snodgrass and Vanderwart1980). A total of 150 pictures were used in the formal practice session, with an additional eleven pictures used in a prior familiarization phase. The training session consisted of two blocks with a break in the middle, each containing 150 pictures. In each block, there were 75 switching trials and 75 non-switching trials. Half of the trials were in L1 (Spanish) and the other half in L2 (English). Each trial began with a fixation point appearing in the middle of the screen for 250 ms. Thereafter, a picture surrounded by a blue or red frame appeared at the center of the screen until a response was given, or for a maximum of 4000 ms. The correspondence between the color of the frame and language was counterbalanced across blocks and participants.
2.3.2. PM task
Participants performed a PM task while EEG brain activity was recorded. We employed an adaptation of the PM task used by West and Bowry (Reference West and Bowry2005). The task consisted of a main task (ongoing activity) that might be interrupted when a PM cue appeared. Specifically, during the ongoing task, colored letters appeared for a 2-back task. To avoid any possible effect of the type of item, for half of the participants, the ongoing task was pressing the “yes” key when the letter presented on the screen matched with the letter appearing two trials before, and the “no” key in all other cases. For the other half, the ongoing task was pressing “yes” when the color of the presented stimulus matched the color that appeared two trials before and pressing the “no” key in all other cases. There was a first block in which participants carried out this ongoing task as a baseline. Importantly, after this baseline block, there was a block where participants had to perform the ongoing task, but, in addition, they were asked to implement the prospective intention. For each participant, the instructions for the prospective task consisted of pressing a different key when the screen presented a given letter or color. Thus, for half of the trials, participants were told that the PM cues were the letters D, H, L and S, and they should press the keys 1, 2, 9 or 0, respectively, when one of these cues appeared. For the other half of the trials, they were instructed to press the 1, 2, 0 and 9 keys when the colors magenta, grey, lime and blue appeared. The order of these two prospective task instructions and the baseline block was counterbalanced across participants. Although there was no specific break between blocks, the experimenter entered the room between them to ensure participants understood the instructions and to clarify any doubts.
Henceforth, trials where the prospective cues were presented will be referred to as “PM” trials because they correspond to the PM task. The remaining trials that did not contain the PM cue, and in which participants performed the ongoing activity will be referred to as “ON” trials. The baseline block consisted of 300 trials where participants were instructed to respond “yes” to 35% of the stimuli and “no” to the remaining 65%. The PM block consisted of 600 trials, where 536 trials corresponded to the ongoing task (ON trials) and 64 trials contained the prospective cues to perform the intention (PM trials). Before these blocks, a practice phase of 20 trials was carried out.
The stimuli were 10 consonants (B, D, F, H, K, L, N, S, V, Z) presented in the red, blue, lime, magenta, yellow, gray, black, maroon, purple, and cyan colors with a 15 mm × 10 mm size. Participants were shown examples of all the colors during the instruction phase, and it was confirmed that they could clearly distinguish them before starting the task. Each trial consisted of the stimulus presentation (centered for 2000 ms) where participants gave a response, and it was followed by a blank screen (1500 ms). The tasks described in this section were programmed using the E-Prime 2.0 software.
2.3.3. EEG recording and pre-processing
We used a Neuroscan Synamps2 (El Paso, TX) system to collect EEG data with the Curry acquisition software (version 7; compumedicsneuroscan.com) and 64 Ag/AgCl electrodes distributed on the scalp. The data processing was performed with EEGLAB 14.1 (Delorme & Makeig, Reference Delorme and Makeig2004), running in a MATLAB environment (version 7.4.0, MathWorks, Natick, MA, USA).
During recording, two pairs of bipolar electrodes were placed vertically and horizontally to record eye movements. The EEG analog signal was amplified and digitized at a sampling frequency of 1000 Hz. The impedance of the electrodes were maintained at <10 kΩ. The ground electrode was placed along the midline in front of the Fz position. The EEG data were bandpass filtered between 0.5 and 1000 Hz during online recording. A high-pass filter of 0.1 Hz and a low-pass filter of 30 Hz were applied offline to the data. Moreover, we applied a notch filter of 50 Hz to remove the external electronic noise in the signal.
Offline EEG preprocessing was performed in the following steps: first, all electrodes were referenced to the average of both mastoids, and a high-pass filter of 0.1 Hz and a low-pass filter of 30 Hz were applied to the data; second, artifacts were removed through visual inspection, identifying channels with a high level of artifacts, which were then interpolated from adjacent electrodes; third, artifact correction was performed using the Independent Component Analysis (ICA) toolbox in EEGLAB for semi-automatic artifact removal; and fourth, epoch rejection was conducted with a cutoff of ±100 μV (<25% per participant).
The average number of interpolated channels was 3.02, and the percentage of rejected epochs after ICA was 3.72%. The temporal windows were aligned to the onset of the ongoing and PM stimuli. The time windows for the ERP analysis included a 200-ms pre-stimulus period used for baseline correction and 1200 ms of post-stimulus activity.
2.4. Design
The experiment followed a 2×2 mixed factorial design using group (switching group and control group) as between-subject factor and type of trial (ongoing, PM) or prospective load (baseline, with PM) as within-subject factors.
3. Results
First, we report the analyses performed on the behavioral data (accuracy and response times) for the language-switching task in the training condition. Second, the analyses performed on the prospective memory task (accuracy and response times) are described. This section includes (1) analyses to assess differences in cue detection and retrieval of the PM intention, and (2) analyses to assess monitoring cost in the ongoing activity. Finally, ERP data analyses are reported with a subsection for each ERP component. Behavioral analyses, including counterbalancing conditions, are included in the Supplementary Material. All statistical analyses were conducted using IBM SPSS Statistics software.
3.1. Language-switching training task
For the language-switching group, we analyzed the data from the naming task. We performed a 2 (language: L1, L2)×2 (switch/non-switch) repeated measures ANOVA on accuracy and response times (RTs). Data cleaning was performed on raw response times, removing data greater than three times the interquartile range. Also, a 200-ms cut-off was applied to remove automatic responses.
For both accuracy and RTs, we averaged participants’ correct responses to the pictures and submitted them to a 2 (language: L1 vs L2)×2 (switch trial: switch/non-switch) repeated measures ANOVA. Finally, in all analyses, Bonferroni correction for multiple comparisons in post hoc tests was applied when appropriate.
For accuracy, the analyses revealed significant main effects of language F(1,27) = 46.94; p < .0001;
![]() $ {\eta}_p^2 $
= 0.635 and type of switch F(1,27) = 28.076; p < .0001;
$ {\eta}_p^2 $
= 0.635 and type of switch F(1,27) = 28.076; p < .0001;
![]() $ {\eta}_p^2 $
= 0.510, indicating better performance in L1 (M = .96, SD = .04) compared to L2 (M = .85, SD = .10), and in non-switch trials (M = .92, SD = .10) compared to switch trials (M = .89, SD = .08). The interaction F(1,27) = 46.94; p < .05;
$ {\eta}_p^2 $
= 0.510, indicating better performance in L1 (M = .96, SD = .04) compared to L2 (M = .85, SD = .10), and in non-switch trials (M = .92, SD = .10) compared to switch trials (M = .89, SD = .08). The interaction F(1,27) = 46.94; p < .05;
![]() $ {\eta}_p^2 $
= 0.243 involving both variables was also significant, showing greater differences between switching (L2 switch: M = .82, SD = .11) and non-switching trials (L2 non-switch: M = .87, SD = .08) in L2 (t(27) = 4.848; p < .0001; d = 0.51) than in L1 (L1 switch: M = .95, SD = .04; L1 non-switch: M = .96, SD = .03 t(27) = 2.854; p < .05; d = 0.48).
$ {\eta}_p^2 $
= 0.243 involving both variables was also significant, showing greater differences between switching (L2 switch: M = .82, SD = .11) and non-switching trials (L2 non-switch: M = .87, SD = .08) in L2 (t(27) = 4.848; p < .0001; d = 0.51) than in L1 (L1 switch: M = .95, SD = .04; L1 non-switch: M = .96, SD = .03 t(27) = 2.854; p < .05; d = 0.48).
For response times, there were no significant main effects or interactions [language F(1,27) = 0.304; p = .586;
![]() $ {\eta}_p^2 $
= 0.012; type of switch F(1,27) = 0.383; p = .541;
$ {\eta}_p^2 $
= 0.012; type of switch F(1,27) = 0.383; p = .541;
![]() $ {\eta}_p^2 $
= 0.015; language by type of switch F(1,27) = 0.151; p = .701;
$ {\eta}_p^2 $
= 0.015; language by type of switch F(1,27) = 0.151; p = .701;
![]() $ {\eta}_p^2 $
= 0.006].
$ {\eta}_p^2 $
= 0.006].
Altogether, the results in the picture-naming task indicated an advantage in both switch and non-switch trials when naming in L1 compared to L2. Interestingly, that contrasts with previous results that showed impaired performance when switching into L1 from L2 (i.e., asymmetric switch cost) due to the inhibition processes needed for language control (e.g., Costa & Santesteban, Reference Costa and Santesteban2004). However, usually, this switch cost in L1 compared to L2 in a switching naming task has been reported in response times, but not in accuracy (Meuter & Allport, Reference Meuter and Allport1999). Furthermore, not all subsequent studies have reproduced asymmetric switch costs (e.g., Christoffels et al., Reference Christoffels, Firk and Schiller2007; Declerck et al., Reference Declerck, Koch and Philipp2012), prompting questions about whether these costs truly indicate language inhibition (see Declerck & Philipp, Reference Declerck and Philipp2015, for a review).
3.2. Prospective memory (PM) task
We have organized the behavioral results for the PM tasks into two main sections. First, we include the analysis regarding the PM cue detection and the retrieval of the prospective intention, and second, the analysis focusing on the cost of PM monitoring in the ongoing task. Within each section, we include analyses for accuracy and RTs. Previously, data trimming was performed by filtering the data following the criteria used by López-Rojas et al. (Reference López-Rojas, Rossi, Marful and Bajo2022); that is, RTs faster than 200 ms were removed. Also, we looked for outlier participants by checking for mean accuracy values greater than three times the interquartile range in the ON task, although we did not have to remove any data for the analysis as a result.
3.2.1. PM cue detection and retrieval of the intention
For these analyses, we compared trials where the PM cue appeared (PM trials) with trials where participants performed the ongoing task (ON trials). A PM response was labeled as correct when the participant pressed the required key upon detecting the PM cue (detection of the PM cue plus retrieval of the intention). Only correct responses to the PM cue (M = .61, SD = .16) were reported for these analyses, since erroneous responses to the PM cue (only detection) were zero. In order to reduce the interference of attentional changes during the experiment, only the ON trials that appeared just before the PM trials were selected (Cejudo et al., Reference Cejudo, López-Rojas, Gómez-Ariza and Bajo2022). For both accuracy and RTs, we averaged each type of trial (ON and PM) and group (switching and control). Thus, a 2 (type of trial: ON, PM)×2 (group: switching, control) repeated measures ANOVA was carried out (see Table 2A). The accuracy analysis showed that the main effect of type of trial F(1,46) = 152.949; p < .0001;
![]() $ {\eta}_p^2 $
= 0.769 was significant, indicating better performance in ON trials (M = .89, SD = .09) compared to PM trials (M = .61, SD = .16). However, no significant effects or interactions involving the group variable were found in this analysis [group F(1,46) = 0.503; p = .482;
$ {\eta}_p^2 $
= 0.769 was significant, indicating better performance in ON trials (M = .89, SD = .09) compared to PM trials (M = .61, SD = .16). However, no significant effects or interactions involving the group variable were found in this analysis [group F(1,46) = 0.503; p = .482;
![]() $ {\eta}_p^2 $
= 0.011; type of trial by group F(1,46) = 0.135; p = 0.715;
$ {\eta}_p^2 $
= 0.011; type of trial by group F(1,46) = 0.135; p = 0.715;
![]() $ {\eta}_p^2 $
= 0.003].
$ {\eta}_p^2 $
= 0.003].
Table 2. Mean score and standard deviations (in brackets) in behavioral data for the switching and control group in the different experimental conditions
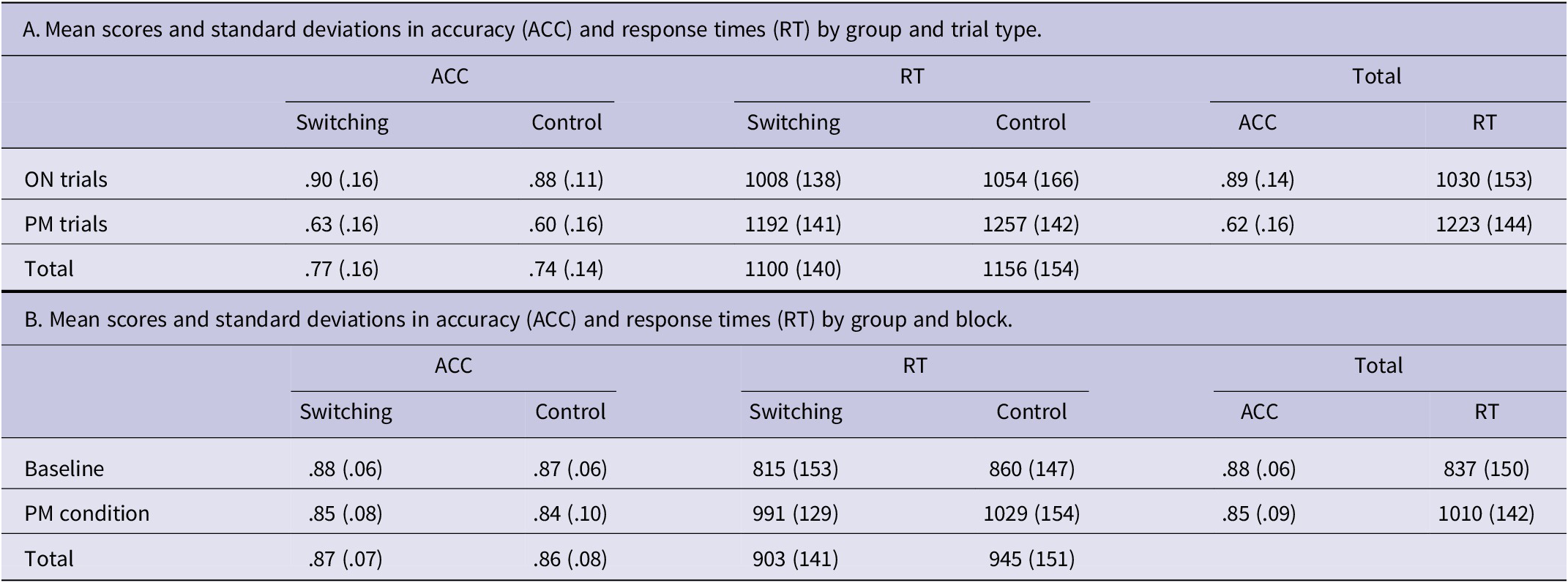
Similarly, for response times, we only found a main effect of type of trial, F(1,46) = 89.520; p < .0001;
![]() $ {\eta}_p^2 $
= 0.661, where ON trials (M = 1030, SD = 153) presented faster response times than the PM trials (M = 1223, SD = 144) [group F(1,46) = 2.230; p = .142;
$ {\eta}_p^2 $
= 0.661, where ON trials (M = 1030, SD = 153) presented faster response times than the PM trials (M = 1223, SD = 144) [group F(1,46) = 2.230; p = .142;
![]() $ {\eta}_p^2 $
= 0.046; type of trial by group F(1,46) = 0.231; p = 0.633;
$ {\eta}_p^2 $
= 0.046; type of trial by group F(1,46) = 0.231; p = 0.633;
![]() $ {\eta}_p^2 $
= 0.005]. Altogether, the behavioral results did not show an effect of the language-switching practice on the performance of the PM intention.
$ {\eta}_p^2 $
= 0.005]. Altogether, the behavioral results did not show an effect of the language-switching practice on the performance of the PM intention.
3.2.2. The cost of PM monitoring in the ongoing task
To investigate monitoring effects, we performed analyses comparing the ON trials in the ongoing baseline block with the ON trials from the block in which the PM intention was implemented. Thus, a 2 (group: switching and control) × 2 (prospective load: baseline, with PM intention) repeated measures ANOVA on accuracy and RTs in the ON trials was carried out (see Table 2B). Note that for these ANOVAs, all ON trials per condition were averaged and included in the analyses.
Results performed on the accuracy data indicated a significant main effect of prospective load, F(1,47) = 8.330; p < .05;
![]() $ {\eta}_p^2 $
= 0.151, with greater accuracy in the baseline condition (M = .88, SD = .06) compared to the PM condition (M = .85, SD = .10). Even so, in accuracy, there were no significant effects of group F(1,47) = 0.378; p = .542;
$ {\eta}_p^2 $
= 0.151, with greater accuracy in the baseline condition (M = .88, SD = .06) compared to the PM condition (M = .85, SD = .10). Even so, in accuracy, there were no significant effects of group F(1,47) = 0.378; p = .542;
![]() $ {\eta}_p^2 $
= 0.008 or interaction between the variables group and prospective load F(1,47) = 0.281; p = .599;
$ {\eta}_p^2 $
= 0.008 or interaction between the variables group and prospective load F(1,47) = 0.281; p = .599;
![]() $ {\eta}_p^2 $
= 0.006. For response times, we found the same pattern of results where the main effect of prospective load reached significance F(1,47) = 8.330; p < .0001;
$ {\eta}_p^2 $
= 0.006. For response times, we found the same pattern of results where the main effect of prospective load reached significance F(1,47) = 8.330; p < .0001;
![]() $ {\eta}_p^2 $
= 0.151, indicating faster response times in the baseline condition (M = 837, SD = 150) compared to the PM condition (M = 1010, SD = 142). No other main effects or interactions were significant [group F(1,47) = 1.183; p = .282;
$ {\eta}_p^2 $
= 0.151, indicating faster response times in the baseline condition (M = 837, SD = 150) compared to the PM condition (M = 1010, SD = 142). No other main effects or interactions were significant [group F(1,47) = 1.183; p = .282;
![]() $ {\eta}_p^2 $
= 0.025; prospective load by group F(1,47) = 0.028; p = 0.869;
$ {\eta}_p^2 $
= 0.025; prospective load by group F(1,47) = 0.028; p = 0.869;
![]() $ {\eta}_p^2 $
= 0.001].
$ {\eta}_p^2 $
= 0.001].
In sum, our data showed an impairment in the performance of the ongoing activity (accuracy and response times) when the PM intention was implemented, compared to the condition without PM intention (baseline condition).
3.3. Electrophysiological data: ERPs
To investigate the modulations associated with language-switching practice in the PM task, we compared the ERPs for hits in PM and ON trials for each group (switching vs. control group). As in the behavioral analysis, we selected the ON trials that appeared immediately before the PM trials. Thus, to study the prospective components of the PM task, we explored the N300 and frontal positivity, components that usually appear together and that have been associated with strategic monitoring processes in cue detection during a PM task. Following visual inspection and based on previous studies, we selected the time window from 200 to 400 ms to analyze both components (see also Cejudo et al., Reference Cejudo, López-Rojas, Gómez-Ariza and Bajo2022; López-Rojas et al., Reference López-Rojas, Rossi, Marful and Bajo2022; West, Reference West2011). The N300 was located over parietal-occipital electrodes (PO5, PO3, POZ, PO4, PO6, O1, OZ, O2) and the frontal positivity over electrodes in the midline frontal region (F3, F1, FZ, F2, F4, FC3, FC1, FCZ, FC2, FC4). In addition, we analyzed two other components that have been related to the retrospective memory components of PM: the P3b and the slow wave component. The P3b component is associated with working memory (WM) updating upon cue detection, and it was registered at 300–400 ms in parietal regions (P3, P1, PZ, P2, P4, PO5, PO3, POZ, PO4, PO6). Finally, to capture the frontal slow waves that have been related to monitoring and evaluation of the retrieved intention, we analyzed the mean amplitude in the time window from 500 to 1200 ms in frontal regions (F3, F1, FZ, F2, F4, FC3, FC1, FCZ, FC2, FC4). After preprocessing the EEG data, one participant was excluded due to high levels of noise in the EEG signals resulting in significant epoch rejection. Thus, data from 28 participants in the switching group and 27 in the control group were entered into the analyses.
For each component, we averaged the mean amplitudes across electrodes and conditions and submitted them to a 2 (group: switching, control) × 2 (type of trial: ON, PM) repeated measures ANOVA.
3.3.1. N300
We averaged the amplitudes per participant and submitted them to a 2 (group: switching, control) × 2 (type of trial: ON, PM) repeated measures ANOVA (see Figure 1). The main effect of type of trial (F(1,53) = 7.725; p < .05;
![]() $ {\eta}_p^2 $
= 0.127; ON trial: M = −0.091, SD = 2.80; PM trial: M = −0.421, SD = 2.76) was significant, with more negative amplitudes in the trials where the PM cue appeared compared to the ON trials. However, the main effect of group F(1,53) = 0.008; p = .930;
$ {\eta}_p^2 $
= 0.127; ON trial: M = −0.091, SD = 2.80; PM trial: M = −0.421, SD = 2.76) was significant, with more negative amplitudes in the trials where the PM cue appeared compared to the ON trials. However, the main effect of group F(1,53) = 0.008; p = .930;
![]() $ {\eta}_p^2 $
= 0.00 was not significant. Most importantly, the interaction type of trial by group F(1,53) = 8.929; p < .05;
$ {\eta}_p^2 $
= 0.00 was not significant. Most importantly, the interaction type of trial by group F(1,53) = 8.929; p < .05;
![]() $ {\eta}_p^2 $
= 0.144 was significant.Footnote
3 Analyses of this interaction showed that there were significant differences between type of trials for the switching group (ON: M = 0.113, SD = 3.030; PM: M = −0.559, SD = 2.956; t(27) = 4.311, p < .0001, d = 0.225), whereas in the control group, the differences between trials did not reach significance (ON: M = −0.301, SD = 2.587; PM: M = −0.277, SD = 2.60; t(26) = −0.140, p = .890, d = −0.009).Footnote
4
$ {\eta}_p^2 $
= 0.144 was significant.Footnote
3 Analyses of this interaction showed that there were significant differences between type of trials for the switching group (ON: M = 0.113, SD = 3.030; PM: M = −0.559, SD = 2.956; t(27) = 4.311, p < .0001, d = 0.225), whereas in the control group, the differences between trials did not reach significance (ON: M = −0.301, SD = 2.587; PM: M = −0.277, SD = 2.60; t(26) = −0.140, p = .890, d = −0.009).Footnote
4
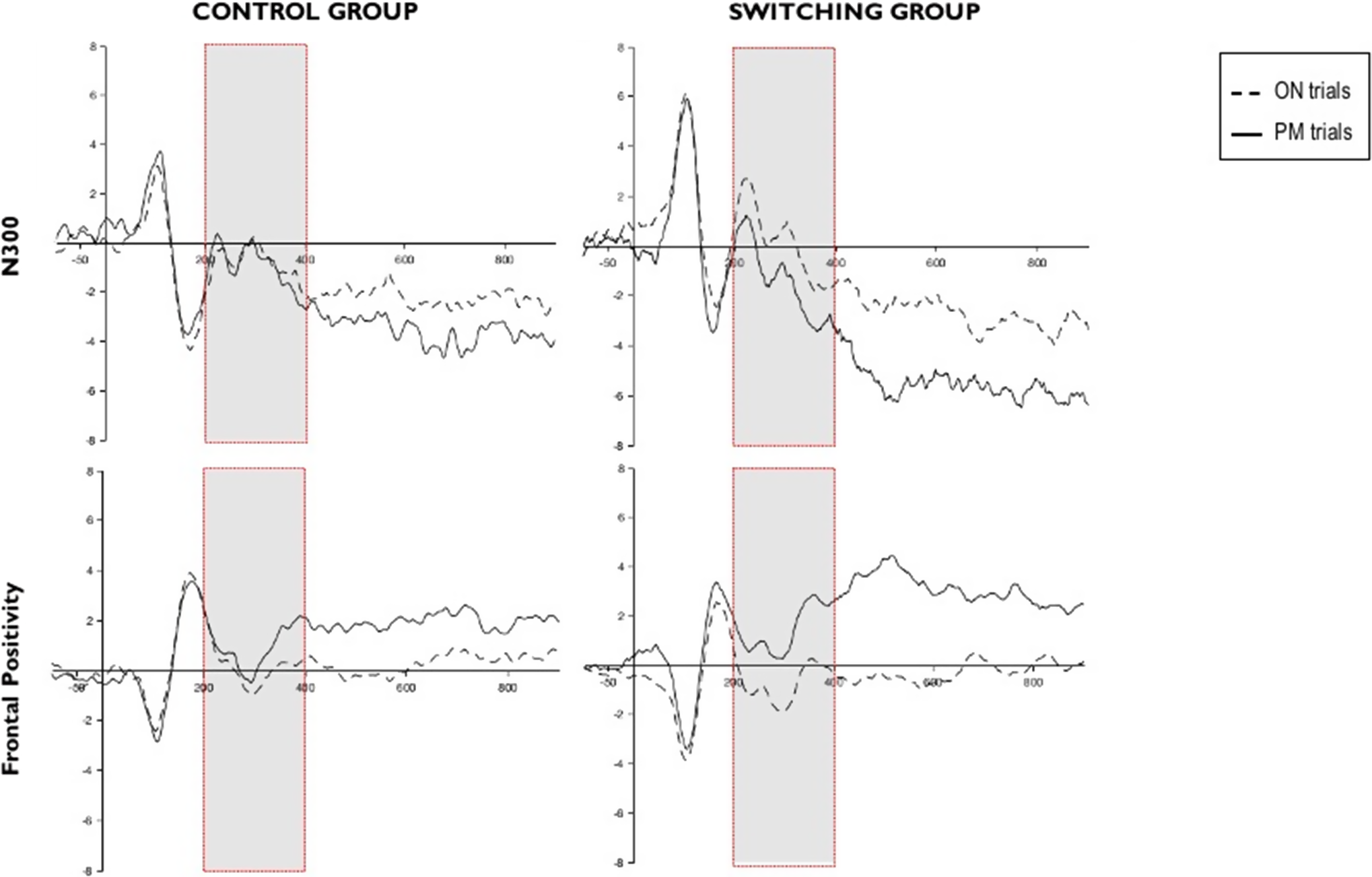
Figure 1. Grand-averaged event-related potentials at brain regions of interest for the N300 (top row) and the frontal positivity (bottom row). Dashed lines represent mean amplitudes in microvolts for ON trials, whereas solid lines represent PM trials. Time windows of interest in each component are framed in red.
3.3.2. Frontal positivity
To study this component, we performed a 2 (group: switching, control) × 2 (type of trial: ON, PM) repeated measures ANOVA (see Figure 1). The main effects of type of trial F(1,53) = 31.241; p < .0001;
![]() $ {\eta}_p^2 $
= 0.371 was significant, indicating greater wave positivity in the PM trials (M = 0.603, SD = 2.053) compared to the ON trials (M = −0.136, SD = 2.222). The main effect of group F(1,53) = 0.001; p = .973;
$ {\eta}_p^2 $
= 0.371 was significant, indicating greater wave positivity in the PM trials (M = 0.603, SD = 2.053) compared to the ON trials (M = −0.136, SD = 2.222). The main effect of group F(1,53) = 0.001; p = .973;
![]() $ {\eta}_p^2 $
= 0.00 was not statistically significant. In contrast, the interaction between the type of trial and group F(1,53) = 7.478; p < .05;
$ {\eta}_p^2 $
= 0.00 was not statistically significant. In contrast, the interaction between the type of trial and group F(1,53) = 7.478; p < .05;
![]() $ {\eta}_p^2 $
= 0.124 reached significance, indicating that the difference between trials was greater in the switching group (ON: M = −0.303, SD = 2.566; PM: M = 0.789, SD = 2.240; t(27) = 4.311, p < .0001, d = −0.445) than in the control group (ON: M = 0.037, SD = 1.830; PM: M = 0.411, SD = 1.861; t(26) = −2.179, p < .05, d = −0.199).
$ {\eta}_p^2 $
= 0.124 reached significance, indicating that the difference between trials was greater in the switching group (ON: M = −0.303, SD = 2.566; PM: M = 0.789, SD = 2.240; t(27) = 4.311, p < .0001, d = −0.445) than in the control group (ON: M = 0.037, SD = 1.830; PM: M = 0.411, SD = 1.861; t(26) = −2.179, p < .05, d = −0.199).
3.3.3. P3b
A repeated measures ANOVA with 2 (group) × 2 (type of trial) was conducted to explore the P3b component. Figure 2 shows mean amplitudes for this component. There was a significant main effect of type of trial F(1,50) = 12.258; p < .05;
![]() $ {\eta}_p^2 $
= 0.197 with ON trials (M = 1.578, SD = 2.535) showing greater positive amplitude compared to PM trials (M = 1.110, SD = 2.597). By contrast, the main effect of group F(1,50) = 0.020; p = .887;
$ {\eta}_p^2 $
= 0.197 with ON trials (M = 1.578, SD = 2.535) showing greater positive amplitude compared to PM trials (M = 1.110, SD = 2.597). By contrast, the main effect of group F(1,50) = 0.020; p = .887;
![]() $ {\eta}_p^2 $
= 0.00 and the type of trial by group F(1,50) = 0.262; p = .611;
$ {\eta}_p^2 $
= 0.00 and the type of trial by group F(1,50) = 0.262; p = .611;
![]() $ {\eta}_p^2 $
= 0.005 were not significant.
$ {\eta}_p^2 $
= 0.005 were not significant.
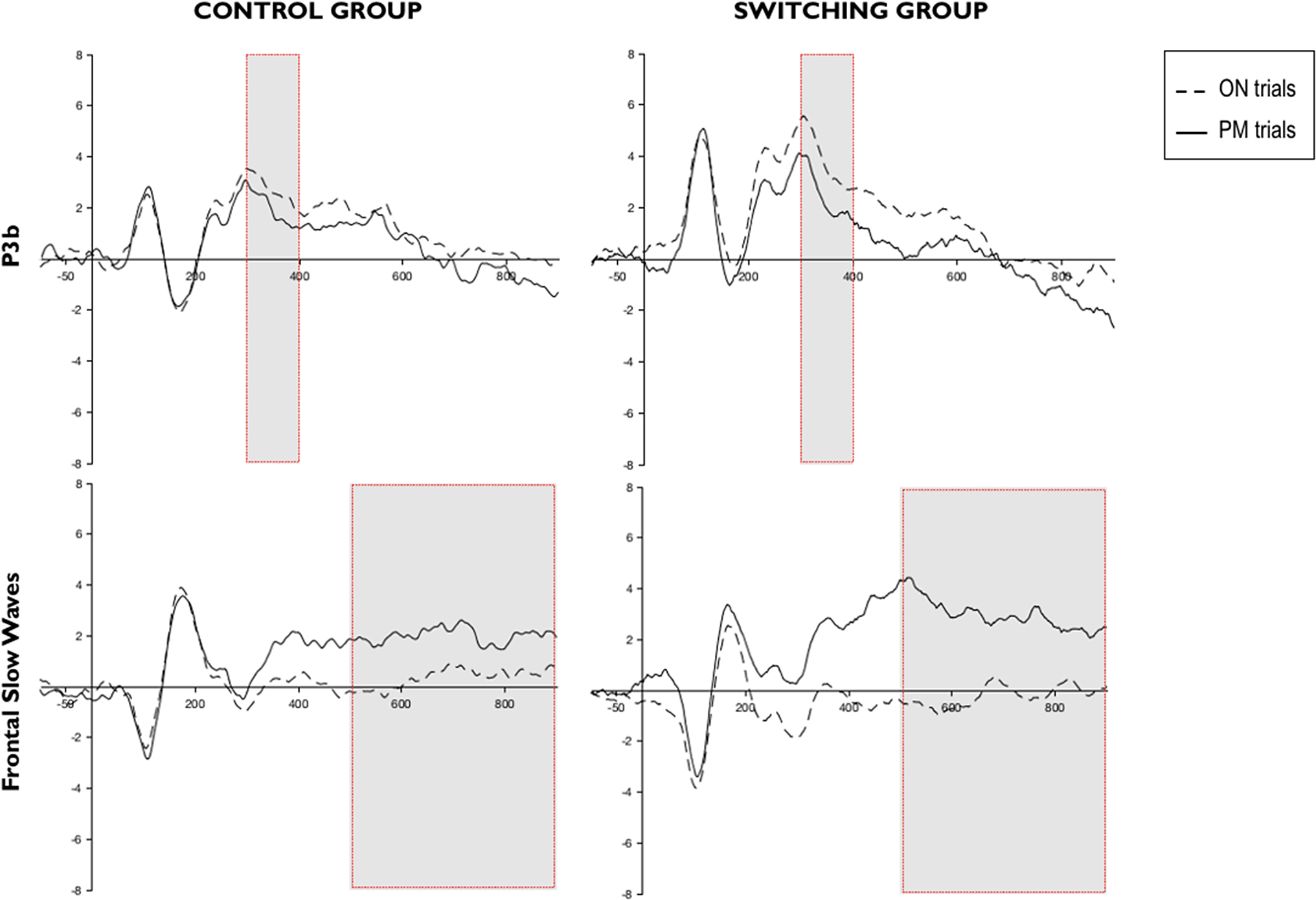
Figure 2. Grand-averaged event-related potentials at brain regions of interest for the P3b (top row) and the frontal slow waves (bottom row). Dashed lines represent mean amplitudes in microvolts for ON trials, whereas solid lines represent PM trials. Time windows of interest in each component are framed in red. While visual inspection suggests larger amplitude differences in the switching group compared to the control group, statistical analyses did not reveal a significant interaction effect.
3.3.4. Frontal slow waves
A 2 (group) × 2 (type of trial) ANOVA for repeated measures was conducted (see Figure 2 for a graphic representation of this component). The main effect of type of trial F(1,50) = 27.230; p < .0001;
![]() $ {\eta}_p^2 $
= 0.353 was significant, indicating lower positive amplitude in ON trials (M = 0.001, SD = 2.454) than in PM trials (M = 1.249, SD = 2.796). In contrast, none of the other effects and interactions reached significance [group F(1,50) = 0.113; p = .738;
$ {\eta}_p^2 $
= 0.353 was significant, indicating lower positive amplitude in ON trials (M = 0.001, SD = 2.454) than in PM trials (M = 1.249, SD = 2.796). In contrast, none of the other effects and interactions reached significance [group F(1,50) = 0.113; p = .738;
![]() $ {\eta}_p^2 $
= 0.002, type of trial by group F(1,50) =3.084; p = .085;
$ {\eta}_p^2 $
= 0.002, type of trial by group F(1,50) =3.084; p = .085;
![]() $ {\eta}_p^2 $
= 0.058].
$ {\eta}_p^2 $
= 0.058].
4. Discussion
Previous research has shown that language experience influences attention, reasoning, etc. However, little research has been directed toward understanding the effect of language experience on prospective remembering (see López-Rojas et al., Reference López-Rojas, Rossi, Marful and Bajo2022, Reference López-Rojas, Csilinkó, Bajo and Marful2023a, Reference López-Rojas, Marful, Pérez and Bajo2023b). The aim of this study was to investigate whether previous practice in language switching in Spanish–English bilinguals modulated their performance in a subsequent PM task, and if that was the case, to identify the PM processes affected by the practice. To this end, late bilingual participants carried out a language-switching task prior to the execution of a PM task, and we compared their performance with an equivalent late bilingual group without previous switching experience. During the task, we recorded brain activity with EEG, in order to qualify the nature of these changes and identify the ERP components associated with different PM processes.
Interestingly, results indicated that practicing language switching did not have evident behavioral effects on PM performance but selectively modified some ERP components associated with PM processing. The lack of effects or interactions of language-switching practice on the behavioral data occurred even though the analyses captured the usual effect of type of trial. Thus, accuracy and RTs clearly showed the usual differences between trials where the ongoing task was performed by itself (ON trials) and those where the PM cues had to be detected and the prospective intention implemented (PM trials). Higher accuracy and faster response times in the ON trials compared to the PM trials (Ballhausen et al., Reference Ballhausen, Schnitzspahn, Horn and Kliegel2017) are expected since correct performance in the PM trials requires the detection of the PM cue, interrupting the ongoing activity, shifting attention to recall the PM intention, and executing it (Kliegel et al., Reference Kliegel, Altgassen, Hering and Rose2011). Similarly, the pattern of results indicated a cost in the ongoing activity when the PM intention was implemented compared to when it was performed in isolation (Marsh et al., Reference Marsh, Hancock and Hicks2002). Again, larger accuracy and faster response times in the baseline condition compared to the condition with PM intention suggest that remembering a future intention while an ongoing activity is being carried out requires reallocating attentional resources, resulting in a performance decrease (Smith, Reference Smith2003). While the usual PM behavioral effects were evident in our data, the absence of modulation by switching practice might be explained by the nature of language switching, which primarily involves high-cognitive processes such as inhibition and monitoring (Calabria et al., Reference Calabria, Baus and Costa2019; Hartanto & Yang, Reference Hartanto and Yang2019; Struys et al., Reference Struys, Woumans, Nour, Kepinska and Van den Noort2019). These processes can engage selectively prospective neural mechanisms without necessarily leading to overt changes in accuracy or reaction times, especially in PM tasks where performance is already high or where ceiling effects may occur (Uttl, Reference Uttl2008).
In this regard, differences in the PM task due to exposure to language switching appeared in the ERP data. Similar to other studies (Grundy & Bialystok, Reference Grundy and Bialystok2018), even in the absence of behavioral modulations, we found differences between the control and switching groups in the ERP analyses. Most importantly, these modulations appeared only in the ERP components associated with prospective processing (i.e., N300 and frontal positivity), whereas no modulations were found in the retrospective components (i.e., P3b and frontal slow waves).
Thereby, we found a larger N300 amplitude for the PM trials than for the ongoing trials (West, Reference West2011), indicating the engagement of detection processes when the PM cue appeared. Similarly, we found a general effect of the type of trial in the frontal positivity component, with more positive wave amplitudes for the PM trials than for the ongoing trials. More importantly, analyses indicated that, for both the N300 and the frontal positivity, participants in the switching group showed greater negative and positive amplitudes, respectively, in the PM trials compared to the ongoing trials. The larger significant differences between PM and ON trials in the language-switching groups in these ERP components and the lack of significant differences in the control group for the N300 suggest that the two groups differ in the degree to which they engage monitoring resources during the PM task. It is important to interpret these effects as being specific to the cognitive demands of language switching, rather than as general consequences of engaging in any task prior to the PM task. This may explain, for example, the lack of group differences in behavioral results, as the brief language-switching practice may not be sensitive enough to affect performance in the nonlinguistic PM task, but does affect neural data.
Altogether, the pattern of results in the prospective components indicates that a short practice in language switching has neural consequences in the strategic monitoring processes involved in the detection of PM cues in the environment. These results agree with the findings by López-Rojas et al. (Reference López-Rojas, Rossi, Marful and Bajo2022), which indicated that bilinguals immersed in a context with frequent switching between languages showed greater N300 compared to bilinguals and monolinguals from a single-language context. Thus, similarly, the N300 component was not evident in our single-context bilinguals in the present study but emerged in the same type of bilinguals after language-switching practice. An intriguing result is the absence of differences between PM and ON in the N300 component within the control group. Notice, however, that the N300 has not always been detected in some PM studies (López-Rojas, et al., Reference López-Rojas, Csilinkó, Bajo and Marful2023a; Wang et al., Reference Wang, Cao, Cui, Shum and Chan2013; West, Reference West2011; West et al., Reference West, Bowry and Krompinger2006; Wilson et al., Reference Wilson, Cutmore, Wang, Chan and Shum2013), highlighting the elusive character of this component and its susceptibility to factors such as the nature of the PM cues or the type of PM task being performed (Cousens et al., Reference Cousens, Cutmore, Wang, Wilson, Chan and Shum2015). Similarly, the effect of the practice group in the frontal positivity component resembles the data of previous studies with tasks that involved language switching (Kuipers & Thierry, Reference Kuipers and Thierry2010). For example, Beatty-Martínez and Dussias (Reference Beatty-Martínez and Dussias2017) found that non-code-switching bilinguals had an enhancement in frontal positivity during code-switching processing relative to unilingual processing. Also, Kaan et al. (Reference Kaan, Kheder, Kreidler, Tomic and Valdes Kroff2020) reported evidence that, in the presence of monolinguals, bilinguals showed greater frontal positivity when a trial with unexpected language switching appeared compared to non-switch trials, indicating the role of this component as a marker of language control in interactional contexts (Beatty-Martínez & Titone, Reference Beatty-Martínez and Titone2021).
Furthermore, our data evidence the transfer of processes from a pure language control task (e.g., naming task) to a more domain-general task (e.g., PM task). Hence, results in the N300 and frontal positivity suggested greater engagement of monitoring and switching processes by the switching group to complete the task. In contrast, the retrospective components (i.e., P3b and frontal slow waves) did not show between-group differences, suggesting that, unlike the prospective components, training in language switching did not affect the memory updating processes involved in PM.
Similar to other previous studies (West, Reference West2011), analyses of the P3b component showed differences between types of trials. Nevertheless, we found greater positivity in the ON trials than in the PM trials, which is different from the more positive amplitude for PM trials relative to ON trials found in other PM studies (West et al., Reference West, Wymbs, Jakubek and Herndon2003). However, our pattern of results is in agreement with López-Rojas et al. (Reference López-Rojas, Rossi, Marful and Bajo2022), where bilinguals with frequent language-switching experience, and bilinguals and monolinguals from a single-language context showed greater P3b wave positivity during the ongoing activity compared to the PM trials. They argued that the different pattern may be related to the monitoring requirements imposed by the task, so that in difficult monitoring conditions participants might engage to a greater extent in working memory and updating processes during the ongoing activity to overcome the monitoring cost associated with retaining and recalling the PM intention from memory. In line with this idea, previous studies showed that increasing the working memory load of the ongoing activity resulted in a reduced P3b in the PM trials (West & Bowry, Reference West and Bowry2005; West et al., Reference West, Bowry and Krompinger2006). Thus, it might be possible that the nature of the ongoing activity in the present study (i.e., an N-back) resulted in a highly demanding working memory condition during the ongoing task that reversed the wave amplitudes in the P3b.
Similarly, the frontal slow waves component (more positive amplitudes in the PM trials compared to the ongoing trials), that was also independent of the between-groups manipulation, was observed. This component indicated the presence of retrieval monitoring processes when a PM cue is detected (Rösler et al., Reference Rösler, Heil and Glowalla1993; West et al., Reference West, Wymbs, Jakubek and Herndon2003). However, the frontal slow waves component has been demonstrated to be sensitive to the retrieval demands of the PM task. Thus, previous studies indicated greater frontal slow waves in more-demanding PM conditions, reflecting the engagement of a more effortful retrospective retrieval (Cona et al., Reference Cona, Bisiacchi and Moscovitch2014). Hence, the absence of differences between groups in our study suggested that participants were similarly engaged in the retrieval processes needed to recall the future intention.
This pattern of results is important because it identifies and dissociates the PM processes influenced by language-switching experience. Whereas language-switching practice influences the prospective components of PM (i.e., N300 and frontal positivity), no modulations were found in the retrospective components (P3b and frontal slow waves). In addition, this dissociation is theoretically consistent, because language switching involves context monitoring and cue detection (Declerck & Philipp, Reference Declerck and Philipp2015; Macizo et al., Reference Macizo, Bajo and Paolieri2012), processes that have also been proposed as involved in PM tasks (Ballhausen et al., Reference Ballhausen, Schnitzspahn, Horn and Kliegel2017; Scullin et al., Reference Scullin, Mullet, Einstein and McDaniel2015). These processes suggest overlapping demands between language switching and PM tasks in terms of monitoring and attentional control, but not in terms of memory.
However, language switching is primarily associated with mechanisms like inhibition (Calabria et al., Reference Calabria, Baus and Costa2019; Green & Abutalebi, Reference Green and Abutalebi2013) and domain-general monitoring (Hartanto & Yang, Reference Hartanto and Yang2019; Struys et al., Reference Struys, Woumans, Nour, Kepinska and Van den Noort2019), rather than relying on memory retrieval processes. Although memory functions such as storage, updating, recognition, or recall may play a supporting role, they are not central to the cognitive mechanisms underlying language switching. This distinction is reflected in our findings, where language training practice modulated ERP components associated with prospective processing but did not engage components linked to the retrieval of intentions from long-term memory. The specific schema retrieved upon cue detection plays a crucial role in modulating retrospective processes. Future studies should focus on these retrospective processes by incorporating, for example, linguistically complex stimuli to retrieve when presented the PM cue. This manipulation will allow the PM task schema to align more closely with language switching, enabling a better examination of shared cognitive mechanisms. Interestingly, the fact that language-switching practice had no effect at a behavioral level but did so at a neural level points to the high sensitivity of EEG to explore fine-grained neurocognitive processes. A number of previous studies have also shown language-related brain differences without evident behavioral effects in bilinguals (Ansaldo et al., Reference Ansaldo, Ghazi-Saidi and Adrover-Roig2015; DeLuca et al., Reference DeLuca, Segaert, Mazaheri and Krott2020; Luk et al., Reference Luk, Anderson, Craik, Grady and Bialystok2010; Rodríguez-Pujadas et al., Reference Rodríguez-Pujadas, Sanjuán, Ventura-Campos, Román, Martin, Barceló, Costa and Ávila2013). The lack of behavioral effects has been suggested as advantageous, since it rules out interpretations due to possible confounds emerging from differences in performance and favors interpretations based on the functional neural modulations of language experience (Grundy et al., Reference Grundy, Anderson and Bialystok2017; Luk et al., Reference Luk, Anderson, Craik, Grady and Bialystok2010; for an opposite argument, see de Bruin et al., Reference De Bruin, Dick and Carreiras2021).
In fact, previous neuroimaging studies on language-switching training have highlighted how different bilingual experiences can produce distinct neurocognitive adaptations (for a recent framework, see DeLuca et al., Reference DeLuca, Segaert, Mazaheri and Krott2020). For example, Kang et al. (Reference Kang, Fu, Wu, Ma, Lu and Guo2017) found that after a short-term language-switching training, bilinguals showed reduced activation in language control brain areas such as the anterior cingulate cortex and the caudate. Moreover, these changes correlated with a reduction in switching costs, indicating a benefit in general conflict monitoring processes engaged in the language-switching task. Similarly, previous studies have shown how bilingual language switching modified the activation in the anterior cingulate, making the control of cognitive conflict more efficient (Abutalebi et al., Reference Abutalebi, Della Rosa, xGreen, Hernandez, Scifo, Keim, Kappa and Costa2012). Hence, due to the significant role of the anterior cingulate cortex in the top-down control processes elicited when a PM cue appears (Botvinick et al., Reference Botvinick, Braver, Barch, Carter and Cohen2001; Cona et al., Reference Cona, Scarpazza, Sartori, Moscovitch and Bisiacchi2015), we suggest that the language-switching practice in our bilingual participants could affect the activation in this area, modulating the strategic monitoring processes involved during the PM task. Further neuroimaging studies should address how variations in the bilingual experience differently modulate the brain regions associated with the prospective and retrospective processes engaged in a PM task (for a meta-analysis, see Cona et al., Reference Cona, Scarpazza, Sartori, Moscovitch and Bisiacchi2015).
Additionally, these findings are in line with a wide body of literature that explores the influence of the interactional context in which bilinguals are immersed on different cognitive outcomes (Beatty-Martínez et al., Reference Beatty-Martínez, Navarro-Torres, Dussias, Bajo, Guzzardo Tamargo and Kroll2020; Hartanto & Yang, Reference Hartanto and Yang2016; Hofweber et al., Reference Hofweber, Marinis and Treffers-Daller2016). From this perspective, studying the cognitive effects of individual differences in the bilingual experience is a promising line of research. Importantly, the pattern of results supports previous studies indicating that language-switching training could impact general cognition beyond pure linguistic tasks (Chen et al., Reference Chen, Ma, Zhang, Li, Zhang, Yuan and Guo2021; Liu et al., Reference Liu, Yang, Jiao, Schwieter, Sun and Wang2019b; Timmer et al., Reference Timmer, Calabria and Costa2019; Zhang et al., Reference Zhang, Kang, Wu, Ma and Guo2015).
Finally, an aspect warranting nuanced consideration is the duration and nature of the language-switching practice employed in this study. The observed effects on relevant ERP processes following the brief practice period on language switching raise questions about whether these effects are inherently tied to bilingualism. First, the use of a passive control group raises the possibility that the practice undertaken by the switching group may have induced heightened general arousal and alertness or enhanced working memory fluency, potentially influencing the observed ERP correlates associated with PM. Second, it could also be argued that similar results could have been observed with a nonlinguistic switching task. Although these arguments have some merit, it is important to note that previous studies investigating the effects of language-switching practice on cognitive abilities have often employed similar methodologies (Chen et al., Reference Chen, Ma, Zhang, Li, Zhang, Yuan and Guo2021; Liu et al., Reference Liu, Yang, Jiao, Schwieter, Sun and Wang2019b; Zhang et al., Reference Zhang, Kang, Wu, Ma and Guo2015). Additionally, Au et al. (Reference Au, Buschkuehl and Jaeggi2020) conducted a meta-analysis focusing on quantifying the difference between active and passive control groups in cognitive interventions, suggesting no meaningful performance difference between the control groups. In addition, our findings replicate previous PM bilingual studies. Specifically, similar ERP modulations (greater amplitude differences between the ongoing and PM trials in the N300 component) have been found for bilinguals immersed in dual-language contexts compared to bilinguals from single-language contexts (López-Rojas et al., Reference López-Rojas, Rossi, Marful and Bajo2022). Hence, the significance of our study lies in its experimental approach, adding evidence to previous data suggesting an impact of prior language-switching practice on PM. However, further studies should be conducted to examine these issues in greater depth.
In sum, this study provides evidence of the immediate impact of language switching on the recall of future intentions. Therefore, we suggest that different patterns of L2 use and exposure could modulate the cognitive processes underlying prospective processing. Additionally, these data agree with previous studies exploring the effect of language-switching practice on cognitive processes such as monitoring or inhibition (Liu et al., Reference Liu, Yang, Jiao, Schwieter, Sun and Wang2019b; Timmer et al., Reference Timmer, Calabria and Costa2019; Zhang et al., Reference Zhang, Kang, Wu, Ma and Guo2015). Future research should explore in greater depth how PM can be modulated by factors related to language switching, such as immersion in different interactional contexts (Kroll et al., Reference Kroll, Dussias and Bajo2018), use of code-switching (Tomić & Valdés Kroff, Reference Tomić and Valdés Kroff2022) or language entropy (Gullifer & Titone, Reference Gullifer and Titone2020).
5. Conclusion
Practice in language switching has a notable effect on the prospective neural correlates of prospective memory. These data show the power of language to modulate cognitive processes such as attention, perception, or long-term memory (Arndt & Beato, Reference Arndt and Beato2017; Bialystok et al., Reference Bialystok, Dey, Sullivan and Sommers2020; Chabal & Marian, Reference Chabal and Marian2015; D’Souza et al., Reference D’Souza, Brady, Haensel and D’Souza2021; Del Maschio et al., Reference Del Maschio, Crespi, Peressotti, Abutalebi and Sulpizio2022) and support previous findings about the role of the interactional context in which bilinguals are immersed in shaping cognitive control (Gullifer et al., Reference Gullifer, Chai, Whitford, Pivneva, Baum, Klein and Titone2018; Hartanto & Yang, Reference Hartanto and Yang2020; Khodos et al., Reference Khodos, Moskovsky and Paolini2021).
Supplementary material
The supplementary material for this article can be found at http://doi.org/10.1017/S1366728925100266.
Data availability statement
The datasets and materials supporting the findings of this study are openly available in the Open Science Framework (OSF) repository at https://osf.io/xkrv9/?view_only=434940d900bf43888dac4581825572c4.
Funding statement
This research was supported by the doctoral research grant FPU17/03378 and by the HORIZON-MSCA-2023-PF-01 program (grant: 101150333 – ToDo-Brain) to Cristina López-Rojas; by grants from the Spanish Ministry of Economy and Competitiveness to Alejandra Marful and to Teresa Bajo (A.M. and T.B: PID2021-127728NB-I00; T.B.: PGC2018-093786-B-I00 30B51801); and by the Junta de Andalucía (A-CTS-111-UGR18 / B-CTS-384-UGR20 / P20_00107) to Teresa Bajo Funding for open access charge was provided by the Universidad de Granada. Additional funding was provided by the Mind, Brain and Behavior Research Center (CIMCYC), University of Granada, through CEX2023-001312-M / AEI/10.13039/501100011033 and UCE-PP2023-11 / UGR.






