Highlights
-
• Bilinguals show different neural responses during emotional reading in L1 versus L2.
-
• Fearful texts elicit stronger activation of the left hippocampus in L1 versus L2.
-
• Lower functional coupling between semantic and limbic areas during L2 fearful text reading.
1. Introduction
Language and emotion are closely interconnected. Although language is our primary way of expressing and sharing emotions and feelings, its role goes beyond merely communicating affective states and is directly involved in how we experience and perceive emotions (Brooks et al., Reference Brooks, Shablack, Gendron, Satpute, Parrish and Lindquist2017; Lindquist, Reference Lindquist2021). Studies of affective linguistic processing have shown that emotional words have the ability to elicit emotional states and are processed differently compared to neutral words (Citron, Reference Citron2012). The salient nature of emotional words gives them a processing advantage over neutral stimuli (Kousta et al., Reference Kousta, Vinson and Vigliocco2009), hypothesized to stem from the emotional associations ascribed to the word during its acquisition (Keuper et al., Reference Keuper, Zwanzger, Nordt, Eden, Laeger, Zwitserlood, Kissler, Junghöfer and Dobel2012). This effect is evidenced by research showing that emotional words are better remembered and recalled (Jay et al., Reference Jay, Caldwell-Harris and King2008; Kensinger & Corkin, Reference Kensinger and Corkin2003), produce higher interference effect during emotional Stroop task (Algom et al., Reference Algom, Chajut and Lev2004; Ben-Haim et al., Reference Ben-Haim, Mama, Icht and Algom2014) and lead to higher autonomic activity (Gray et al., Reference Gray, Hughes and Schneider1982; Manning & Melchiori, Reference Manning and Melchiori1974). On the other hand, the current evidence shows that emotional states or contexts also influence language processes, with studies showing better acquisition of novel words under positive contexts (Frances et al., Reference Frances, de Bruin and Duñabeitia2020; Wang et al., Reference Wang, Ganushchak, Welie and van Steensel2024).
In neuroimaging studies, emotional words have been shown to produce an enhanced neural response across various brain regions, including limbic, striatal and prefrontal areas (see Citron, Reference Citron2012 for review). While substantially less evidence exists for emotional processing of more complex verbal stimuli, such as short texts, a previous fMRI (functional magnetic resonance imaging) study showed that listening to emotional passages effectively engages the affective system, resulting in higher neural activity in the limbic regions and the ventromedial prefrontal cortex (vmPFC) (Ferstl et al., Reference Ferstl, Rinck and von Cramon2005). Furthermore, reading of negative narratives has been shown to elicit activation in the left amygdala, bilateral prefrontal, temporal and occipital cortex, dorsal striatum and cerebellum (Altmann et al., Reference Altmann, Bohrn, Lubrich, Menninghaus and Jacobs2012), whereas reading of positive sentences has been related with increased activity in the ventral striatum and medial prefrontal cortex (mPFC) (Bohrn et al., Reference Bohrn, Altmann, Lubrich, Menninghaus and Jacobs2013; Kim et al., Reference Kim, Somerville, Johnstone, Polis, Alexander, Shin and Whalen2004).
Bilingual speakers represent a unique opportunity for studying the interaction between language and emotion. In unbalanced bilinguals, the reduced emotionality of the non-native language is a widely documented phenomenon that has been extensively covered in psychophysiological and neuroimaging research (Caldwell-Harris, Reference Caldwell-Harris2014; Harris et al., Reference Harris, Gleason, Ayçiçeǧi and Pavlenko2006; Jończyk, Reference Jończyk and Jończyk2016b; Pavlenko, Reference Pavlenko2012). Evidence from a range of methods, including pupillometry (Thoma & Baum, Reference Thoma and Baum2019; Toivo & Scheepers, Reference Toivo and Scheepers2019), electrodermal activity (Caldwell-Harris & Ayçiçeği-Dinn, Reference Caldwell-Harris and Ayçiçeği-Dinn2009; Harris et al., Reference Harris, Ayçiçeği and Gleason2003), event-related potentials (Jończyk et al., Reference Jończyk, Boutonnet, Musiał, Hoemann and Thierry2016; Opitz & Degner, Reference Opitz and Degner2012) and functional neuroimaging techniques (Chen et al., Reference Chen, Lin, Chen, Lu and Guo2015; Del Maschio et al., Reference Del Maschio, Sulpizio, Bellini, Del Mauro, Giannachi, Buga, Fedeli, Perani and Abutalebi2024; Hsu et al., Reference Hsu, Jacobs and Conrad2015) provides strong support for the reduced emotional resonance during second language (L2) processing. More specifically, the differential emotional response during L2 use has been implicated in various psychological processes, such as decision-making (Costa et al., Reference Costa, Vives and Corey2017), attention allocation (Fan et al., Reference Fan, Xu, Wang, Zhang, Yang and Liu2016; Winskel, Reference Winskel2013), emotion regulation (Dylman & Bjärtå, Reference Dylman and Bjärtå2019; Ortigosa-Beltrán et al., Reference Ortigosa-Beltrán, Jaén, Costumero and García-Palacios2024; Vives et al., Reference Vives, Costumero, Ávila and Costa2021) and fear acquisition (García-Palacios et al., Reference García-Palacios, Costa, Castilla, Del Río, Casaponsa and Duñabeitia2018). Furthermore, in a study by Hayakawa and Keysar (Reference Hayakawa and Keysar2018) the authors demonstrated that L2 use reduces the vividness of mental imagery, a crucial factor in emotional experiences during reading (Wicken et al., Reference Wicken, Keogh and Pearson2021) and argued that this reduction may account for the lower emotionality often observed in L2 (Hayakawa & Keysar, Reference Hayakawa and Keysar2018).
With regard to emotional reading specifically, previous research suggests that emotionally valenced linguistic stimuli affect bilinguals differently in their first language (L1) and L2. Multiple studies have demonstrated a reduced emotional resonance when processing linguistic emotional content in L2 compared to L1. For example, studies measuring skin conductance responses (SCR) have consistently shown lower electrodermal activity in response to taboo words and emotional phrases in L2 compared to L1, indicating a diminished autonomic response (Caldwell-Harris & Ayçiçeği-Dinn, Reference Caldwell-Harris and Ayçiçeği-Dinn2009; Harris et al., Reference Harris, Ayçiçeği and Gleason2003). Similarly, Jankowiak and Korpal (Reference Jankowiak and Korpal2018) found reduced SCR during the reading of emotional narratives in L2, further supporting the idea of attenuated physiological arousal in the non-native language. In addition to SCR findings, research using pupillometry has corroborated these results, reporting smaller pupil dilation during the reading of emotional sentences in L2, suggesting reduced emotional engagement (Iacozza et al., Reference Iacozza, Costa and Duñabeitia2017). More recently, Thoma et al. (Reference Thoma, Hüsam and Wielscher2023) replicated these findings, showing that bilinguals exhibit heightened emotional reactivity to emotional content in L1 versus L2, as evidenced not only by differences in pupil size but also by the strength of visceral responses measured through grip force. The reduced emotionality of L2 was also highlighted in the study by Woumans et al. (Reference Woumans, Van der Cruyssen and Duyck2020) where Dutch–English bilinguals perceived crime scenarios as less severe when reading them in L2 versus L1. These findings align with the results of Segalowitz et al. (Reference Segalowitz, Trofimovich, Gatbonton and Sokolovskaya2008), who used the Implicit Affect Association Task and found that although L2 words are recognized with similar efficiency to L1 words, their affective valence is processed less automatically in L2. Electroencephalography (EEG) studies of emotional word processing in bilinguals also suggest that the affective valence of L2 words is processed in a less immediate way due to delayed lexical access (Chen et al., Reference Chen, Lin, Chen, Lu and Guo2015; Opitz & Degner, Reference Opitz and Degner2012).
Neuroimaging research of emotional reading in bilinguals remains limited, representing an important gap in explaining the neurobiological bases of this phenomenon. The study by Chen et al. (Reference Chen, Lin, Chen, Lu and Guo2015) used a combined EEG and fMRI modality to examine differences in neural activity in a group of late unbalanced Chinese–English bilinguals when processing positive, neutral and negative words. The fMRI results showed reduced activation for L1 emotional words in the left middle occipital gyrus and the left cerebellum, which was interpreted as less effortful processing in L1. Furthermore, the authors found increased activation in the left cerebellum for L2 emotional words, which was interpreted as a possible enhancement of semantic processing to facilitate the integration and retrieval of L2 emotional words. However, no significant L1/L2 differences were observed in the activity of brain regions directly associated with emotional processing during text comprehension, such as the limbic system or mPFC (Altmann et al., Reference Altmann, Bohrn, Lubrich, Menninghaus and Jacobs2012; Ferstl et al., Reference Ferstl, Rinck and von Cramon2005). Another study used the same paradigm to investigate language differences in the functional connectivity of the brain regions associated with negative word reading (Dang et al., Reference Dang, Ma, Yuan, Fu, Chen, Zhang, Lu and Guo2023). The results showed that L1 negative word reading involved two routes of processing: a dorsal route from the inferior frontal gyrus to the medial frontal cortex and posterior cingulate, and a ventral route from the inferior frontal gyrus to the amygdala, inferior temporal gyrus and thalamus. L2 negative word reading showed a similar connectivity pattern; however, it showed an extra connection between the medial frontal cortex and thalamus in the dorsal route and a lack of connectivity between the inferior frontal gyrus and amygdala in the ventral route. The authors interpreted these findings as evidence in favor of neural assimilation and accommodation, where the L1 network is partially utilized during negative word reading in L2. Finally, a study conducted by Hsu et al. (Reference Hsu, Jacobs and Conrad2015) investigated differences in neural activity in a group of late German–English proficient bilinguals when reading short negative, positive and neutral passages from Harry Potter books. The results revealed stronger neural responses to the happy versus neutral condition in the bilateral amygdala and the left precentral cortex that were restricted to L1 reading. Thus, these results support the L1 emotional advantage hypothesis, suggesting that reading emotion-laden texts in the native language provides a stronger and more differentiated emotional experience than reading in a second language. Despite this evidence, the available literature examining the neural pathways involved in emotional reading in a non-native language remains limited, and new empirical data are necessary to draw definitive conclusions. Furthermore, previous research has primarily focused on comparing neural activity between L1 and L2 during emotional linguistic processing, without considering these differences from a connectivity perspective to understand the functional mechanisms behind them.
However, it is important to note that the L2 effect on emotional resonance is not always present and that L2 emotionality can approach that of the native language in specific contexts and given sufficient immersion and L2 use (Brouwer, Reference Brouwer2019; Degner et al., Reference Degner, Doycheva and Wentura2012; Eilola et al., Reference Eilola, Havelka and Sharma2007; Sutton et al., Reference Sutton, Altarriba, Gianico and Basnight-Brown2007). Nonetheless, the majority of studies conducted with unbalanced bilinguals provide consistent evidence that emotional words and sentences have less emotional impact when processed in L2, giving L1 an advantage when processing affective stimuli (Caldwell-Harris, Reference Caldwell-Harris2014). This phenomenon is a crucial aspect of understanding everyday interactions in L2, as bilinguals might comprehend the message but fail to interpret its emotional and social cues.
Several theories have been proposed to explain the reduced emotionality in L2 and how this effect may be modulated by bilingual experience–based factors. While some of them emphasize the role of early acquisition (Pavlenko, Reference Pavlenko2012) or the context in which the language was acquired and used (Harris et al., Reference Harris, Gleason, Ayçiçeǧi and Pavlenko2006), others place emphasis on the frequency of use (Puntoni et al., Reference Puntoni, De Langhe and Van Osselaer2009). Despite differences in the proposed causal factors, it appears that all of them point to the fact that affective and semantic representations are less grounded in L2 due to limited experience with the language, particularly in highly emotional contexts, resulting in a less efficient coupling between the semantic and affective representations in L2 and decreased emotionality and embodiment of the non-native language (Jończyk, Reference Jończyk and Jończyk2016a; Pavlenko, Reference Pavlenko2012; Sheikh & Titone, Reference Sheikh and Titone2016). A crucial region for semantic processing is the anterior temporal lobe (ATL) (Visser et al., Reference Visser, Jefferies and Lambon Ralph2010). The ATL has been proposed as a multimodal semantic hub responsible for integrating conceptual knowledge from modality-specific regions (Patterson et al., Reference Patterson, Nestor and Rogers2007). Recent neurobiological models suggest that the ATL’s semantic function is graded according to its long-range cortical connectivity, with the most anterior and dorsal part being involved in social/emotional representations (Ralph et al., Reference Ralph, Jefferies, Patterson and Rogers2017). In agreement with this view, previous evidence has shown that left temporal pole activity associated with reading meaningful sentences is biased toward social/emotional content, as compared to social content alone or nonsocial (i.e., object) content (Mellem et al., Reference Mellem, Jasmin, Peng and Martin2016). Furthermore, the anterior part of the right superior temporal gyrus is recruited during social emotional processing in a context-independent manner (Zahn et al., Reference Zahn, Moll, Paiva, Garrido, Krueger, Huey and Grafman2009); however, its functional connectivity with specific sentiment-related areas increases during the processing of particular sentiments (Green et al., Reference Green, Ralph, Moll, Stamatakis, Grafman and Zahn2010). Previous evidence has shown that the ATL encodes semantic information irrespective of the language used in bilinguals (Correia et al., Reference Correia, Formisano, Valente, Hausfeld, Jansma and Bonte2014). However, the results of a recent fMRI study suggest that ATL functional connectivity is reduced during L2 processing (Zhang et al., Reference Zhang, Yang, Wang and Li2020). This study compared the neural correlates for L1 and L2 in monolingual native English speakers and late unbalanced Chinese–English bilinguals during a semantic proximity task involving triads of English words. They observed that L1 processing engaged an integrated brain network connecting left ATL with language and sensorimotor areas, but L2 processing failed to show engagement between the left ATL and sensorimotor regions, suggesting that, at least in unbalanced bilinguals, L2 is represented differently from L1 in the semantic/conceptual system.
The present study aimed to investigate the neural mechanisms underlying the differences in patterns of neural activity and functional connectivity in late unbalanced bilinguals during the processing of emotional texts between L1 and L2. Thus, the main objective was twofold. First, our goal was to investigate between-language differences in neural activity during the reading of emotional texts. Based on previous research on emotional verbal processing (Ferstl et al., Reference Ferstl, Rinck and von Cramon2005; Hamann & Mao, Reference Hamann and Mao2002; Herbert et al., Reference Herbert, Ethofer, Anders, Junghofer, Wildgruber, Grodd and Kissler2009; Hsu et al., Reference Hsu, Jacobs and Conrad2015), we hypothesized to see higher activity in brain regions involved in emotional text reading, including the amygdala, mPFC, ventral striatum, inferior parietal lobe, and occipito-temporal cortex during L1 emotional reading. Second, in order to elucidate the functional network underlying these differences, we examined the functional connectivity of the ATL with emotion-specific areas. Based on previous studies (Green et al., Reference Green, Ralph, Moll, Stamatakis, Grafman and Zahn2010; Zahn et al., Reference Zahn, Moll, Paiva, Garrido, Krueger, Huey and Grafman2009), we hypothesized higher connectivity between the ATL and fear-related areas during the reading of fearful texts in L1 compared to L2 and higher connectivity between the ATL and reward-related areas during the reading of happy texts in L1 compared to L2.
2. Materials and methods
2.1. Participants
Thirty-three unbalanced Spanish (L1)–English (L2) bilinguals were initially recruited for this study. Of these, 10 participants presented data quality issues: six presented excessive in-scanner motion (see image acquisition and preprocessing section), three presented low performance on post-scan comprehension/recognition tasks (accuracy below 70% in any of the post-scan tasks) and one participant exhibited both high motion and poor task performance. Given that poor behavioral performance in the post-scan tasks raises concerns about attentional engagement during the fMRI task, participants presenting this issue were excluded from all analyses. Additionally, participants with excessive head motion were excluded from the fMRI analyses. Thus, the final sample for behavioral analyses included 29 participants (15 females, mean age = 22.83, SD = 3.64), and the final sample for fMRI analyses included 23 participants (11 females, mean age = 22.30, SD = 3.39).
All participants were right-handed, had normal or corrected-to-normal vision and reported no history of traumatic head injury or neurological disorders. They were native speakers of Spanish who had acquired English in a formal educational setting, had not spent more than 12 months in an English-speaking country and had at least an intermediate level of English proficiency.
Assessment of English proficiency was carried out using the LexTALE vocabulary test, with a mean score of 69.83 (SD = 7.17), which has been related to a B2 (upper intermediate) level according to the Common European Framework (CEF) for language levels (Lemhöfer & Broersma, Reference Lemhöfer and Broersma2012). Information regarding the participants’ demographic and linguistic data can be found in Table 1.
Table 1. Demographic and linguistic information of participants
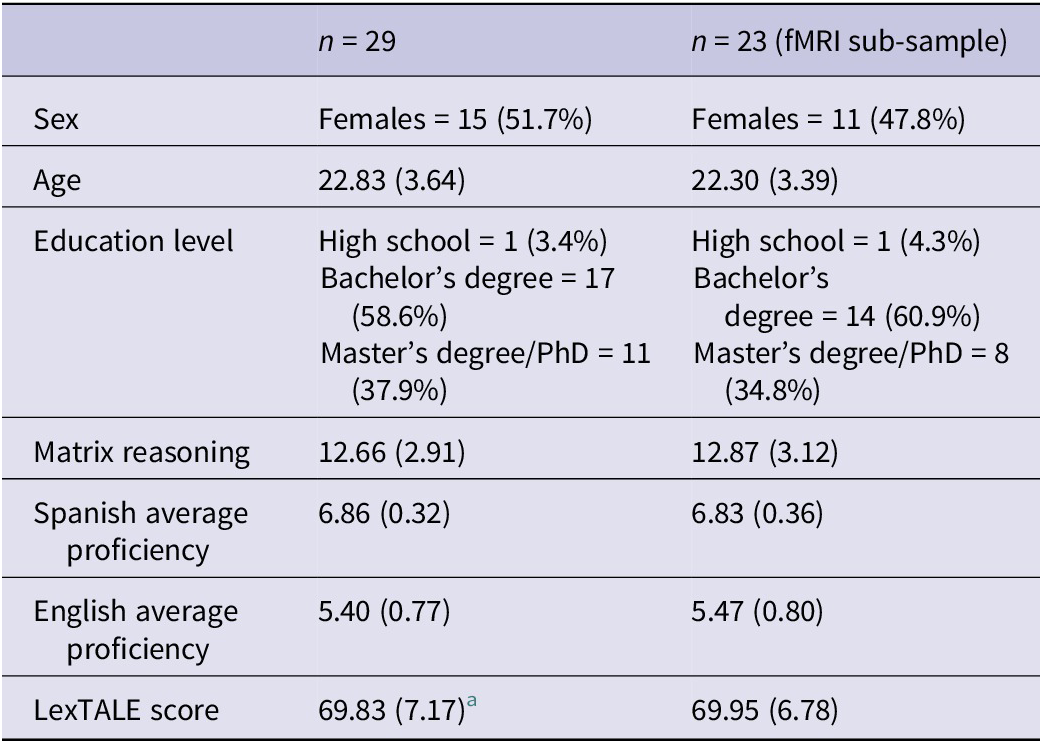
Note: The table shows mean values (quantitative variables) or number of participants (categorical variables) with standard deviation or percentage in parentheses. Proficiency scores reflect self-reported scores provided by the participants on a scale from 1 to 7. Matrix reasoning shows scaled scores from the WAIS-IV subtest.
a One participant did not complete the LexTALE questionnaire, thus the statistic is based on n = 28.
Prior to scanning, all participants provided written consent to participate in the study by signing an informed consent form. The study protocol was approved by the Universitat Jaume I Ethics Committee and was conducted following all applicable regulations. Participants received compensation for their participation.
2.2. Stimuli
To prepare the stimuli, various fiction books were screened to find the appropriate texts that depicted either strongly positive (happy), negative (fearful) or very neutral scenes. In order to minimize the potential effect of familiarity, we deliberately avoided selecting passages from widely recognized or popular books. In addition, we ensured that the texts did not include any proper names, location names or other specific references that could evoke prior associations or familiarity with the content of the selected passages. The initial sample consisted of 300 texts translated into Spanish, Catalan and English that were submitted for rating by 26 individuals in their respective native languages (13 native Spanish and 13 native Catalan speakers) on the following four dimensions: imageability scaled from 1 (low imageability) to 5 (high imageability); valence scaled from −3 (very negative) to 3 (very positive); arousal scaled from 1 (very calming) to 7 (very arousing); fearfulness scaled from 1 (not scary at all) to 5 (very scary) and happiness scaled from 1 (not happy at all) to 5 (very happy). After standardizing the values, 16 texts were chosen for each condition (happy, fearful, neutral). Fearful texts were selected based on arousal and fearfulness ratings, happy texts based on happiness and valence and neutral texts based on valence and arousal. The final sample consisted of 48 happy, fearful and neutral texts, which were randomly divided into two subsets of 24, each containing 8 passages per emotional condition. During the fMRI task, each participant read one subset in their L1 and the other in L2. For the purposes of this study, L1 was Spanish and L2 was English. Relative word frequency was obtained for each passage using NIM software (Guasch et al., Reference Guasch, Boada, Ferré and Sánchez-Casas2013). Within each language, texts across the three conditions were matched for the number of letters, words, sentences and relative word frequency. The descriptive statistics for each stimulus condition are listed in Supplementary Table S1. Stimuli used in this study are available at Open Science Framework (https://osf.io/vksuz).
2.3. fMRI reading task
The fMRI task for this experiment was adapted from the silent reading task used by Hsu et al. (Reference Hsu, Jacobs and Conrad2015) and consisted of a single run where participants read a total of 48 text passages, with 8 passages for each of the 6 experimental conditions (happy, fearful and neutral texts in L1 and L2). The task was administered using E-Prime 3.0 (https://pstnet.com/products/e-prime) via magnetic resonance imaging (MRI)-compatible goggles (VisuaStim Digital, Resonance Technology Inc.). A fixed pseudorandom sequence was generated to balance all possible language switches (L1–L1, L1–L2, L2–L1, L2–L2) and emotional conditions (happy, fearful, neutral), which was identical for all participants. Stimuli were divided into two lists (List 1 and List 2), each containing 48 texts, with 24 texts in L1 and 24 in L2. The texts that appeared in L1 in List 1 appeared in L2 in List 2, and vice versa. List assignment was counterbalanced across participants, while text selection within each condition was randomized for each participant. Each passage was presented in black font at the center of a white screen for 15 seconds. After each text, a black fixation cross was presented for 5 seconds before the next stimulus appeared. The total task duration was 16 minutes. Prior to the scanning session, the participants received detailed instructions on performing the task and practiced with six texts (one for each condition) that were different from the texts used in the fMRI task.
Immediately after the scanning session, in order to assess involvement in the task, the participants were required to complete text comprehension and recognition tasks on a laptop outside the scanner. During the comprehension task, participants were asked specific yes/no questions about the passages’ content. This task consisted of a total of 18 questions, of which 12 were related to the texts that subjects read in the scanner and 6 were unrelated. During the recognition task, they were presented with a series of texts that included passages that they had seen during the fMRI task (remembered texts) and new texts they had never seen before (new texts). Participants were asked whether they recognized each text or not. The recognition task included 54 passages, with 36 texts that the subjects read during the fMRI task and 18 new texts. Behavioral responses were used to explore the effects of language and condition on reaction times (RT) and percentage of correct answers (ACC) during the recognition task.
2.4. Image acquisition and processing
Neuroimaging data were acquired on a 3 T General Electric Signa Architect MRI scanner. For each participant, a 3D structural MRI was acquired using a T1-weighted Brain Volume (BRAVO) Imaging sequence (300 sagittal slices; TR/TE = 7.4/2.9 ms; matrix = 240×240; flip angle = 8°; voxel size = 0.5×0.47×0.47 mm; interslice gap = 0 mm; inversion time = 600 ms). Functional data were acquired using a multiband gradient-echo T2*-weighted echo-planar imaging sequence (482 volumes, 45 axial slices tilted at approximately 10° from the AC/PC plane; multiband factor = 2; TR/TE = 2000/23.5 ms; matrix = 64 × 64; flip angle = 80; voxel size = 3.75×3.75×3 mm; interslice gap = 0 mm).
Functional images were processed using the Statistical Parametric Mapping software package (SPM12; Wellcome Trust Centre for Neuroimaging, London, UK) and MATLAB (version R2022b, MathWorks, Natick, MA). The preprocessing steps included: reorientation to the standard MNI (Montreal Neurological Institute, Montreal, Canada) template; head motion correction; field inhomogeneity artefact correction using the Hysco toolbox (http://www.diffusiontools.com/documentation/hysco.html); coregistration of the anatomical image to the mean functional image; segmentation of structural image; spatial normalization of the functional images to the MNI space with a 3 mm3 resolution using parameters from the segmentation; followed by spatial smoothing with a 6-mm full-width-at-half-maximum (FWHM) Gaussian kernel. ArtRepair toolbox (Mazaika et al., Reference Mazaika, Whitfield-Gabrieli and Reiss2007) was used for the detection and repair of artifacts due to movement during scanning. Participants with more than one voxel size of displacement in any of the six directions or more than 15% of repaired volumes were excluded from fMRI analyses. The general linear model (GLM) was defined for each participant by including regressors for each of the six experimental conditions convolved with the canonical hemodynamic response function. Six motion realignment parameters were included as nuisance regressors. To account for low-frequency drifts, a high-pass filter (263 s) was applied.
2.5. Statistical analyses
In order to compare the brain activity elicited by our task stimuli with the existing literature on emotional reading, we first analyzed brain activity patterns associated with happy versus neutral and fear versus neutral reading in L1 and L2, separately. Then, in order to test our hypothesis of differential neural activity between the two languages during the emotional reading task, we studied the interaction effect between language and emotionality separately for each emotional condition (i.e., [L1 emotional condition – L1 neutral condition] – [L2 emotional condition – L2 neutral condition], with emotional condition being either happy or fearful). For all analyses, we first estimated the corresponding contrast beta weights in each individual participant model (first level) and then conducted group analyses (second level) consisting of whole-brain one-sample t-tests (voxel-wise p < .001; FWE cluster-corrected at p < .05).
Next, we performed a region of interest (ROI) connectivity analysis using the generalized psychophysiological interaction (gPPI) approach (McLaren et al., Reference McLaren, Ries, Xu and Johnson2012) to investigate how the interaction of language and emotionality affected functional connectivity patterns of the ATL with emotion-specific regions for positive and negative valence. Specifically, we focused on the amygdala and nucleus accumbens (NAcc) since these regions have been proposed as core regions for the processing of fearful (Costafreda et al., Reference Costafreda, Brammer, David and Fu2008; Lindquist et al., Reference Lindquist, Satpute, Wager, Weber and Barrett2016) and rewarding (Bartra et al., Reference Bartra, McGuire and Kable2013; Liu et al., Reference Liu, Hairston, Schrier and Fan2011) stimuli, respectively, and have been shown to be recruited during emotional reading (Altmann et al., Reference Altmann, Bohrn, Lubrich, Menninghaus and Jacobs2012; Bohrn et al., Reference Bohrn, Altmann, Lubrich, Menninghaus and Jacobs2013; Dang et al., Reference Dang, Ma, Yuan, Fu, Chen, Zhang, Lu and Guo2023; Hamann & Mao, Reference Hamann and Mao2002; Lewis et al., Reference Lewis, Critchley, Rotshtein and Dolan2007). All mask ROIs were left lateralized and defined using the Automated Anatomical Labeling (AAL, Tzourio-Mazoyer et al., Reference Tzourio-Mazoyer, Landeau, Papathanassiou, Crivello, Etard, Delcroix, Mazoyer and Joliot2002) template implemented in the WFUPickAtlas toolbox (Maldjian et al., Reference Maldjian, Laurienti, Kraft and Burdette2003). The ATL mask was created by combining the AAL-based superior temporal pole and middle temporal pole masks. We first obtained the time series for each ROI represented by the average of the voxels within the ROI masks at each volume. Then, a gPPI statistical model, which included six condition regressors, the ATL time-series as the seed area, six PPI regressors and motion parameters as nuisance regressors, was defined and estimated using amygdala and NAcc time series as dependent variables. The estimated beta weight values for the PPI regressors were subsequently combined to perform language/emotionality interaction contrast (using happy conditions for the NAcc analysis and fearful conditions for the amygdala analysis). Then, we performed second-level one-sample t-test analyses (p < .05) to test our hypothesis of higher connectivity between ATL and emotional regions during emotional text reading in L1. Furthermore, whole-brain voxel-wise exploratory analyses were performed to study potential differences in the ATL functional connectivity during the interaction between language and emotionality in brain regions for which we did not have a priori hypotheses (voxel-wise p < .001; FWE cluster-corrected at p < .05).
Finally, behavioral data from the text recognition task were analyzed in R using mixed-effects models (lme4 package; Bates et al., Reference Bates, Mächler, Bolker and Walker2015). For reaction times (RT), a linear mixed-effects model was fitted with language (L1, L2), emotionality (happy, fearful, neutral) and their interaction as fixed effects, and with random intercepts for both participants and items. For accuracy (hits), a generalized linear mixed-effects model with a binomial distribution and logit link was used, incorporating the same fixed and random effects structure. Significance was evaluated at p < .05, and effect sizes were reported using odds ratios (for hits) and partial eta-squared (for RT).
3. Results
3.1. Behavioral analysis
For accuracy, the generalized linear mixed-effects model did not reveal any significant main effects or interactions. However, there was a trend-level effect of emotionality (z = 1.68, p = .093); the odds ratio for correctly recognizing fearful versus happy texts was 2.30 (95% CI [0.87, 6.09]), indicating a moderate effect size. This suggests that participants may have been more likely to correctly recognize fearful texts compared to happy ones, although this effect did not reach statistical significance.
For reaction times, the linear mixed-effects model revealed a large main effect of language (t(85.70) = −2.32, p = .023, ηp2 = .20), indicating that participants responded significantly faster in L1 compared to L2. No other main effects or interactions reached statistical significance. Descriptive statistics for each condition are reported in Table 2.
Table 2. Descriptive statistics for reaction times and percentage of correct answers for the post-scan recognition task for each experimental condition (n = 29)
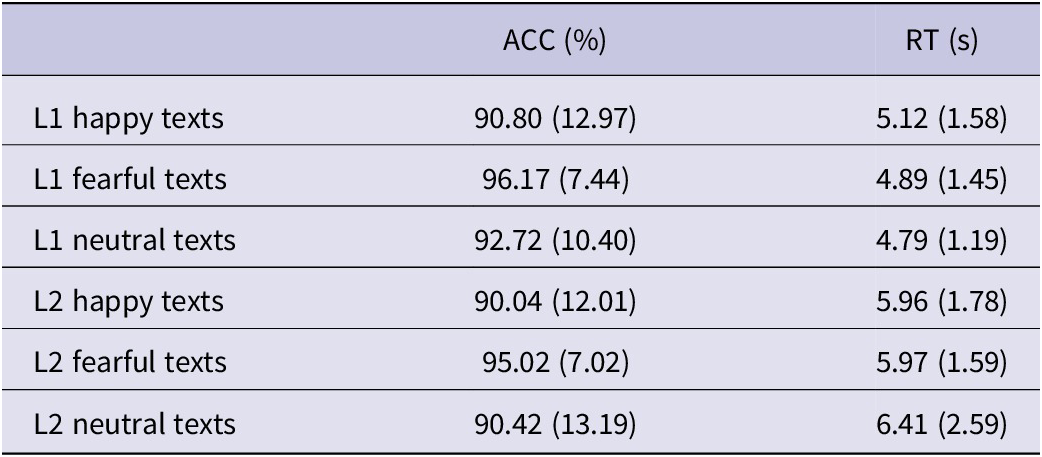
Note: The table shows mean values with standard deviation in parentheses.
3.2. Task-related activation results
To gain new insights into the neural activity patterns during emotional text reading, we first conducted whole-brain analyses comparing happy and fearful texts to neutral ones, separately for L1 and L2.
Compared to the neutral condition, the reading of happy texts in L1 elicited activation across a number of brain regions, including the vmPFC, anterior cingulate cortex, precuneus, posterior cingulate cortex, right supramarginal gyrus and right superior temporal gyrus. In L2, significant activation was restricted to the mPFC and anterior cingulate cortex (see Figure 1A and Supplementary Table S2).
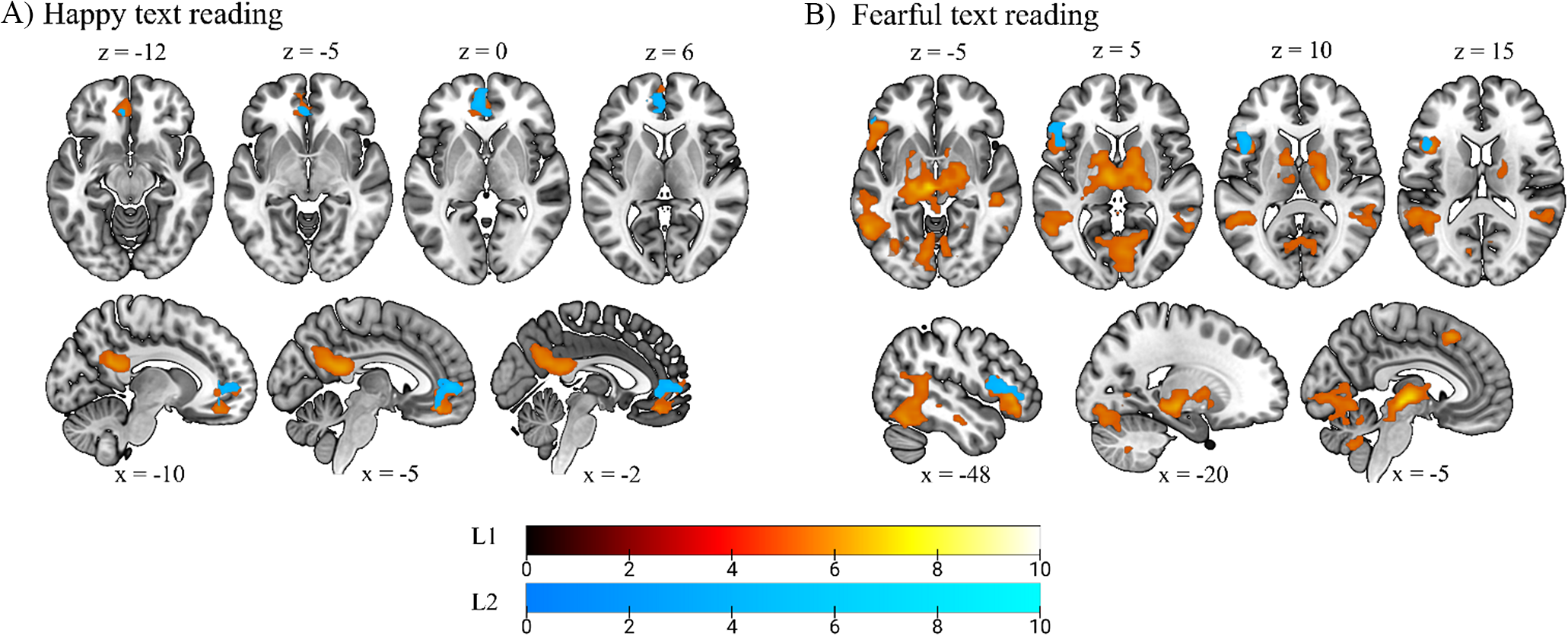
Figure 1. Effect of emotion on voxel-wise brain activity (p < .05, FWE cluster-corrected, with a height threshold of p < .001). Color bars represent t value. Regions highlighted in red indicate activation during L1 reading, and regions highlighted in blue correspond to L2. (A) Voxel-level whole-brain analysis of neural activity during the reading of happy versus neutral texts, showing areas with overlapping activity between L1 and L2 in the mPFC. (B) Voxel-level whole-brain analysis of neural activity during the reading of fearful versus neutral texts, showing areas with overlapping activity between L1 and L2 in the left inferior frontal gyrus.
The reading of fearful texts in L1 showed significant activation across the left hippocampus, left inferior occipital gyrus, left fusiform gyrus, left inferior frontal gyrus, left supplementary motor area, bilateral middle temporal gyrus, bilateral lingual gyrus, calcarine sulcus, thalamus and cerebellum. In L2, the only area that was significantly more active during the reading of fearful texts was left inferior frontal gyrus pars triangularis and pars opercularis (see Figure 1B and Supplementary Table S3).
The whole-brain analysis of the interaction effect of language and emotionality for the fearful condition showed significant activation in the posterior left hippocampus extending into the parahippocampal gyrus (left: −18, −34, −7; t = 5.38, cluster size = 72; p < .05, FWE cluster-corrected) driven by higher activity during the processing of fearful versus neutral texts in L1 (Figure 2). No significant language/emotionality interaction effect was found for the happy texts.
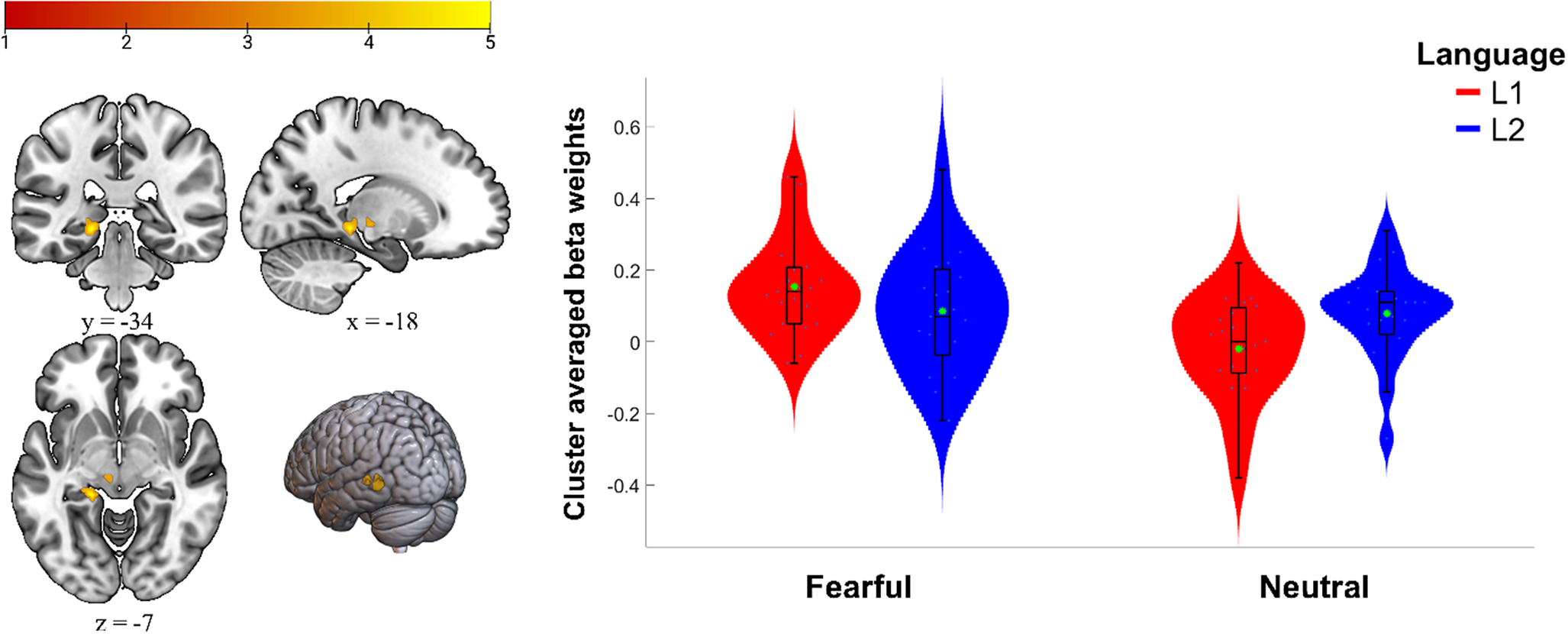
Figure 2. Results of the whole-brain analysis for the language (L1, L2) × emotionality (fearful, neutral) interaction. The left side displays the activation in the left hippocampus (p < .05, FWE cluster-corrected, with a height threshold of p < .001). Color bar represents t value. The right side displays a violin plot showing the cluster-averaged responses for each condition separately (for illustrative purposes only). The green asterisk shows the arithmetic mean.
3.3. Functional connectivity results
Next, we employed gPPI analysis to explore the seed-to-seed functional connectivity between the left ATL and the left amygdala during fearful text reading and the functional connectivity between the left ATL and the left NAcc during happy text reading. Our results showed a large language/emotionality interaction effect (t(22) = 2.54, p = .009, ɳp2 = .23) for the processing of fearful texts (Figure 3). Post-hoc analysis confirmed that the left ATL/left amygdala coupling was significantly higher during the processing of fearful texts in L1 (M = 0.57, SD = 0.46) compared to L2 (M = 0.41, SD = 0.37); (t(22) = 2.45, p = .011, with a moderate effect size, d = 0.51, 95% CI [0.07, 0.94]). The inspection of the beta weight distribution for each condition separately revealed a potential outlier in our data (see Figure 3); however, this participant was not an outlier in the distribution of the differences between conditions for the interaction contrast. Furthermore, the results remained significant when excluding this participant from the analysis (t(21) = 2.25, p = .018, ɳp2 = .19), still indicating a large effect. The analysis investigating the functional connectivity between the ATL and NAcc during happy text reading did not reach significance; however, it showed a trend with a moderate effect size (t(22) = 1.61, p = .06, ɳp2 = .11), suggesting that approximately 11% of the variance in the ATL and NAcc connectivity may be attributable to the experimental conditions.
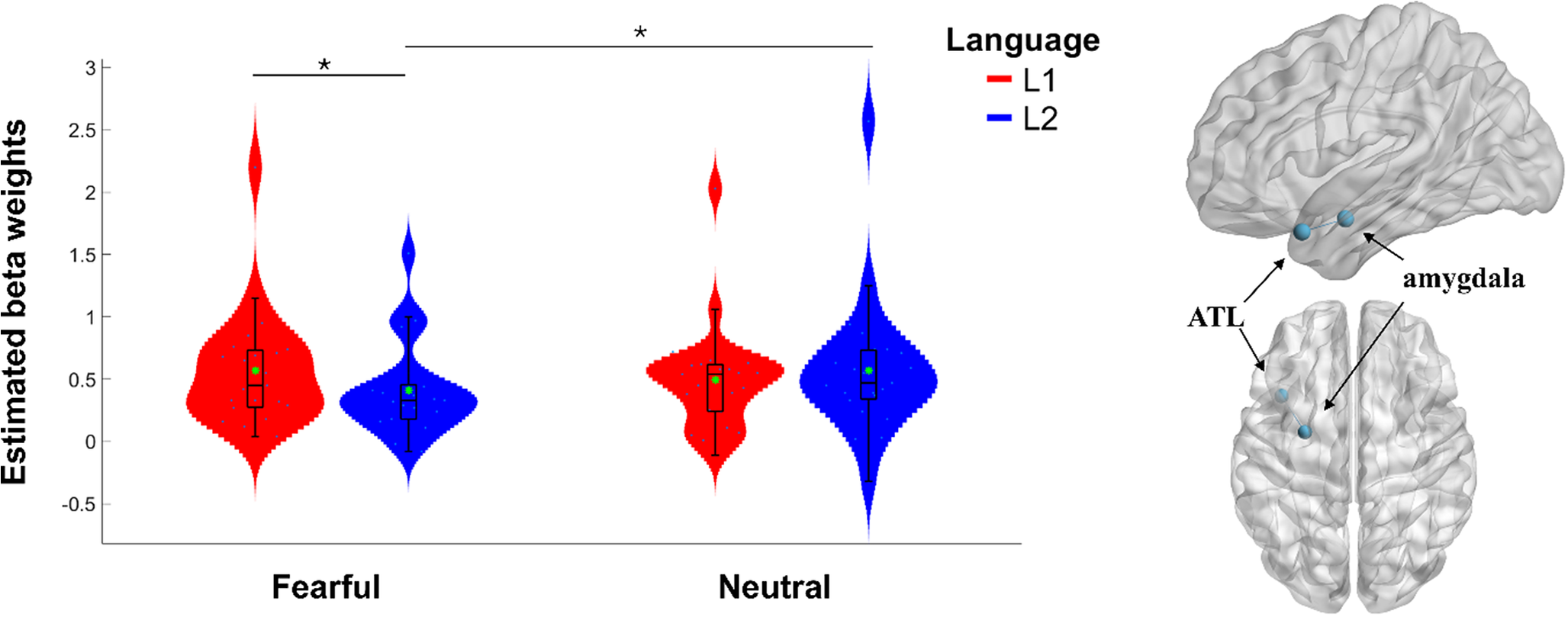
Figure 3. gPPI connectivity between the left ATL and left amygdala during the processing of fearful texts in L1 and L2. The left side displays estimated beta weights for gPPI regressors. The green asterisk shows the arithmetic mean. *p-value < .05. The figure on the right illustrates the connectivity between the left ATL and the left amygdala.
Finally, whole-brain gPPI analyses investigating interaction effect for the fearful condition showed a trend toward significance in the connectivity of the left ATL with the left intracalcarine cortex (left: −6, −94, 5; t = 5.47; cluster size = 49, p = .051, FWE cluster-corrected). No results were found for the interaction effect during happy text reading.
4. Discussion
This study used a silent reading task to examine bilingual differences in the processing of happy, fearful and neutral short fiction passages. Our findings showed different patterns of neural activity between happy and fearful text reading in L1, in addition to a significant language-by-emotionality interaction effect on the activity of the left hippocampus during the reading of fearful texts. The gPPI functional connectivity analysis revealed lower coupling between the left ATL and the left amygdala during the reading of fearful texts in L2, suggesting reduced integration between semantic and emotional systems during foreign language processing.
First, we explored the effect of emotional reading on neural activity separately for each language. Our findings revealed distinctive brain responses during the reading of happy and fearful texts compared to neutral ones in L1. Similar to the results of Hsu et al. (Reference Hsu, Jacobs and Conrad2015), we found activation of the superior temporal gyrus during the reading of happy texts, while increased activity was observed in the left hippocampus, the left inferior frontal gyrus and the cerebellum (vermis) during the reading of fearful texts. Across the two languages (L1 and L2), the vmPFC/anterior cingulate cortex showed increased activity during the reading of happy texts, which aligns with previous research associating this region with the processing of positive linguistic stimuli (Kim et al., Reference Kim, Somerville, Johnstone, Polis, Alexander, Shin and Whalen2004). For the reading of fearful texts, the common activation for L1 and L2 was located in the left inferior frontal gyrus. The left inferior frontal gyrus has been linked with narrative comprehension (Ferstl et al., Reference Ferstl, Neumann, Bogler and von Cramon2008; Mar, Reference Mar2011) and sentence-level semantic integration (Zhu et al., Reference Zhu, Zhang, Wang, Xiao, Huang and Chen2009), and it also plays a role in emotion regulation (Frank et al., Reference Frank, Dewitt, Hudgens-Haney, Schaeffer, Ball, Schwarz, Hussein, Smart and Sabatinelli2014; Picó-Pérez et al., Reference Picó-Pérez, Alemany-Navarro, Dunsmoor, Radua, Albajes-Eizagirre, Vervliet, Cardoner, Benet, Harrison, Soriano-Mas and Fullana2019). Overall, our results agree with previous studies investigating neural substrates of emotional text reading (Altmann et al., Reference Altmann, Bohrn, Lubrich, Menninghaus and Jacobs2012; Ferstl et al., Reference Ferstl, Rinck and von Cramon2005; Hsu et al., Reference Hsu, Jacobs and Conrad2015), demonstrating that the task effectively engaged neural systems associated with processing of emotion-laden linguistic stimuli.
4.1. Language differences in the left hippocampus during fearful text reading
The results of the whole-brain one-sample t-test of the language/condition interaction effect on neural activity provided evidence of differential emotional response in L2, which was evidenced by lower activity in the left hippocampus during the processing of fearful texts in L2. Prior research has shown memory enhancement effects for emotional information, particularly for negative and arousing stimuli (Kensinger, Reference Kensinger2009). These results were partially supported by our behavioral data, which showed a trend suggesting that participants were more likely to correctly recognize fearful texts. The hippocampus is crucial for aversive learning and emotional memory formation (Costa et al., Reference Costa, Lozano-Soldevilla, Gil-Nagel, Toledano, Oehrn, Kunz, Yebra, Mendez-Bertolo, Stieglitz, Sarnthein, Axmacher, Moratti and Strange2022) and forms part of the emotion conceptualization network (Lindquist et al., Reference Lindquist, Wager, Kober, Bliss-Moreau and Barrett2012). Furthermore, previous research has shown hippocampal activation during the reading of negative and fearful texts (Altmann et al., Reference Altmann, Bohrn, Lubrich, Menninghaus and Jacobs2012; Hsu et al., Reference Hsu, Jacobs and Conrad2015). Hippocampal activity during aversive memory encoding has been reported to be modulated by the activity in the amygdala (Cahill & McGaugh, Reference Cahill and McGaugh1998; Richter-Levin & Akirav, Reference Richter-Levin and Akirav2000; Zheng et al., Reference Zheng, Anderson, Leal, Shestyuk, Gulsen, Mnatsakanyan, Vadera, Hsu, Yassa, Knight and Lin2017), which is primarily involved in the processing of salient information and activates in response to negative stimuli in general, and more specifically, in response to negative verbal stimuli (Hamann & Mao, Reference Hamann and Mao2002). Furthermore, as shown by Richardson et al. (Reference Richardson, Strange and Dolan2004), the strength of emotional memory encoding depends on the strength of the interaction between the amygdala and the hippocampus. Thus, the decreased activity in the left hippocampus might suggest that negative texts are not eliciting the same level of processing within the limbic system, indicating reduced emotional impact when processing negative and fearful texts in L2 context. The observed decreased neural response to fearful narratives in L2 is consistent with findings of decreased L2 emotionality from previous studies employing diverse methodologies, such as pupillometry (Thoma et al., Reference Thoma, Hüsam and Wielscher2023), skin conductance response (Jankowiak & Korpal, Reference Jankowiak and Korpal2018) and EEG (Jończyk et al., Reference Jończyk, Boutonnet, Musiał, Hoemann and Thierry2016). Overall, these converging findings across different experimental paradigms reinforce the idea that emotional processing is attenuated in L2.
While our results show that fearful texts in L2 are processed less intensely in the hippocampus, supporting the existing evidence of attenuated emotional response in the non-native language, we did not observe significant language/condition interaction for the happy texts. Evidence from previous studies exploring between-language differences in emotional reading suggests that the valence of the emotional stimuli may modulate the L2 effect (Jończyk et al., Reference Jończyk, Boutonnet, Musiał, Hoemann and Thierry2016; Sheikh & Titone, Reference Sheikh and Titone2016). For example, an eye-tracking study of sentence reading in French–English bilinguals showed that only negative words were susceptible to emotional disembodiment during L2 reading, which the authors attributed to a potential positivity bias in L2 use, where bilinguals tend to use their second language in more positive contexts and therefore draw upon their positive experiences to provide emotional grounding for L2 words (Sheikh & Titone, Reference Sheikh and Titone2016). Similar effects attributable to positivity bias were seen in an EEG study where Spanish-native participants performed a lexical decision task in German, showing a processing advantage for positive, but not negative words (Conrad et al., Reference Conrad, Recio and Jacobs2011). Another study investigating electrophysiological correlates of emotion word processing in Spanish–English bilinguals also reported a positivity bias in L2, with unbalanced bilinguals showing greater neural responses to positive words than to negative ones in their second language (Vélez-Uribe & Rosselli, Reference Vélez-Uribe and Rosselli2021). Moreover, studies investigating language and emotion interaction during decision-making found enhanced response to positive feedback in L2 compared to L1 in a gambling task (He et al., Reference He, Margoni, Wu and Liu2021; Zheng et al., Reference Zheng, Mobbs and Yu2020). Together, these results support the existence of positivity bias during L2 processing, which may explain the lack of significant language differences for positive reading in our study. Interestingly, in the study of emotional reading in bilinguals conducted by Chen et al. (Reference Chen, Lin, Chen, Lu and Guo2015), positive words were processed more intensely in both languages, as evidenced by faster response times (for L1 positive versus neutral words) and higher accuracy (for L2 positive words versus neutral and negative words). However, the neuroimaging results of the study suggested that this processing advantage may rely on different neural mechanisms (Chen et al., Reference Chen, Lin, Chen, Lu and Guo2015). Similarly, the results of the study investigating bilingual differences during emotional passage reading conducted by Hsu et al. (Reference Hsu, Jacobs and Conrad2015) showed that the emotional processing advantage was restricted to L1 positive passages only, which contrasts with the results obtained in this study. This discrepancy could be attributed to variations in task design and the specific nature of the emotional content. For instance, Hsu et al. (Reference Hsu, Jacobs and Conrad2015) used emotional passages from Harry Potter, which included fantastical elements, whereas our study used more realistic, contemporary texts from less recognizable sources. Differences in content characteristics, such as text familiarity or verisimilitude, could therefore contribute to the observed variability in emotional processing across studies.
4.2. Different L1/L2 connectivity patterns in the ATL during fearful text reading
In order to test the hypothesis that the L2 effect during emotional processing arises from inefficient coupling between semantic and affective representations, we investigated functional connectivity between the left ATL and the left amygdala and NAcc using the gPPI connectivity analysis. Crucially, our results showed a language and condition interaction effect on the functional connectivity between the ATL and the amygdala during the processing of fearful texts, with significantly higher coupling during L1 compared to L2 reading. The processing and comprehension of emotional passages, compared to single-word reading, require complex multimodal integration, including syntactic, semantic, affective and episodic information in order to create a meaningful representation of their content (Ferstl et al., Reference Ferstl, Rinck and von Cramon2005, Reference Ferstl, Neumann, Bogler and von Cramon2008), which invariably activates an extended network of modality-specific brain areas. The ATL has been proposed as the region that integrates this multi-modal conceptual information to form semantic representations (Ralph et al., Reference Ralph, Jefferies, Patterson and Rogers2017). Specifically, the controlled semantic cognition model proposed by Ralph et al. (Reference Ralph, Jefferies, Patterson and Rogers2017) suggests that semantic representations are mediated by the integration of modality-specific information encoded in unimodal areas into the ATL, which serves as a transmodal hub. In addition, the ATL is involved in the representation and retrieval of social knowledge (Olson et al., Reference Olson, McCoy, Klobusicky and Ross2013), which plays an important part in narrative comprehension (Clark, Reference Clark1985). On the other hand, the amygdala plays a crucial role in the processing of negative affect (Barrett et al., Reference Barrett, Bliss-Moreau, Duncan, Rauch and Wright2007) and forms part of the mentalizing network that activates during story processing (Mar, Reference Mar2011). The ATL and the amygdala have been shown to be connected both structurally (Abivardi & Bach, Reference Abivardi and Bach2017) and functionally (Sonkusare et al., Reference Sonkusare, Nguyen, Moran, van der Meer, Ren, Koussis, Dionisio, Breakspear and Guo2020). A study conducted by Sonkusare et al. (Reference Sonkusare, Nguyen, Moran, van der Meer, Ren, Koussis, Dionisio, Breakspear and Guo2020), using intracranial EEG in epileptic patients, showed that both the ATL, specifically the temporal pole, and the amygdala presented a synchronized response to multimodal affective stimuli. Notably, this functional connectivity was observed to go from the ATL to the amygdala, while being modulated by the valence of the emotional stimuli. In addition to the language effect shown in the connectivity between the ATL and the amygdala during fearful text reading, a statistical trend was observed for the same effect on the connectivity between the ATL and the NAcc during happy text reading. Together, these results align with the evidence provided by Green et al. (Reference Green, Ralph, Moll, Stamatakis, Grafman and Zahn2010), showing that ATL functional connectivity is dynamically adapted during the processing of particular emotions.
Previous findings show that the ATL semantic network is less integrated during L2 processing as compared to L1, even in highly proficient bilinguals (Zhang et al., Reference Zhang, Yang, Wang and Li2020). A study by Jeong et al. (Reference Jeong, Li, Suzuki, Sugiura and Kawashima2021) further supports this, suggesting that L2 acquisition in a classroom context, as in our study, contributes to the lower semantic integration seen in L2 processing. This study found that native Japanese speakers who had never studied Korean exhibited more accurate and quicker responses when words were learned in a social context, resulting in more extensive brain activation that included primary unimodal regions.
Given these previous findings, the lower connectivity between the ATL and emotional areas during L2 processing demonstrated in our study might imply a less enriched representation of L2 in the semantic system. This hypothesis would align with the current models of the L2 effect on emotion (Harris et al., Reference Harris, Gleason, Ayçiçeǧi and Pavlenko2006; Pavlenko, Reference Pavlenko2012), which suggest a lower grounding of L2 due to lower emotional experiences using that language. Prior evidence suggests that bilinguals tend to exhibit reduced sensitivity to negatively valenced stimuli in L2 (Jończyk et al., Reference Jończyk, Boutonnet, Musiał, Hoemann and Thierry2016, Reference Jończyk, Naranowicz, Bel-Bahar, Jankowiak, Korpal, Bromberek-Dyzman and Thierry2025; Wu & Thierry, Reference Wu and Thierry2012). Thierry and Wu (Reference Thierry and Wu2007) demonstrated that reading in L2 leads to spontaneous co-activation of L1. However, in their subsequent study, they showed that this effect is valence-dependent and that this co-activation was not present during the processing of negative words in L2 (Wu & Thierry, Reference Wu and Thierry2012). These findings led the authors to propose the existence of a bottom-up cognitive suppression mechanism that may involve basal ganglia and limbic circuits (amygdala, medial temporal lobe) that block access to L1 representations during the L2 processing of negative content (Wu & Thierry, Reference Wu and Thierry2012). The results of this study may suggest an alternative mechanism. If the unconscious access to L1 translation equivalents by L2 words (Thierry & Wu, Reference Thierry and Wu2007) relies on the richness of semantic representations, the reduced connectivity between the semantic and emotional regions during negative word processing would impede the automatic co-activations. Future studies are needed to confirm this hypothesis.
5. Limitations
A limitation of this study is the sample size, which had to be reduced to ensure data quality by excluding subjects with excessive movement or poor task performance. While the limited sample size may have affected our ability to detect additional effects that could have emerged with a larger cohort, it is important to note that our main results remain significant even after accounting for potential outliers and correcting for multiple comparisons. In fact, we observed several trends that, if confirmed, could be highly relevant to understanding the effects of bilingualism on emotional processing. These findings will need to be corroborated in future studies with larger samples.
6. Conclusions
In conclusion, this study provides further evidence of differential emotional processing between L1 and L2 in unbalanced bilinguals. By employing a silent reading task of short fiction passages with varying emotional content, we extend previous evidence of reduced emotional resonance in L2 with a more ecologically valid narrative context. The significant language/emotionality interaction effect observed in the left hippocampus during the processing of fearful texts suggests lower emotional processing within the limbic system in L2. Moreover, the observed differences in functional connectivity between the left ATL and the amygdala during the processing of fearful narratives point to a less effective integration of emotional representations in the semantic system during foreign language processing. Together, these findings contribute to our understanding of the neural mechanisms underlying reduced emotional resonance in a foreign language, highlighting the critical role of semantic integration in bilingual emotional processing.
Supplementary material
The supplementary material for this article can be found at http://doi.org/10.1017/S1366728925100187.
Data availability statement
The stimuli used in this study, task paradigm in e-prime format, first-level processed MRI images and behavioral data are available online at https://osf.io/vksuz. Any other data or resources that support the findings of this study are available from the corresponding author upon reasonable request.
Acknowledgments
This work was supported by the project PID2019-105077RJ-I00 funded by the MCIN/AEI/10.13039/501100011033, by the project CIAICO/2022/180 funded by the Conselleria de Educación, Universidades y Empleo and by the Ramón y Cajal fellowship (RYC2021-033809-I) funded by MCIN/AEI/10.13039/501100011033 and the NextGenerationEU/PRTR, awarded to VC. MV was supported by the Grant RYC2019-028370-I funded by MICIU/AEI/10.13039/501100011033 and by “ESF Investing in your future.”
Competing interests
The authors declare none.
Ethics statement
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008.







