Depression is the leading cause of disability worldwide, and a chronic or recurrent course results in substantial personal, social and economic consequences.Reference Birnbaum, Kessler, Kelley, Ben-Hamadi, Joish and Greenberg1,2 Although many effective antidepressant treatment strategies are available, approximately one-third of patients with major depressive disorder achieve remissionReference Gaynes, Warden, Trivedi, Wisniewski, Fava and Rush3,Reference Rush, Trivedi, Wisniewski, Nierenberg, Stewart and Warden4 so there is an urgent need for the development of new and more effective treatments. Meta-analyses have shown a rapid and robust antidepressant effect of the N-methyl-d-aspartate receptor antagonist ketamine in patients with treatment-resistant depression.Reference Caddy, Amit, McCloud, Rendell, Furukawa and McShane5,Reference Han, Chen, Zou, Zheng, Li and Wang6 These promising results have led to growing media interest in using ketamine.Reference Zhang, Hong, Husain, Harris and Ho7 Although doctors are legally allowed to prescribe ketamine there is relatively scarce information on the consequences of repeated and longer-term use in depression. However, the media continue to describe ketamine as a new therapeutic breakthrough for depression, which has encouraged many patients to request ketamine treatment. Commercial clinics in the USA are offering it on a large scale despite not being approved for depression.Reference Sanacora, Frye, McDonald, Mathew, Turner and Schatzberg8,Reference Wilkinson, Toprak, Turner, Levine, Katz and Sanacora9 Some hospitals in England also use it, but it is not currently available from the National Health Service (NHS) outside a handful of centres.
The Ketamine Advocacy Network is a patient organisation whose aim is for ketamine treatment to be widely available.10 Many patients are also eager to try this experimental treatment, sometimes prior to conventional evidence-based antidepressant treatment.Reference Veraart, Smith-Apeldoorn, Trueman, de Boer, Schoevers and McShane11 Researchers have taken a more conservative approach that the clinical implementation of ketamine treatment is premature and should wait for better evidence on its effects and side-effects.Reference Loo12,Reference Schatzberg13 Consensus statements and ethical discussions also advocate a cautious expansion of use.Reference Sanacora, Frye, McDonald, Mathew, Turner and Schatzberg8,Reference Singh, Morgan, Curran, Nutt, Schlag and McShane14 This is partially because of the fact that both popular and research accounts indicate that ketamine has been used recreationally in nightclubs and dance parties,Reference Curran and Morgan15,Reference Cysell16 but may also be because of its potential to develop tolerance and craving. One important group absent from these discussions is the patients themselves. This paper explores the attitudes of (a) individuals who are considering ketamine as an antidepressant option; (b) individuals who have used ketamine as an antidepressant; (c) people with experience of illegal drug use and addiction, and (d) patient advocates and carers, to inform policy and practical decisions about its clinical use.
Method
Design
This is a mixed-methods study exploring ketamine prescription for depression using focus groups and an online questionnaire in the form of voting questions. This took place as part of a consultation day on the 22 August 2018. The focus groups asked participants for their opinions on the best way for ketamine and similar drugs to be used in the UK, and to share their priorities on options for how these could be prescribed and monitored.
Participants and recruitment
We invited patients using mental health services – some with experience of using ketamine and some without; carers; people with experience of illegal drug use and addiction, and advocates to discuss their views about ketamine as a potential treatment for depression.
All individuals eligible who replied to the invitation or advert were allocated a place at the event. Individuals were screened to ensure they met the participant criteria of being:
(a) a patient with experience of depression;
(b) a carer of a patient with experience of depression;
(c) a person with experience of addiction.
As part of the registration process people were asked about their experience of depression and ketamine. This information was only used when allocating participants to the focus groups.
Forty-four participants joined us for the day. Participants were patients (n = 38, 20 with self-disclosed experience of ketamine, of which 15 had experience of ketamine in a medical setting), advocates (n = 3), carers (n = 3) and some participants had dual roles.
The carers registered themselves as having no experience of using ketamine and were simply participating as a carer for someone who has experienced ketamine treatment or a recreational user. The three advocates have experience as peer support workers and two help raise awareness on harm reduction and support people with experience of depression, addiction and drug use. Two also have experience of using ketamine recreationally. The dual roles included one participant identifying as a patient/carer, and 26 participants identifying as patient/advocate. There were 14 men, 22 women and 8 participants with no gender specified. Participants were not asked to provide their date of birth or age when registering.
Recruitment was by five channels: patients with an interest in the ‘ketamine for depression service’ at Oxford Health NHS Foundation Trust (n = 30); the Service User Advisory Group (n = 6), Young Person's Mental Health Advisory Group (n = 3) and Addictions Service User Research Group (n = 1) at the Maudsley Biomedical Research Centre; ‘DrugWise Daily’ (n = 4) website advert (www.dsdaily.org.uk).
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008. Participants acknowledged their consent to discuss the topic when registering for the event. Participants were offered payment of £75.00 plus travel expenses for their time and contribution.
Procedure
The event was structured into three sessions, with voting incorporated into session three. Each discussion session was preceded by a short presentation that offered participants the opportunity to ask questions to ensure all participants had enough prerequisite information about the topics to enable a varied discussion. The information from these presentations can be found in supplementary Table 1 (available online at https://doi.org/10.1192/bjo.2019.52). Because of the sensitivities we did not audio record any discussion session, but participants were able to make written notes and scribes summarised the points raised and if specific to patients or carers noted that in the record. After each focus group, the scribe's notes were summarised back to the group to ensure all participants agreed that the notes were accurate and relevant.
Sessions 1 and 2 were structured around a focus group topic guide. Session 3 focused on the voting questions.
In each session there were seven discussion groups, of 5 to 8 participants each with a facilitator and scribe, one of whom was a patient–researcher, and the other person was a graduate research assistant working in mental health research. The groups remained the same for all discussions in session 1 but then individuals were reallocated for sessions 2 and 3. The topic guide and voting questions are shown in Appendices 1 and 2. Participants were also invited to feedback in writing in response to the following: ‘What would be your ideal prescribing solution?’
Analysis
The focus group data were imported into QSR International's NVivo 9 software, a qualitative data analysis program. The data consisted of each scribe's notes as well as the participant's notes from the feedback sheets. Each set of notes from the seven notetakers were combined into a large document for analysis.
Using an exploratory framework analysis,Reference Pope, Ziebland and Mays17 two patient-researchers (A.W. and H.G.) independently identified themes from the data and constructed a thematic framework. They met to reach consensus about the codes to be used and generated the final framework. As the focus groups were mixed between participants and the facilitators were masked to the participants’ ketamine/depression background, the difference in opinion between groups is not available. Voting data were analysed for frequencies of response. Further detail of the methods used is provided in supplementary Fig. 1.
Results
Ten key themes were identified that incorporated 13 subthemes (Fig. 1). They are described and where appropriate supported by the voting answers in session 3. When participants signed up to the discussion day, and in the course of the discussions, participants disclosed their drug use (legal and illegal) as well as whether they had ever been prescribed ketamine.
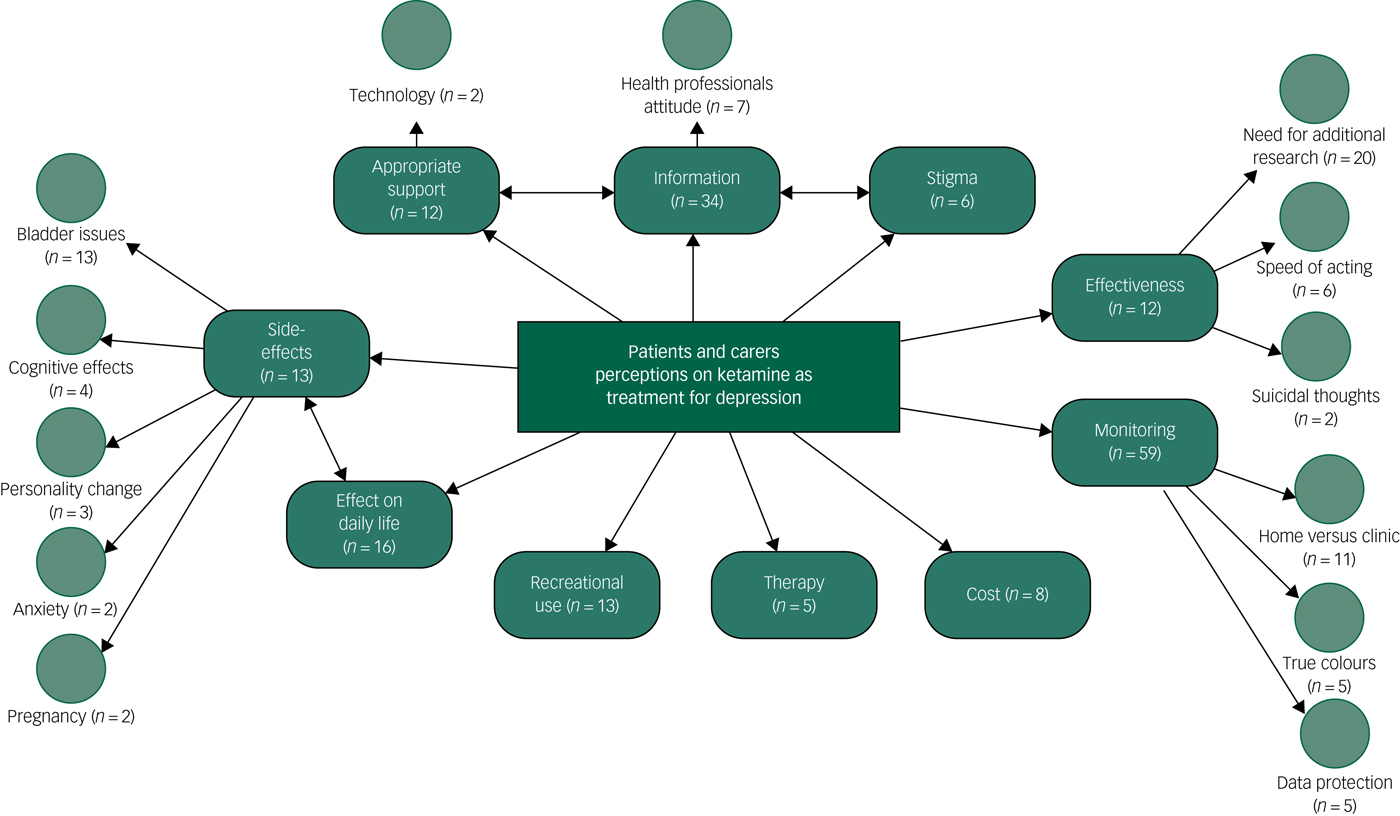
Fig. 1 Patients’ and carers’ perceptions on the use of ketamine as a treatment for depression.
Monitoring
Participants demonstrated a keen interest in the discussion around monitoring ketamine and in the voting the majority of participants felt extra monitoring was required (63%, n = 28). This involved a discussion about how ketamine usage can be regularly reviewed to ensure personal safety (for example dosage, side-effects and dependency; discussed further below), how ketamine could be administered and how this administration can be monitored as part of a national monitoring system. When participants discussed how much monitoring was needed the answers ranged from infrequent to a very intensive monitoring regime. Most participants felt that administering ketamine in the clinic for the first few times and then being allowed to take it at home to self-administer was the most desirable outcome. It is, however, worth mentioning that some participants expressed concerns around the risks of home administration:
‘Not sure how I feel about people taking esketamine home – if it's that potent – could take it all or pile it up.’
There was a range of views for what monitoring system was acceptable with 48% (n = 21) opting for a national system but 29% (n = 13) did not agree and 23% (n = 10) were unsure.
In the discussion it was clear that if a national monitoring system was in place then it should be anonymous and confidential, and completely protect patients’ privacy. Any monitoring should also not disadvantage patients such as those needing ketamine but with a previous history of addiction. Generally, monitoring should be mainly for the benefit of the patients, and to gather anonymous data for the research purposes, but not for the benefit of pharmaceutical or insurance companies.
Information
Nearly all participants thought that there was not enough information about ketamine as a treatment option. This included information about side-effects, long-term effectiveness, costs and what experiences are normal or not when taking ketamine. Family members and carers also expressed a wish for more information about ketamine and its side-effects, which can cause distress and miscommunication.
Participants were also concerned about health professionals' lack of information about using ketamine as a treatment for depression. They said they often feel guilty and not well understood by health professionals, particularly by general practitioners (GPs), when disclosing ketamine as a drug of choice to treat depression. Participants also hoped that action would be taken to normalise ketamine as a drug that changes people's lives for the better and allows them to feel happy and that educating health professionals about ketamine was a high priority.
Importantly almost 80% (n = 35) of participants suggested that patients should be asked to provide information about their use of ketamine, their mood and quality of life in order to be prescribed ketamine. They also felt that health information is too often not shared that can cause disruption and negatively affect the patient and their care.
Effect on daily life
Participants were worried about long travel times to the clinic for administering ketamine and how disruptive and costly it can be, not only to themselves, but also their partners and carers. Participants were also concerned about their ability to drive, work and take care of their children responsibly after ketamine administration.
Side-effects
A common worry was the presence of ketamine-related side-effects. Participants were concerned about becoming more anxious following ketamine and how that anxiety can further affect depression. Urinary tract infection and other bladder problems were often mentioned as concerns as well as the effects on cognitive processes – particularly on their memory. Participants also wondered whether ketamine would influence their personality.
Participants felt that despite currently unknown long-term side-effects, potential benefits outweighed potential risks (such as bladder issues or personality changes). Most participants were willing to take those risks to alleviate depressive symptoms, improve their daily life functioning and rekindle their relationships with their loved ones. However, side-effects seemed to be acceptable if they did not have a negative influence on their relationships with loved ones. This involved side-effects that could change a patients' personality and cognitive abilities that would have an impact on their behaviour. This in turn would make them a burden on their families or change their relationships. Dose and frequency of using ketamine raised discussions about how increasing tolerance could lead to dependency. Lastly, there was a concern about taking ketamine while pregnant.
Recreational use
Most focus groups discussed the dangers of recreational use of ketamine and participants varied in their experience of recreational use. Some felt comfortable buying ‘off the streets’ and others could not even imagine buying ‘off the streets’ even though they knew that ketamine could help with their depression. The inexperienced group also said that they would not buy ‘off the street’ because of concerns about the purity of ketamine and potential legal consequences. Although it was acknowledged that there may be a risk of migrating from one recreational drug to another, the general feeling was that this risk is low and similarly the risk of hoarding and selling on illegally was also low.
Effectiveness
Participants commented on the considerable gaps in current research about ketamine and its long-term effects. Despite that, participants thought that ketamine was an effective method of treating, and managing, depression. For instance, a few participants with experience of taking ketamine mentioned how ketamine could prevent or significantly minimise the presence of suicidal thoughts.
One of the most frequently mentioned advantages of using ketamine as a treatment for depression was that it relieves depressive symptoms rapidly, as noted by participants who have had experience with taking ketamine. Some participants shared that they received ketamine as a ‘one-off’ dose clinically when feeling helpless and desperately needed to alleviate depressive symptoms, whereas others who took ketamine recreationally also confirmed that ketamine has a rapid effect.
Appropriate support
The majority of participants felt that support when given ketamine could be provided by appropriately training healthcare professionals or carers/family members so that the burden of pastoral care is not solely with a psychiatrist. However, participants were not in agreement about whether patients should attend a mental health clinic to take ketamine (41% (n = 18) in favour and 38% (n = 17) against). Participants felt that health professionals and/or family members should be able to give this support. One participant noted that the costs of a national system could be reduced if health professionals or family members were able to provide this support rather than a doctor or nurse:
‘When taking ketamine, I think that the person should have someone with them in a clinic, but that second person can be a carer or a friend or peer support worker, or a professional with lived experience of ketamine but they do not need to be a qualified healthcare professional’.
Cost
The cost of ketamine was thought to be a vital factor when considering its use, specifically in relation to those patients who would need it long term. Participants discussed the difference in the cost of ketamine via an NHS prescription (which is £8.80) versus the cost of ketamine ‘on the streets’. Some participants felt that if they were prescribed regular ketamine for long-term use, they would consider buying ketamine ‘off the street’ if it was cheaper.
Stigma
There were a considerable number of participants who felt that there is stigma around using ketamine to treat depression, following its bad reputation as a horse tranquiliser and hallucinogenic drug taken in nightclubs. One group discussed the media's negative portrayal of ketamine, with the underlying message being that if licenced, it could lead to addiction. It was noted that ketamine is stigmatised more despite ‘other drugs used medically are also sold on the streets’.
Therapy
Some participants said that ultimately, talking therapy is equally as crucial as taking ketamine for recovering from depression and improving their day-to-day functioning. Participants felt that this should not be neglected for ketamine-only treatment. As one participant mentioned in the follow-up survey: ‘talk therapy/counselling should be a part of the process’.
Prescribing options
Participants were asked to vote for their preferred option for prescribing ketamine and also had the option to expand on the question, ‘What would be your ideal prescribing solution?’ (Appendix 2).
Most participants agreed that a psychiatrist should prescribe ketamine, or in some instances a GP, providing their knowledge of ketamine was sufficient (Fig. 2). Some suggested that in the beginning it should be a psychiatrist prescribing ketamine, and after a certain period of monitoring and assessing side-effects, this responsibility could then be transferred to the GP. Mostly, participants felt that there should be some system of monitoring in place, for example through questionnaires. However, it is noteworthy that there was no consensus on the frequency of monitoring: it ranged from daily through weekly monitoring to monthly monitoring. As one participant said:
‘If there was easier access to psychiatrists they should make the assessment and prescription. As access is poor, then GPs need to be far better educated on the matter and patients allowed a secondary opinion from another GP, if their own GP is initially reluctant to prescribe and they feel very strongly that they should be taking ketamine. Collection from pharmacies, taken at home and optional questionnaires.’
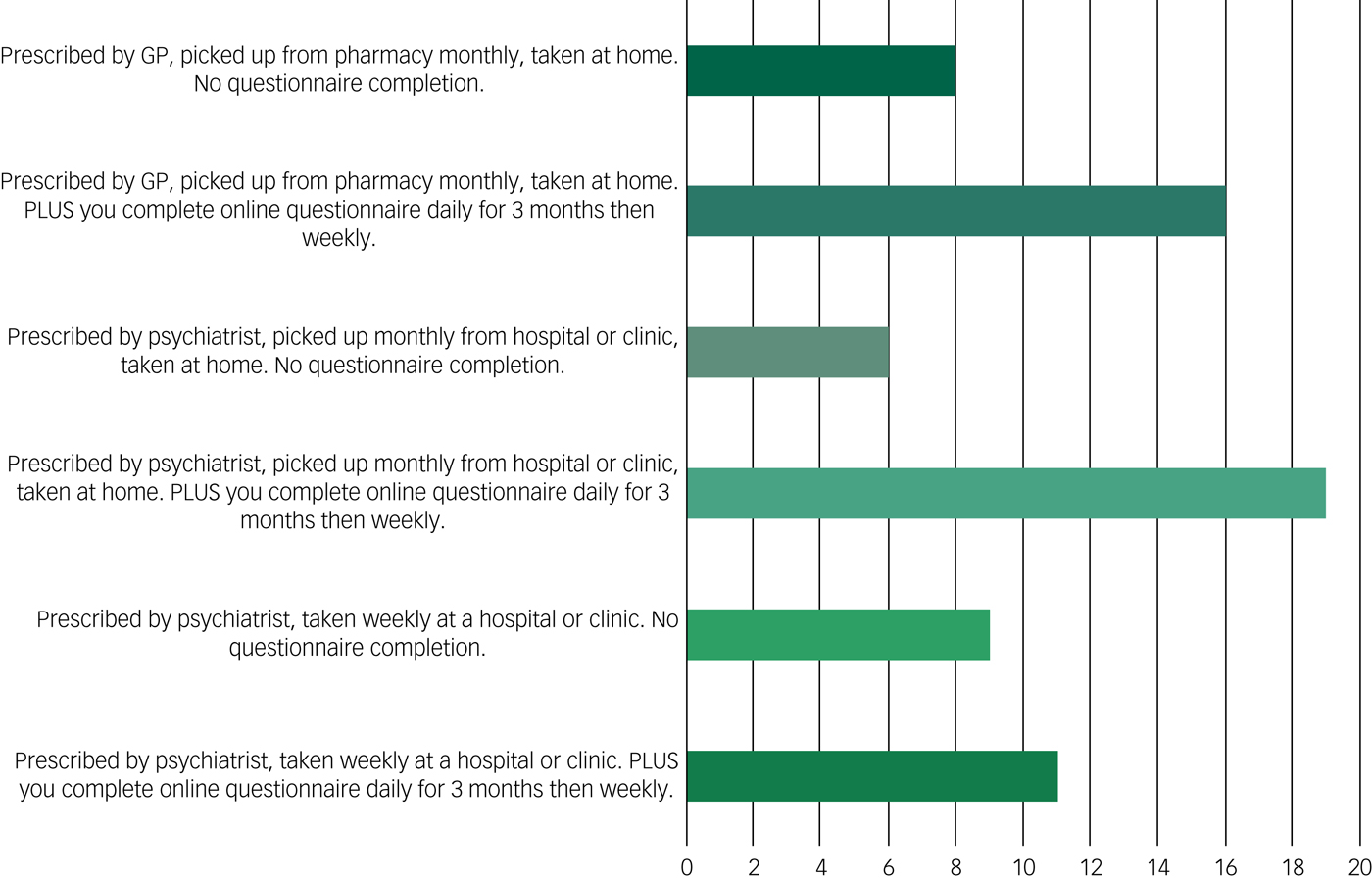
Fig. 2 Responses to the options offered to the question ‘Which prescribing option would be best for ketamine as a treatment for depression?’ (n = 69). Participants were allowed to select more than one option, and they selected prescribing options they felt comfortable with.
Discussion
Main findings
Although there was a range of views on almost all the topics discussed there were still some general guidance that can be drawn from the consultation. First, patients want better evidence on long-term use. This was particularly focused on safety and side-effects. Second, there is a recognition that monitoring is required, both to gather this evidence, and to ensure safe use and administration. However, there was some scepticism about the control of such monitoring by drug companies or even by the NHS because of privacy issues and the potential misuse of data. When provided with options for monitoring intervals there was not much agreement about how often. Finally, it was thought that the best way to manage the competing need of controlling risks but ensuring access was for patients to gain experience of ketamine through initial clinic-based administration, followed by taking it at home. This was balanced by a wish to see ketamine made widely available in a medical setting and scepticism that secondary care, as it is currently configured, can deliver this.
Practical issues of access were important: repeated travelling to remote clinics and lack of sufficiently informed medical staff were key barriers. A vicious circle was identified in which low availability was both caused by and contributed to stigma.
Strengths and limitations
This study is not without its limitations. The focus groups were not audio recorded, and the analysis was reliant upon hand-written notes, which limits the depth of our understanding of certain issues. Although the wide variety of experience was a strength of the study, we only had 3 carers and 3 advocates out of the 44 participants. This may have affected our results and the next generation of studies might recruit homogeneous groups so that the differences in views might be more in focus. A limitation on the qualitative method is that we did not continue to recruit new participants and so analyse data until no new themes arose (saturation). To minimise interpretation bias and to ensure that the qualitative data was accurate and relevant, we embedded a member checking process at the end of each focus group. A strength of the study was that patient–researchers facilitated the focus groups and analysed the qualitative data. We also ensured a diverse sample in terms of age and experiences to capture a wide scope of opinions.
Implications
Taken together, these results present an important overview of the current perceptions of ketamine as a treatment for depression. To meet the aspirations of these ‘experts by experience’ requires: education of health service staff about the use of ketamine; the development of monitoring structures that treat patients as collaborating partners; and wide access through the development of specialist services that can manage both clinic- and home-based treatment.
Funding
This paper represents independent research part funded by the National Institute for Health Research (NIHR) Biomedical Research Centre at South London and Maudsley NHS Foundation Trust and King's College London (IS-BRC-1215-20018). The views expressed are those of the authors and not necessarily those of the NHS, the NIHR or the Department of Health and Social Care. T.W. acknowledges the financial support of the NIHR Senior Investigator Award. We also acknowledge funding from NIHR Oxford Health Biomedical Research Centre, NIHR University College London Hospitals Biomedical Research Centre, University of Exeter, and the NIHR Collaboration for Leadership on Applied Health Research and Care Oxford.
Acknowledgements
We would also like to acknowledge Professor Val Curran and Professor Celia Morgan for their advice in setting up with focus groups. Data availability: all authors have ongoing access to the study data. All data is fully anonymised. This data can be made available on request.
Supplementary material
Supplementary material is available online at https://doi.org/10.1192/bjo.2019.52.
Appendix 1

Appendix 2






eLetters
No eLetters have been published for this article.