Schizophrenia is one of the most devastating psychiatric disorders to affect children and adolescents. Although extremely rare before the age of 10, the incidence of schizophrenia rises steadily through adolescence to reach its peak in early adult life. The clinical severity, impact on development and poor prognosis of child- and adolescent-onset schizophrenia reinforce the need for early detection, prompt diagnosis and effective treatment.
The current concept of schizophrenia in children and adolescents evolved from a different perspective held during much of the 20th century. Until the early 1970s, the term childhood schizophrenia was applied to children who would now be diagnosed with autism. Kolvin’s landmark studies distinguished children with early-onset (autistic) symptoms beginning in the first 2 years of life from children with a relatively ‘late-onset’ psychosis with onset of symptoms after age 6 or 7, which closely resembled adult schizophrenia (Reference Hollis, Rutter, Bishop and PineHollis 2008). Importantly, in ICD-9 (1978) and DSM-III (1980) the separate category of childhood schizophrenia was removed, and the same diagnostic criteria for schizophrenia were applied across the age range. The validity of the diagnosis of schizophrenia in childhood and adolescence is supported by follow-up studies into adulthood that show a high level of diagnostic stability (Reference HollisHollis 2000).
This article focuses on children and young people who meet ICD-10 (World Health Organization 1992) or DSM-5 (American Psychiatric Association 2013) diagnostic criteria for schizophrenia. I use the term ‘adolescent schizophrenia’ as short-hand to refer to child and adolescent cases with onset up to 17 years of age. I examine evidence for continuities and discontinuities between adolescent schizophrenia and adult-onset schizophrenia in terms of aetiology, premorbid features, clinical presentation, course and outcome, and treatment response. My goal is to summarise what is currently known about adolescent schizophrenia and to indicate the extent and limitations of the evidence base for clinical diagnosis, management and treatment.
Epidemiology
Incidence and prevalence
Reference Gillberg, Wahlstrom and ForsmanGillberg et al (1986) calculated age-specific prevalences for all psychoses (including schizophrenia, schizophreniform disorder, affective psychosis, atypical psychosis and drug psychoses) using Swedish case-register data on 13- to 18-year-olds with psychotic illnesses. In 41% of cases the diagnosis was schizophrenia. At 13 years of age, the prevalence for all psychoses in the general population was 0.9 per 10 000, showing a steady increase during adolescence, reaching a prevalence of 17.6 per 10 000 at age 18 years.
Gender ratio
Males are over-represented in many clinical studies of childhood-onset schizophrenia (Reference Russell, Bott and SammonsRussell 1989; Reference Spencer and CampbellSpencer 1994). However, other studies of predominantly adolescent-onset schizophrenia have described an equal gender ratio (Reference HollisHollis 2000).
Aetiology and risk factors
Genetics
Twin studies have suggested the heritability of schizophrenia to be as high as 83% (Reference Cannon, Kaprio and LonnqvistCannon 1998). However, one of the most significant implications of twin, adoption and family studies in schizophrenia is that they challenge the idea that what is inherited is a categorical psychiatric disorder. Similar to autism, genetic studies in schizophrenia have shown that the genetic liability to schizophrenia extends to schizotypal personality disorders and other conditions viewed as lying on the broader schizophrenia spectrum (Reference Erlenmeyer-Kimling, Squires-Wheeler and AdamoErlenmeyer-Kimling 1995). These results suggest that what is inherited in schizophrenia is likely to be underlying neurodevelopmental and psychological traits that interact with environmental factors to determine liability to the disorder.
Candidate genes in childhood-onset schizophrenia
The National Institute of Mental Health (NIMH) study of childhood-onset schizophrenia has contributed much to the literature. It reported an association between two overlapping genes G70 and G32 on chromosome 13 (13q33.2) and childhood-onset schizophrenia (Reference Addington, Gornick and SpornAddington 2004). Another intriguing finding of the study is an association between the dysbindin-1 gene on chromosome 6 (DTNBP1 6p22.3) and poor premorbid social and academic adjustment (Reference Rapoport, Addington and FrangouRapoport 2005). In addition, polymorphisms of the GAD1 (glutamic acid decarboxylase) gene have been associated with both childhood-onset schizophrenia and abnormal frontal grey matter loss (Reference Addington, Gornick and DuckworthAddington 2005). Finally, neuregulin 1 susceptibility haplotypes have been associated with abnormal developmental trajectories for both grey and white matter in this population (Reference Addington, Gornick and ShawAddington 2007).
Cytogenetic abnormalities
The NIMH study also revealed a high rate of previously undetected cytogenetic abnormalities, including velocardiofacial syndrome (VCFS) in 4 of the 80 young people (5%) involved (Reference Sporn, Addington and ReissSporn 2004a). Velocardiofacial syndrome is associated with progressive cortical grey matter loss in children and adolescents who are not yet showing psychotic symptoms, suggesting that one or more genes mapping to chromosome region 22q11 is responsible for a high-risk phenotype (Reference Sporn, Addington and ReissSporn 2004a). The overall high rate of various cytogenetic abnormalities (seen in 10% of the NIMH sample) suggests the possibility of more subtle genomic instability similar to that seen in autism (Reference Rapoport, Addington and FrangouRapoport 2005). This idea is supported by the high rate of small structural deletions/duplications that disrupt genes seen in early-onset schizophrenia (Reference Walsh, McClellan and McCarthyWalsh 2008).
Cannabis and schizophrenia
Acute intoxication with cannabis and other illicit substances, such as stimulants and hallucinogens, can precipitate psychotic symptoms or exacerbate existing psychotic illness. Cannabis confers an overall twofold increased risk of later schizophrenia (Reference Arseneault, Cannon and WittonArseneault 2004). In the Dunedin cohort, Reference Arseneault, Cannon and PoultonArseneault et al (2002) showed that the association was strongest for the youngest cannabis users, with 10.3% of those who were using cannabis at 15 years of age developing schizophreniform disorder by the age of 26. So far, cannabis use has not been directly implicated in adolescent schizophrenia – possibly because of the relatively lower prevalence of cannabis use in younger adolescents and a short duration between exposure and psychotic outcome. However, cannabis use is associated with earlier age at onset of schizophrenia in adults (Reference Arendt, Rosenberg and FoldagerArendt 2005). Studies of gene–environment interaction (Reference Caspi, Moffitt and CannonCaspi 2005), taken together with human (Reference Dean, Bradbury and CopolovDean 2003) and animal (Reference Pistis, Perra and PillollaPistis 2004) neuropharmacological studies, suggest that cannabis may enhance the risk of schizophrenia in vulnerable individuals during a critical period of adolescent brain development.
Neurobiology of schizophrenia
Structural brain abnormalities
The brain changes reported in childhood-onset schizophrenia appear to be very similar to those described in adult schizophrenia, supporting the idea of an underlying neurobiological continuity. Children with childhood-onset schizophrenia (onset at less than 13 years of age) in the NIMH study had smaller brains, with larger lateral ventricles and reduced prefrontal lobe volume, than healthy young people (Reference Jacobsen and RapoportJacobsen 1998). As in adult studies, reduced total cerebral volume is associated with negative symptoms of schizophrenia (Reference Alaghband-Rad, Hamburger and GieddAlaghband-Rad 1997). Childhood-onset patients have a higher rate of developmental brain abnormalities than controls, including an increased frequency of cavum septum pellucidum (Reference Nopoulos, Giedd and AndreasenNopoulos 1998). In patients with adolescent schizophrenia there is evidence of ventricular enlargement and reduced volume of the prefrontal cortex and thalamus (Reference James, Smith and JayaloesJames 2004).
Progressive brain changes
Longitudinal imaging studies in adolescent schizophrenia show a fourfold greater reduction in cortical volume than in healthy adolescents (Reference Rapoport, Giedd and BlumenthalRapoport 1999). Most strikingly, the pattern of the exaggerated grey matter loss is identical to the pattern of normal development, suggesting amplification of a normal developmental process (Reference Rapoport and GogtayRapoport 2007). Progressive changes appear to be time-limited to adolescence, and the rate of volume reduction in frontal and temporal structures is associated with premorbid developmental impairment and baseline symptom severity, declining as individuals reach adult life (Reference Giedd, Jefferies and BlumenthalGiedd 1999).
The clinical phases of schizophrenia
Premorbid social and developmental impairments
Adolescent schizophrenia is associated with poor premorbid functioning and early developmental delays (Reference Alaghband-Rad, McKenna and GordonAlaghband-Rad 1995; Reference HollisHollis 1995 Reference Hollis2003). Similar types of developmental and social impairments in childhood have been reported in adult-onset schizophrenia, but premorbid impairments appear to be more common and severe in adolescent schizophrenia. In the Maudsley study of adolescent schizophrenia (Reference HollisHollis 2003) significant early delays were particularly common in the areas of language (20%), reading (30%) and bladder control (36%). A consistent characteristic in the premorbid phenotype is impaired sociability, with about a third of individuals with adolescent schizophrenia having significant premorbid difficulties in social development affecting the ability to make and keep friends.
Premorbid IQ in adolescent schizophrenia is reduced and lower than in adult schizophrenia (Reference Alaghband-Rad, McKenna and GordonAlaghband-Rad 1995; Reference HollisHollis 2000). In about a third of child- and adolescent-onset cases, the young person has an IQ below 70, with the whole distribution of IQ shifted down compared with both adolescent affective psychoses and adult schizophrenia.
Premorbid psychopathology
A diverse range of clinical diagnoses, including attention-deficit hyperactivity disorder (ADHD), conduct disorder, anxiety, depression and autism spectrum disorders, may precede the diagnosis of schizophrenia in children and adolescents (Reference Schaeffer and RossSchaeffer 2002). However, there is a lack of any specific premorbid diagnosis that could practically aid early clinical identification of those at high risk of schizophrenia. A more promising line of research has demonstrated a strong link between self-reported psychotic symptoms in childhood and later schizophrenia (Reference Poulton, Caspi and MoffittPoulton 2000). In the Dunedin cohort study, psychotic symptoms at age 11 increased the risk of schizophreniform disorder at age 26 but not of other psychiatric diagnoses. Relative to the rest of the cohort, those identified at age 11 with ‘strong’ psychotic symptoms also had significant impairments in motor development, receptive language and IQ (Reference Cannon, Caspi and MoffittCannon 2002). Although none of these individuals met criteria for a diagnosis of schizophrenia during adolescence, it appears that isolated or attenuated psychotic symptoms, in combination with pan-developmental impairment, constitute a significant high-risk premorbid phenotype.
Is it possible to identify young people ‘at risk’ of psychosis and schizophrenia?
Research has examined the feasibility of detecting and treating young people in the ‘at-risk’ stage, prior to the development of psychosis. This approach rests on three assumptions:
-
1 it is possible to detect such people
-
2 these people will be at markedly increased risk of later psychosis
-
3 an effective intervention will reduce this risk.
There is evidence to support assumptions 1 and 2 in people with a strong family history of psychosis, who are therefore at high genetic risk (Reference Miller, Lawrie and HodgesMiller 2001), and in those reporting particular perceptual abnormalities (Reference Klosterkotter, Hellmich and SteinmeyerKlosterkotter 2001).
Various criteria have been developed in an attempt to identify a ‘high-risk’ phenotype. Features typically include: transient or attenuated psychotic symptoms; a decline in psychosocial functioning; and enhanced familial risk of psychosis. Early studies conducted in specialist centres suggested that up to 50% of ‘high-risk’ individuals made the transition to a firm diagnosis of a psychotic disorder within 12 months (Reference Yung, Philips and YuenYung 2003). However, more recent studies suggest that transition rates are considerably lower, at 10–15% (Reference Fusar-Poli, Bonoldi and YungFusar-Poli 2012), and that the ‘high-risk’ phenotype is clinically heterogeneous and unstable over time (Reference van Os and Murrayvan Os 2013). Mood disorder and substance misuse are particularly prevalent in these samples. Given the low rate of transition to frank psychosis in these help-seeking and functionally impaired individuals, treating the presenting problems (e.g. depression, substance misuse and paranoia) may be a more sensible strategy than intervening with the primary purpose of preventing the onset of psychosis.
Prodromal symptoms and onset of psychosis
Before the onset of psychosis, young people typically enter a prodromal phase characterised by a gradual but marked decline in social and academic functioning that precedes active psychotic symptoms. An insidious deterioration prior to the onset of psychosis is typical of the presentation of adolescent schizophrenia, and is more common in schizophrenia than in affective psychoses (Reference Hollis, Rutter, Bishop and PineHollis 2008). Non-specific behavioural changes such as social withdrawal, declining school performance, and uncharacteristic and odd behaviour begin, on average, over a year before the onset of positive psychotic symptoms. In retrospect, it can be seen that these behavioural changes were often early negative symptoms, which had their onset well before positive symptoms such as hallucinations and delusions.
Early recognition of the disorder is difficult, as premorbid cognitive and social impairments gradually shade into prodromal symptoms before the onset of active psychotic symptoms. Prodromal symptoms can include odd ideas, eccentric interests, change in affect, and unusual or bizarre perceptual experiences. Although these features can also occur in schizotypal personality disorder and autism spectrum disorder, in a schizophrenic prodrome there is usually progression to more severe dysfunction.
Diagnosis of schizophrenia in children and adolescents
Clinical characteristics
Even if strict adult definitions of schizophrenia (DSM-5 or ICD-10) are applied, there are age-dependent variations in phenomenology. Adolescent schizophrenia is characterised by a more insidious onset, negative symptoms, greater disorganisation (incoherence of thought and disordered sense of self), hallucinations in different modalities and, for relatively fewer patients, systematised or persecutory delusions (Reference Green, Padron-Gayol and HardestyGreen 1992).
A wide variety of anomalous perceptual experiences may occur at the onset of an episode of schizophrenia, leading to a sense of fear or puzzlement which may constitute a delusional mood and herald a full psychotic episode. These anomalous experiences may include the sense that familiar places and people and their reactions have changed in some subtle way. These experiences may result from a breakdown between perception and memory (of familiar places and people) and associated affective responses (salience given to these perceptions). For example, a young person at the onset of illness may study their reflection in the mirror for hours because it looks strangely unfamiliar, or misattribute threatening intent to an innocuous comment, or experience family members or friends as being unfamiliar, leading to a secondary delusional belief that they have been replaced by a double or alien. In summary, some clinical phenomena in schizophrenia can be understood in terms of a loss of normal contextualisation and coordination of cognitive and emotional processing.
Clinical assessment
The assessment of a child or adolescent with possible schizophrenia should include a detailed history, mental state and physical examinations and laboratory tests. A baseline psychometric assessment is also desirable. A detailed understanding of specific cognitive deficits in individual cases of adolescent schizophrenia can be particularly helpful in guiding education and rehabilitation. The neurological examination should focus on abnormal involuntary movements and other signs of extrapyramidal dysfunction.
Physical investigations
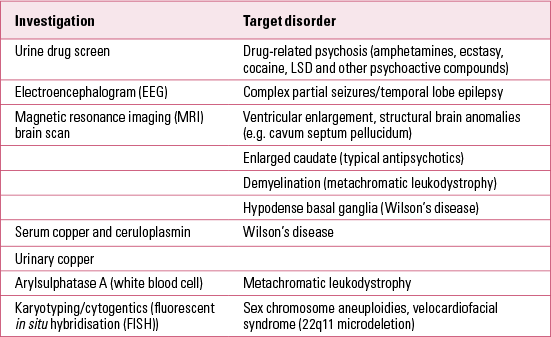
The potential range of physical investigations and laboratory tests in suspected cases of child- and adolescent-onset schizophrenia are listed in Table 1. It is usual to obtain a full blood count, liver and thyroid function tests and a drug screen (urine or hair analysis). The high yield of cytogenetic abnormalities reported in childhood-onset schizophrenia (Reference Nicholson, Giedd and LenaneNicholson 1999) suggests the value of cytogenetic testing, including karyotypying for sex chromosome aneuploidies and fluorescent in situ hybridisation (FISH) for 22q11.2 deletion syndrome (22q11DS or velocardiofacial syndrome).
TABLE 1 Physical investigations in child- and adolescent-onset psychoses
Developmental issues in assessment
The child’s cognitive level will influence their ability to understand and express complex psychotic symptoms such as passivity phenomena, thought alienation and hallucinations. In younger children, careful distinctions have to be made between developmental immaturity and psychopathology. For example, distinguishing true hallucinations from normal subjective phenomena like dreams and communication with imaginary friends may be difficult for younger children. Developmental maturation can also affect the spatial localisation of hallucinations. Internal localisation of hallucinations is more common in younger children and makes these experiences more difficult to subjectively differentiate from inner speech or thoughts. Formal thought disorder may also appear very similar to the pattern of illogical thinking and loose associations seen in children with immature language development. Negative symptoms can appear very similar to non-psychotic language and social impairments, and can also be easily confused with anhedonia and depression.
Differential diagnosis
Psychotic symptoms in children and adolescents are diagnostically non-specific, occurring in a wide range of functional psychiatric and organic brain disorders. The differential diagnosis of children and adolescents with suspected schizophrenia is summarised in Box 1. Referral for a neurological opinion is recommended if neurodegenerative disorder is suspected (see below).
BOX 1 Differential diagnosis of schizophrenia in childhood and adolescence
Psychoses
Affective psychoses (bipolar/major depressive disorder)
Schizoaffective disorder
Atypical psychosis
Developmental disorders
Autism spectrum disorders (Asperger syndrome)
Developmental language disorder
Schizotypal personality disorder
Organic conditions
Drug-related psychosis (amphetamines, ecstasy, lysergic acid diethylamide (LSD), phencyclidine (PCP))
Complex partial seizures (temporal lobe epilepsy)
Wilson’s disease
Metachromatic leukodystrophy
Affective, schizoaffective and ‘atypical’ psychoses
The high rate of positive psychotic symptoms found in adolescent-onset major depression and mania can lead to diagnostic confusion. Affective psychoses are most likely to be misdiagnosed as schizophrenia if a Schneiderian concept of schizophrenia is applied, with its emphasis on first-rank symptoms. Because significant affective symptoms also occur in about one-third of patients with first-episode schizophrenia, it may be impossible to make a definitive diagnosis on the basis of a single cross-sectional assessment. In DSM-5 the distinction between schizophrenia, schizoaffective disorder and affective psychoses is determined by the relative predominance and temporal overlap of psychotic symptoms (hallucinations and delusions) and affective symptoms (elevated or depressed mood). Given the difficulty in applying these rules with any precision, there is a need to identify other features to distinguish between schizophrenia and affective psychoses. Irrespective of the presence of affective symptoms, the most discriminating symptoms of schizophrenia are an insidious onset and the presence of negative symptoms (Reference Hollis, Rutter, Bishop and PineHollis 2008). Similarly, complete remission from a first psychotic episode within 6 months of onset is the best predictor of a diagnosis of affective psychosis (Reference Hollis, Rutter, Bishop and PineHollis 2008). Schizoaffective and atypical psychoses are diagnostic categories with low predictive validity and little longitudinal stability (Reference HollisHollis 2000).
Autism spectrum disorders
Some children with autism or Asperger syndrome have social and cognitive impairments that overlap closely with the premorbid phenotype described in schizophrenia. Furthermore, children on the autism spectrum can also develop psychotic symptoms in adolescence. In the NIMH childhood-onset schizophrenia sample, 19 individuals (25%) had a lifetime diagnosis of autism spectrum disorder; of these, 1 was diagnosed with autism, 2 with Asperger syndrome and 16 with pervasive developmental disorder not otherwise specified (PDD-NOS) (Reference Sporn, Addington and GogtaySporn 2004b). Although some children with autism spectrum disorders show a clear progression into classic schizophrenia, others show a more episodic pattern of psychotic symptoms without the progressive decline in social functioning and negative symptoms characteristic of adolescent schizophrenia.
Often it is only possible to distinguish between schizophrenia and an autism spectrum disorder by taking a careful developmental history that details the age at onset and pattern of autistic impairments in communication, social reciprocity and interests/behaviours.
Neurodegenerative disorders
Rare neurodegenerative disorders with onset in late childhood and adolescence can mimic schizophrenia. The most important examples are Wilson’s disease (hepatolenticular degeneration) and metachromatic leukodystrophy. These disorders usually involve significant extrapyramidal symptoms (e.g. tremor, dystonia and bradykinesia) or other motor abnormalities (e.g. unsteady gait) and a progressive loss of skills (dementia) that can aid the distinction from schizophrenia. Suspicion of a neurodegenerative disorder is one of the clearest indications for brain magnetic resonance imaging (MRI) in adolescent psychoses. Adolescents with schizophrenia show relative reduction in grey matter and sparing of white matter. In contrast, metachromatic leukodystrophy is characterised by frontal and occipital white matter destruction and demyelination. In Wilson’s disease, hypodense areas are seen in the basal ganglia, together with cortical atrophy and ventricular dilatation. The pathognomonic Kayser–Fleisher ring in Wilson’s disease begins as a greenish-brown crescent-shaped deposit in the cornea above the pupil (this is most easily seen during slit lamp examination). In Wilson’s disease there is increased urinary copper excretion, and reduced serum copper and serum ceruloplasmin levels. The biochemical marker for metachromatic leukodystrophy is reduced arylsulfatase-A (ASA) activity in white blood cells. This enzyme deficiency results in a deposition of excess sulfatides in many tissues, including the central nervous system.
Treatments
Pharmacological treatments
Because of the very small number of trials of antipsychotics conducted with child and adolescent patients, it is necessary to extrapolate most evidence on drug efficacy from studies in adults. However, it should be noted that children and adolescents show a greater sensitivity to a range of antipsychotic-related adverse events, including extrapyramidal side-effects (EPSE) and treatment resistance with traditional antipsychotics (Reference Kumra, Jacobsen and LenaneKumra 1998), and weight gain, obesity and metabolic syndrome with atypical antipsychotics (Reference de Hert, Dobbelaere and Sheridande Hert 2011).
Head-to-head comparisons of atypical and typical antipsychotics (e.g. risperidone v. olanzapine v. haloperidol) in adolescents with schizophrenia have reported similar efficacy against psychotic symptoms, but a differing profile of adverse effects (Reference Sikich, Frazier and McLellenSikich 2008). These findings broadly replicate results from the NIMH-funded Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) pragmatic study that found no overall difference in effectiveness between typical and atypical antipsychotics in adults, whereas there were differences in tolerability and side-effect profiles (Reference Lieberman, Stroup and McEvoyLieberman 2005). In younger patients (children and adolescents), EPSE are more common with haloperidol and high-dose risperidone than with olanzapine. Weight gain and obesity are most common with olanzapine, less common with risperidone and least common with haloperidol. Sedation is greater with olanzapine and haloperidol than with risperidone (Reference Toren, Ratner and LaorToren 2004). Further evidence is emerging that children and adolescents experience more rapid and serious weight gain on olanzapine and risperidone than do adults (Reference de Hert, Dobbelaere and Sheridande Hert 2011). Morbid obesity (body mass index BMI >90th percentile) is found in up to 50% of adolescents and young people chronically treated with atypical antipsychotics (Reference Theisen, Linden and GellerTheisen 2001). Complications of obesity include hyperglycaemia (type 2 diabetes), hyperlipidemia and hypercholesterolemia. It is recommended that dietary advice (reducing carbohydrate intake) combined with regular exercise should be prescribed before initiating antipsychotics in children and adolescents.
Baseline investigations and monitoring
Before starting treatment with antipsychotic medication a physical examination should include height, weight (BMI), cardiovascular examination, including pulse and blood pressure, and a neurological examination for evidence of abnormal movements. Baseline laboratory investigations include prolactin, fasting blood glucose and plasma lipids. Weight should be recorded weekly for the first 6 weeks on antipsychotic medication, repeated at 12 weeks and then every 6 months. Physical examination, laboratory investigations and review of adverse effects should be repeated 6 monthly while a young person is receiving antipsychotic medication (National Institute for Health and Care Excellence 2013).
Summary
Choice of antipsychotic is determined largely by the profile of adverse effects, as most drugs, with the possible exception of clozapine, show similar efficacy. The growing awareness of the adverse effect profiles of different drugs and greater sensitivity to these effects in children and adolescents means that drug choice should be a collaborative exercise, tailored to the needs and preferences of the young person and their family.
Psychosocial interventions
Family interventions
Psychosocial family interventions have a number of principles in common. First, it is useful for families to understand schizophrenia as an illness, as patients are then less likely to be seen as responsible for their symptoms and behaviour. Second, the family is not implicated in the aetiology of the illness. Instead, the burden borne by the family in caring for a disturbed or severely impaired young person is acknowledged. Third, the intervention is offered as part of a broader multimodal package that includes drug treatment.
An important issue when working with parents of children and adolescents with schizophrenia is to recognise that the illness typically results in a bereavement process for the loss of their ‘normal’ child. Parents will often value a clear diagnosis of schizophrenia, as it can provide an explanation for previously unexplained perplexing and disturbed behaviour. Understanding schizophrenia as a disorder of the developing brain can also relieve feelings of guilt commonly expressed by parents and carers.
Cognitive–behavioural therapy
Cognitive–behavioural therapy (CBT) has been shown to improve the short-term (6-month) outcome of adults with schizophrenia who have antipsychotic-resistant positive symptoms (Reference Turkington and KingdonTurkington 2000). The National Institute for Health and Care Excellence (NICE) clinical guideline recommends that children and young people with psychosis and schizophrenia should be offered family interventions and individual CBT in conjunction with antipsychotic medication (National Institute for Health and Care Excellence 2013). This recommendation reflects extrapolation of evidence and guidance in adult schizophrenia, as direct evidence is currently lacking for the benefit of family interventions or CBT in adolescent schizophrenia.
Course and outcome
Short-term course
Adolescent schizophrenia characteristically runs a chronic course, with only a minority of cases making a full symptomatic recovery from the first psychotic episode. The Maudsley follow-up study (Reference Hollis, Rutter, Bishop and PineHollis 2008) found that only 12% of young people with schizophrenia admitted to hospital were in full remission on discharge, compared with 50% of those with affective psychoses. The short-term outcome of schizophrenia presenting in early life appears to be worse than that of a first episode in adulthood. If full recovery does occur, then it is most likely within the first 3 months of onset of psychosis. In the Maudsley study, adolescent-onset patients who were still psychotic after 6 months had only a 15% chance of achieving full remission, whereas over half of those who had active psychotic symptoms for less than 3 months made a full recovery (Reference Hollis, Rutter, Bishop and PineHollis 2008). The clinical implication is that the course over the first 6 months of illness is the best predictor of remission.
Long-term outcome
A number of long-term follow-up studies of child- and adolescent-onset schizophrenia all describe a typically chronic, unremitting long-term course, with severely impaired functioning in adult life (Reference Hollis, Rutter, Bishop and PineHollis 2008). However, the generally poor outcome of early-onset schizophrenia conceals considerable heterogeneity. In most studies, about one-fifth of young patients have a good outcome with only mild impairment, whereas at the other extreme about one-third are severely impaired, requiring intensive social and psychiatric support.
Prognostic factors
The predictors of poor outcome in adolescent-onset schizophrenia include premorbid social and cognitive impairments, a prolonged first psychotic episode, extended duration of untreated psychosis and the presence of negative symptoms (Reference Hollis, Rutter, Bishop and PineHollis 2008).
Organisation of treatment services
It is a paradox that patients with child- or adolescent-onset schizophrenia have the most severe form of the disorder, yet they often receive inadequate and poorly coordinated services. Possibly this is because the responsibility for schizophrenia is seen to lie with adult psychiatric services, which have a remit that typically does not extend to patients under 18 years of age. In the UK, services for adolescents with psychosis and schizophrenia are provided by community-based child and adolescent mental health services (CAMHS) or, in some areas, by early intervention in psychosis (EIP) teams, which provide services for young people from age 14 upwards. The NICE clinical guideline for psychosis and schizophrenia in children and young people recommends that the assessment of young people with psychosis in EIP services should include access to a psychiatrist with training in child and adolescent mental health (National Institute for Health and Care Excellence 2013). However, there remain significant gaps in the provision of comprehensive services for adolescents with schizophrenia, including access to crisis and assertive outreach services and psychosocial interventions.
Conclusions
The past decade has seen a dramatic growth in our understanding of the clinical course and neurobiological underpinnings of adolescent schizophrenia. It is now clear that adult-based diagnostic criteria have validity in this age group and the disorder has clinical and neurobiological continuity with schizophrenia in adults. Adolescent schizophrenia is a severe variant of the adult disorder associated with greater premorbid impairment, a higher familial risk, and more severe clinical course and poorer outcome. The poor outcome of children and adolescents with schizophrenia has highlighted the need to target early and effective treatments and develop specialist services for this group.
MCQs
Select the single best option for each question stem
-
1 Twin studies have suggested that heritability in schizophrenia:
-
a is under 40%
-
b is between 40–50%
-
c is 50–60%
-
d may extend to schizotypal personality disorder
-
e none of the above.
-
-
2 Concerning the relation between cannabis and schizophrenia:
-
a cannabis use confers a fivefold increase in the risk of schizophrenia
-
b cannabis is directly implicated in adolescent schizophrenia
-
c cannabis is associated with later age at onset of schizophrenia in adults
-
d in the Dunedin study 20% of cannabis users aged 15 developed schizophrenia at age 26
-
e acute cannabis intoxication can precipitate psychotic symptoms.
-
-
3 Of the brain changes and developmental difficulties in schizophrenia:
-
a the brain changes differ considerably from those in adults
-
b individuals with schizophrenia have larger brains than normal
-
c ventricular enlargement is only detectable after the age of 26
-
d the three developmental impairments documented include language, reading and bladder control
-
e about two-thirds of young people with schizophrenia have an IQ below 70.
-
-
4 Differential diagnoses include all but:
-
a Wilson’s disease
-
b phenylketonuria
-
c developmental language disorder
-
d complex partial seizures
-
e metachromatic leukodystrophy.
-
-
5 Concerning the prognosis of adolescent schizophrenia:
-
a the majority of individuals make a full symptomatic recovery
-
b short-term outcome is better in youth than in adulthood
-
c if full recovery does occur, it is most likely within the first month
-
d prognosis is poor if the first episode is prolonged
-
e prognosis is independent of the presence of negative symptoms.
-
MCQ answers
| 1 | d | 2 | e | 3 | d | 4 | b | 5 | d |



eLetters
No eLetters have been published for this article.