Polyphenolic compounds are found in a wide variety of human foods, including fruits, vegetables, nuts, seeds, tea and honey(1). Flavonoids are a large and complex group of polyphenols that share a basic three-ring chemical structure, with two aromatic centres and a central oxygenated heterocyclic ring(Reference Manach, Williamson and Morand2). The most prominent flavonoids in fruits and vegetables are flavonols, and, of these, quercetin (3,3′,4′,5,7-pentahydroxyflavone) is the most commonly consumed in the human diet(Reference Manach, Williamson and Morand2). The physiological effects of dietary flavonols such as quercetin are of current interest due to their in vitro anti-oxidative(Reference Číž, Pavelková and Gallová3, Reference Dias, Porawski and Alonso4), anti-inflammatory(Reference Comalada, Camuesco and Sierra5, Reference Nair, Mahajan and Reynolds6) and anti-pathogenic(Reference Vrijsen, Everaert and Boeyé7, Reference Chiang, Chiang and Liu8) activities.
Total dietary flavonol intake estimates for US adults range from 13 to 22 mg/d, with quercetin representing about 75 %(Reference Chun, Chung and Song9, Reference Sampson, Rimm and Hollman10). Following absorption from food or supplements, elimination of quercetin is slow, with a reported half-life ranging from 11 to 28 h(Reference Manach, Williamson and Morand2). Quercetin conjugates are widely distributed in the organ tissues of rats after supplementation, where they may be biologically active(Reference de Boer, Dihal and van der Woude11). Despite earlier concerns, long-term supplementation with high doses of quercetin has not been linked to any adverse effects in rodents or human subjects(Reference Harwood, Danielewska-Nikiel and Borzelleca12, Reference Utesch, Feige and Dasenbrock13). Moreover, epidemiological studies have positively correlated flavonoid intake with decreased incidence of CHD(Reference Hertog, Feskens and Hollman14) and common human cancers(Reference Neuhouser15). The potential beneficial health effects of supplementation with quercetin deserve further attention and are the subject of the present study.
In vitro and animal studies indicate that quercetin supplementation has the potential to exhibit multiple immunomodulatory effects including augmentation of neutrophil chemotaxis and respiratory burst activity(Reference Akbay, Basaran and Undeger16), macrophage function(Reference Oršolić and Bašić17, Reference Yu, Lai and Yang18) and natural killer (NK) cell lytic activity(Reference Yu, Lai and Yang18, Reference Exon, Magnuson and South19). NK cells and granulocytes are dominant players in early host defence against pathogens; thus, their potential augmentation by quercetin could have important health benefits. NK cells, which comprise 10–15 % of circulating lymphocytes in humans, play a central role in initial host resistance to viral, bacterial and parasitic infections due to their ability to lyse target cells without prior sensitisation(Reference Bancroft20, Reference Cooper, Fehniger and Caligiuri21). Also critical to optimal cellular immune function are the phagocytic and microbicidal activities of granulocytes, of which polymorphonuclear leucocytes, or neutrophils, are the most abundant. Neutrophils are swiftly recruited to infection sites, where they engulf pathogens in a process called phagocytosis(Reference Kobayashi, Voyich and Burlak22). Phagocytosis, in turn, stimulates the production of reactive oxygen species. This respiratory burst, also known as the oxidative burst, is responsible for the oxygen-dependent intracellular killing of ingested microbes. Several animal and in vitro studies have shown that quercetin augments NK cell activity (NKCA) as well as granulocyte oxidative burst activity (GOBA) and phagocytosis(Reference Akbay, Basaran and Undeger16, Reference Yu, Lai and Yang18, Reference Exon, Magnuson and South19), but few studies have examined the effects of quercetin supplementation on innate immune function in human subjects.
Two short-term human studies previously conducted by our laboratory found no differences in NKCA or GOBA following ingestion of aglycone quercetin at 1000 mg/d for 3 weeks compared with placebo(Reference Henson, Nieman and Davis23, Reference Nieman, Henson and Gross24), although one study found that quercetin supplementation reduced the incidence and severity of respiratory infections in endurance athletes after 3 d exhaustive exercise(Reference Nieman, Henson and Gross24). However, these studies were primarily focused on quercetin's acute effects on post-exercise immune function perturbations in highly trained athletes, and as such were limited in their application to non-athletes. The present study seeks to examine the chronic effects of quercetin consumption on innate immune function in the general population. A secondary objective of the study was to assess the influence of quercetin on markers of inflammation.
Chronically elevated inflammatory cytokines such as IL-6 and TNF-α have been correlated with increased risk of rheumatoid arthritis(Reference Koch, Kunkel and Strieter25) and CHD(Reference Danesh, Kaptoge and Mann26). Quercetin, which has been shown to exert strong in vitro anti-inflammatory effects, acts specifically through the NF-κB signalling pathway to decrease expression of IL-6 and TNF-α(Reference Comalada, Camuesco and Sierra5, Reference Nair, Mahajan and Reynolds6). Supplementation with quercetin could potentially be used to reduce chronic expression of these inflammatory cytokines, thereby lowering disease risk. Although quercetin exerts strong in vitro anti-inflammatory effects, few supplementation trials have been conducted to examine the effects of quercetin supplementation on circulating markers of inflammation. A recent study by Egert et al. investigated whether 150 mg quercetin/d for 6 weeks would decrease plasma TNF-α in overweight and obese subjects, but no such effect was found(Reference Egert, Bosy-Westphal and Seiberl27). The authors postulated that the dosage of quercetin used in their study was insufficient to exert significant anti-inflammatory effects in the blood compartment. The present study will examine whether longer-term supplementation with quercetin at higher dosages has an influence on markers of inflammation in adult female subjects.
It is currently unknown whether long-term quercetin supplementation exhibits an immunomodulatory effect in humans, or whether these potential effects are dose-dependent. The objective of the present study was to measure the influence of 12-week aglycone quercetin supplementation in two doses (500 and 1000 mg/d) on measures of innate immune function (NKCA, GOBA and granulocyte phagocytosis) and markers of inflammation (plasma IL-6 and TNF-α) in adult, community-dwelling female subjects. Dosages and supplementation periods used were chosen based on animal data and prior work in our laboratory(Reference Davis, Murphy and Carmichael28, Reference Nieman, Henson and Davis29).
Methods
Subjects
A total of 120 females, aged 30–79 years, were recruited via mass advertising. Subjects had to be healthy and non-institutionalised, and women were excluded if pregnant or lactating. Subjects agreed to avoid any other supplements containing quercetin; no other restrictions were placed on diet, supplement usage or medications. The present study was conducted according to the guidelines laid down in the Declaration of Helsinki and all procedures involving human subjects were approved by the institutional review board of Appalachian State University. Written informed consent was obtained from all subjects.
Research design
Subjects were randomised to one of three groups: 500 mg quercetin/d (Q-500; n 38), 1000 mg quercetin/d (Q-1000; n 40) or placebo (n 42). Supplements were administered utilising double-blinded procedures. Subjects ingested two soft chew supplements twice daily (upon awakening, and between 14.00 hours and the last meal of the day) during the 12-week study period. Supplements were prepared by Nutravail Technologies (Chantilly, VA, USA) with Quercegen Pharma (Newton, MA, USA), and were soft, individually wrapped chews (5·3 g/piece) that contained either 125 or 250 mg quercetin, 125 or 250 mg vitamin C (ascorbic acid and sodium ascorbate), 5 or 10 mg niacin, and 20 kcal (84 kJ) of sugars in a carnauba wax, soya lecithin, maize starch, glycerine and palm oil base coloured with Food, Drug and Cosmetic Act (FD&C) yellow no. 5 and no. 6. A series of HPLC measurements was conducted to determine that the amount of quercetin in each chew was stable and accurate. Placebo supplements were prepared in the same way minus the quercetin, vitamin C and niacin. Data from Quercegen Pharma (unpublished results, personal communication) indicate that bioavailability of quercetin is enhanced with vitamin C and niacin; hence, the present study tested whether or not soft chews with or without the combination of quercetin, vitamin C and niacin had an influence on the outcome measures. Subjects started supplementing after the first blood sample and continued for 12 weeks. At 2 weeks before the first laboratory visit for the study, subjects provided demographic and lifestyle habit information via a survey posted on the web using SurveyMonkey.com (Portland, OR, USA). Information on dietary patterns was obtained through a semi-quantitative FFQ for food groups including fruit, vegetables, cereals, meat, dairy and fat. Exercise habits were assessed through answers to categorical questions dealing with both leisure-time and work activities. Physical fitness levels were self-reported using a ten-point Likert scale, with one corresponding to low fitness and ten to high fitness. Subject height was measured with a stadiometer, and BMI and body composition were determined using a Tanita bioelectrical impedance scale (Tanita, Arlington Heights, IL, USA). Before and after the 12-week supplementation period, subjects came to the laboratory in the morning (07.00 to 09.00 hours) after an overnight fast to donate blood samples. Blood samples were taken from an antecubital vein with subjects in a seated position. Plasma quercetin levels and leucocyte subset cell counts were analysed for all samples. NKCA and lymphocyte subsets were analysed for seventy-four subjects, and GOBA and phagocytosis were assessed in sixty-four subjects. There was some overlap between the cohorts: eighteen subjects were tested for both NKCA and GOBA/phagocytosis.
Plasma quercetin
Total plasma quercetin (quercetin and its primary conjugates) was measured following solid-phase extraction via reversed-phase HPLC with UV detection as previously described(Reference Nieman, Henson and Davis30, Reference Nieman, Henson and Maxwell31). Quercetin conjugates were hydrolysed by incubating 500 μl plasma samples with 10 μl 10 % dl-dithiothreitol solution, 50 μl 0·58 m-acetic acid, 50 μl of a mixture of β-glucuronidase–arylsulfatase and crude extract from Helix pomatia (Roche Diagnostics Corporation, Indianapolis, IN, USA) for 2 h at 37°C. Chromatographic analysis was performed using the Ultimate 3000 HPLC-PDA system (Dionex Corporation, Sunnyvale, CA, USA) with a Gemini C18 column (Phenomenex, Torrance, CA, USA). Three quality control samples, using human plasma samples spiked with quercetin at concentrations of 1·0, 1·5 and 3·0 μmol/l, were assayed in duplicate, with an intra-assay CV of 12·5 %.
Leucocyte differential
A complete blood count with leucocyte differential was analysed in the clinical laboratory of the Watauga Medical Center (Boone, NC, USA) using standard clinical laboratory equipment and quality standards.
Plasma IL-6 and TNF-α
High-sensitivity ELISA kits were used to measure total plasma concentrations of IL-6 and TNF-α in accordance with the manufacturer's protocol (R&D Systems, Inc., Minneapolis, MN, USA). All samples and provided standards were analysed in duplicate. The minimum detectable concentrations of IL-6 and TNF-α were < 0·039 pg/ml and < 0·106 pg/ml, respectively. Pre- and post-supplementation samples were analysed on the same assay plate to decrease interkit assay variability, and the intra-assay CV for all variables was less than 10 %. Data were analysed with SOFTmax software (Molecular Devices, Sunnyvale, CA, USA).
Lymphocyte subsets
Lymphocyte subset data (%T, %B and %NK) were obtained by flow cytometry. Briefly, peripheral blood mononuclear cells were isolated from heparinised whole blood by density gradient centrifugation with Fico/Lite. The cells were washed twice and re-suspended to 1 ml in Roswell Park Memorial Institute (RPMI)-1640 supplemented with 10 % heat-inactivated fetal calf serum, penicillin, streptomycin and l-glutamine (complete-RPMI). A sample (100 μl) of cells was removed and stained for 15 min with 10 μl CYTO-STAT® tetraCHROME™. In addition to CD45-fluorescein isothiocyanate (FIFC), the fluorescent conjugates used were as follows: CD56-phycoerythrin (RD1), CD19-phycoerythrin-Texas-Red®-X (ECD) and CD3-phycoerythrin-Cy5 (PC5) (Beckman Coulter, Fullerton, CA, USA). CYTOSTAT® tetraCHROME™ is a combination of four murine monoclonal antibodies, each conjugated to a specific fluorochrome, used to differentiate lymphocyte subsets based on their cell surface markers. The lymphocytes were gated using intracellular complexity (side scatter) and FITC fluorescence intensity (CD45+), and flow cytometric dot plots of CD19-ECD v. CD3-PC5 and CD56-RD1 v. CD3-PC5 were produced. In this manner, the percentage of lymphocytes that were NK cells (CD56+ CD3− ), B-cells (CD19+ CD3− ) and T-cells (CD3+ CD19− ) was determined for each subject. Absolute numbers of each cell type were then calculated using the complete blood count data to allow group comparison of circulating cell counts.
Natural killer cell activity assay
NKCA was assessed using a modification of a flow cytometric assay(Reference Nieman, Henson and Gross24, Reference Chang, Gusewitch and Chritton32). Peripheral blood mononuclear cells (effector cells) were isolated from heparinised blood by density gradient centrifugation with Fico/Lite, washed twice, and re-suspended to 3·75 × 106 cells/ml. K562 cells in log phase (target cells, 1 × 106 cells/ml) were labelled for 20 min at 37°C in 5 % CO2 with 10 μl 3 mm-DiO solution (3,3′-dioctadecyloxacarbocyanine perchlorate (Sigma Chemical Co., St Louis, MO, USA) in dimethylsulfoxide) per 1 ml cell suspension. After labelling, the target cells were washed and re-suspended in complete-RPMI to a concentration of 5 × 105 cells/ml. Effector and target cells were combined in four 15 × 75 mm (5 ml) tubes to yield effector:target ratios of 60:1, 30:1, 15:1 and 7·5:1 with final volumes of 0·9 ml. Given the proven reproducibility, high sensitivity and large number of cells used in this flow cytometric assay, a single assay tube was used for each effector:target ratio(Reference Chang, Gusewitch and Chritton32). Control tubes received target cells with no effectors. All tubes received 0·1 ml of a solution of propidium iodide (500 μg/ml; Sigma Chemical Co., St Louis, MO, USA) in RPMI, after which the tubes were vortexed, pelleted by centrifugation for 2 min at 1200 rpm, and incubated for 2 h at 37°C in 5 % CO2. Following incubation, the tubes were vortexed briefly, placed in an ice-water-bath and analysed by flow cytometry within 45 min. Analyses of samples were performed using a Beckman Coulter FC-500 flow cytometer with CXP software (Fullerton, CA, USA). DiO-labelled target cells emit a green fluorescence that can be detected on fluorescence channel 1 and compromised cells that have taken up propidium iodide emit a red fluorescence that can be detected on fluorescence channel 3. The percentage of target cells that were compromised was determined for each tube. The results were acceptable if the spontaneous lysis of target cells (percentage non-viable target cells in the control tube) was ≤ 5 %. NK cell-induced lysis of target cells was determined by subtracting the spontaneous lysis of target cells from the percentage of non-viable target cells in tubes containing both effectors and targets at each effector:target ratio. Results were normalised by conversion to lytic units, calculated as the number of effector cells required to lyse 20 % of 5000 target cells, and reported as the number of lytic units contained in 107 cells(Reference Bryant, Day and Whiteside33).
Measurement of granulocyte phagocytosis and oxidative burst activity
Simultaneous measurement of granulocyte phagocytosis and oxidative burst activity was performed using a modified flow cytometric assay(Reference Perticarari, Presani and Banfi34). For each sample, 100 μl heparinised whole blood were dispensed into two 15 × 75 mm (5 ml) tubes. To each tube, 10 μl hydroethidine working solution (10 μg hydroethidine/ml in PBS–glucose; Invitrogen Corporation, Carlsbad, CA, USA) were added. The tubes were vortexed briefly, incubated in a 37°C water-bath for 15 min, and cooled in a 4°C ice-water-bath for 12 min. After the hydroethidine-loaded blood samples were cooled, 20 μl of working bacteria–FITC solution (Staphylococcus aureus labelled with FITC, diluted in PBS to 1·33 × 108 particles/ml; Invitrogen Corporation, Carlsbad, CA, USA) were added to both tubes and vortexed briefly. Tube 2 (test) was transferred to a 37°C water-bath, and tube 1 (control) was left in the ice. The tubes were incubated for 20 min, placed in an ice-water-bath, and 100 μl ice-cold Quench Solution (0·025 % Trypan blue in 0·1 m-citrate buffer, pH 4·0) were added to each tube. The tubes were vortexed for 10 s and incubated for 1 min to quench the FITC fluorescence of any non-internalised bacteria, after which the cells were washed twice with ice-cold PBS and re-suspended in 50 μl cold fetal bovine serum. Samples were processed on a Q-Prep™ Workstation (Beckman Coulter, Inc., Fullerton, CA, USA), which lysed the erythrocytes and stabilised and fixed the leucocytes. Tubes were stored at room temperature in the dark until flow cytometric analysis, which was performed within 24 h of blood collection for all samples.
Analysis of samples was performed using a Beckman Coulter FC-500 flow cytometer with CXP software (Fullerton, CA, USA). FITC emits a green fluorescence; therefore, cells that phagocytose the FITC-labelled bacteria are detected as fluorescence channel 1-positive. Similarly, cells stimulated by the bacteria will undergo a respiratory burst, oxidising the non-fluorescent hydroethidine to ethidium bromide, which emits a red fluorescence that can be detected on fluorescence channel 3. After gating on the granulocytes using forward scatter and side scatter, the mean fluorescence intensity (x-mean) for each channel was determined and shifts in x-mean were calculated by subtracting the control (4°C) x-mean from the test (37°C) x-mean for fluorescence channel 1 and fluorescence channel 3. Typically, 5000 granulocytes were counted for each reaction tube. The mean intra-assay CV was < 7 % for phagocytosis and < 10·5 % for GOBA.
Statistical analysis
All statistical analyses were performed using SPSS PC version 16.0 software (SPSS Inc., Chicago, IL, USA). Data are expressed as mean values with their standard errors. Data were analysed using a 3 (group) × 2 (time) repeated-measures ANOVA, between-groups design, with post hoc analysis using independent t tests that contrasted pre-to-post-supplementation changes of Q-500 and Q-1000 with placebo. Self-reported fitness levels, BMI, body composition and lifestyle data were correlated with NKCA using Pearson correlations. Subject characteristics were contrasted between groups using one-way ANOVA. For all tests, P < 0·05 was considered significant.
Results
Subject characteristics
Subjects were Caucasian females, aged 30–79 years, with a large variance in body mass and composition (Table 1). No changes in body mass and composition were noted over the course of the study, and interaction effects indicated no group differences (data not shown). With all subjects combined, no significant correlations were found between subject age and any of the pre-study measures of innate immune function (all P>0·4) or inflammation (all P>0·2).
Table 1 Subject characteristics*
(Mean values with their standard errors)

Q-500, 500 mg quercetin/d; Q-1000, 1000 mg quercetin/d.
* The study population was 120 females aged 30–79 years.
Plasma quercetin
Plasma quercetin increased significantly above placebo levels after the 12-week supplementation with 500 or 1000 mg aglycone quercetin/d (group × time interaction effect; P < 0·001) (Fig. 1). No significant correlations were found between plasma quercetin levels and dietary variables (data not shown).

Fig. 1 Plasma quercetin levels at baseline (![]() ) and after 12 weeks of supplementation (□) with quercetin at 500 mg/d (Q-500) or 1000 mg/d (Q-1000) compared with placebo (n 120). Values are means, with standard errors represented by vertical bars. There was a group × time interaction (P < 0·001). * Significant change from pre-study compared with placebo (P ≤ 0·05).
) and after 12 weeks of supplementation (□) with quercetin at 500 mg/d (Q-500) or 1000 mg/d (Q-1000) compared with placebo (n 120). Values are means, with standard errors represented by vertical bars. There was a group × time interaction (P < 0·001). * Significant change from pre-study compared with placebo (P ≤ 0·05).
Leucocyte subsets
The pattern of change over time was not significantly different between groups for total leucocytes (P = 0·306), total lymphocytes (P = 0·867) or neutrophil counts (P = 0·193) (Table 2).
Table 2 Blood leucocyte subset cell counts and plasma inflammatory markers at baseline and after 12 weeks of supplementation with quercetin at 500 mg/d (Q-500) or 1000 mg/d (Q-1000) compared with placebo*
(Mean values with their standard errors)

* Pre- and post-study blood samples were obtained from all subjects (n 120) after an 8 h fast.
Plasma IL-6 and TNF-α
The 12-week quercetin supplementation at 500 or 1000 mg/d had no effect on plasma concentrations of IL-6 (P = 0·812) or TNF-α (P = 0·208) (Table 2). For all subjects combined, a positive correlation was found between BMI and IL-6 (r 0·23; P = 0·010), whereas the relationship between BMI and TNF-α did not reach significance (r 0·131; P = 0·153).
Granulocyte oxidative burst activity and phagocytosis
The pattern of change over time was not significantly different between groups for granulocyte respiratory burst activity (P = 0·602) or phagocytosis (P = 0·990) (Figs. 2 and 3). No correlations were found between any outcome measures and GOBA or phagocytosis. For all subjects combined, GOBA increased pre- to post-supplementation (P < 0·001).

Fig. 2 Granulocyte phagocytosis of fluorescein isothiocyanate (FITC)-labelled Staphylococcus aureus at baseline (![]() ) and after 12 weeks of supplementation (□) with quercetin at 500 mg/d (Q-500) or 1000 mg/d (Q-1000) compared with placebo. Data are expressed as shifts in mean FITC fluorescence from 4°C to 37°C (n 64). Values are means, with standard errors represented by vertical bars. There was no group × time interaction (P = 0·990).
) and after 12 weeks of supplementation (□) with quercetin at 500 mg/d (Q-500) or 1000 mg/d (Q-1000) compared with placebo. Data are expressed as shifts in mean FITC fluorescence from 4°C to 37°C (n 64). Values are means, with standard errors represented by vertical bars. There was no group × time interaction (P = 0·990).

Fig. 3 Granulocyte oxidative burst activity following incubation with Staphylococcus aureus at baseline (![]() ) and after 12 weeks of supplementation (□) with quercetin at 500 mg/d (Q-500) or 1000 mg/d (Q-1000) compared with placebo. Data are expressed as shifts in mean ethidium bromide fluorescence from 4°C to 37°C (n 64). Values are means, with standard errors represented by vertical bars. There was no group × time interaction (P = 0·602).
) and after 12 weeks of supplementation (□) with quercetin at 500 mg/d (Q-500) or 1000 mg/d (Q-1000) compared with placebo. Data are expressed as shifts in mean ethidium bromide fluorescence from 4°C to 37°C (n 64). Values are means, with standard errors represented by vertical bars. There was no group × time interaction (P = 0·602).
Lymphocyte subsets
Circulating counts of B, T or NK cells were not influenced by 12-week supplementation with quercetin at 500 or 1000 mg/d (Table 3).
Table 3 Blood lymphocyte subset cell counts for subjects with natural killer cell activity data (n 74) at baseline and after 12 weeks of supplementation with quercetin at 500 mg/d (Q-500) or 1000 mg/d (Q-1000) compared with placebo*
(Mean values with their standard errors)
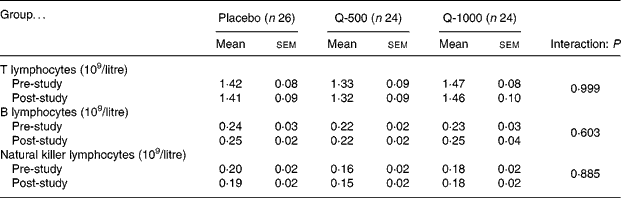
* Pre- and post-study blood samples were analysed for lymphocyte subsets using flow cytometry.
Natural killer cell activity
Pre- and post-supplementation data for percentage lysis of K562 target cells by NK cells showed no significant differences between groups across all effector:target ratios (Table 4). The pattern of change over time for total lytic activity of NK cells was not significantly different between treatment groups (P = 0·696) (Fig. 4). Although NKCA tended to increase pre- to post-supplementation for all subjects combined, this apparent time effect did not reach significance (P = 0·163). When pre- and post-study measures were averaged for all subjects combined (n 74), NKCA was correlated positively with self-reported physical fitness level (r 0·24; P = 0·032) and negatively with BMI (r − 0·25; P = 0·035) and body fat percentage (r − 0·38; P = 0·001) (Fig. 5).
Table 4 Natural killer cell activity at baseline and after 12 weeks of supplementation with quercetin at 500 mg/d (Q-500) or 1000 mg/d (Q-1000) compared with placebo expressed as percentage lysis at four effector:target (E:T) cell ratios*
(Mean values with their standard errors)
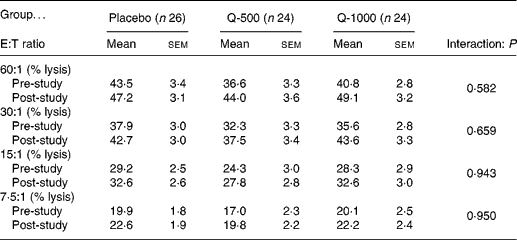
* Natural killer cells (effectors) were incubated with K562 cells (targets) and analysed by flow cytometry for seventy-four subjects; percentage non-spontaneous lysis of target cells at each E:T ratio is presented here.

Fig. 4 Natural killer (NK) cell activity (NKCA) of human subjects at baseline (![]() ) and after 12 weeks of supplementation (□) with quercetin at 500 mg/d (Q-500) or 1000 mg/d (Q-1000) compared with placebo (n 74). NKCA is expressed as the number of lytic units contained in 107 cells, where a lytic unit is defined as the number of NK cells required to lyse 20 % of 5000 K562 target cells. Values are means, with standard errors represented by vertical bars. There was no group × time interaction (P = 0·696).
) and after 12 weeks of supplementation (□) with quercetin at 500 mg/d (Q-500) or 1000 mg/d (Q-1000) compared with placebo (n 74). NKCA is expressed as the number of lytic units contained in 107 cells, where a lytic unit is defined as the number of NK cells required to lyse 20 % of 5000 K562 target cells. Values are means, with standard errors represented by vertical bars. There was no group × time interaction (P = 0·696).

Fig. 5 Relationship between body fat percentage and natural killer cell activity (NKCA) of human subjects (r − 0·38; P = 0·001). Data points average pre- and post-study measures for all subjects with NKCA data (n 74).
Discussion
This is the first human clinical trial to investigate the long-term effects of aglycone quercetin supplementation on measures of innate immune function and inflammation in adult female subjects recruited from the community. These subjects tended to be overweight individuals (mean BMI>25·0 kg/m2) with mean pre-study plasma IL-6 and TNF-α concentrations that were slightly elevated compared with values reported for lean, healthy women in other studies(Reference Bastard, Jardel and Bruckert35, Reference Rexrode, Pradhan and Manson36). The data indicate that the 12-week supplementation with 500 or 1000 mg quercetin/d was not associated with changes in NKCA, GOBA, phagocytosis, or plasma IL-6 and TNF-α in this population.
Quercetin is a powerful antioxidant(Reference Číž, Pavelková and Gallová3) that exerts in vitro anti-inflammatory effects via inhibition of NF-κB signalling in a variety of cells including macrophages and peripheral blood mononuclear cells(Reference Comalada, Camuesco and Sierra5, Reference Nair, Mahajan and Reynolds6). Homo- and heterodimers of NF-κB are located in the cytoplasm of unstimulated immune cells, where they are tightly bound to IκB inhibitory proteins(Reference Nair, Mahajan and Reynolds6). When immune cells are stimulated, the IκB proteins are phosphorylated, ubiquitylated and degraded, freeing the NF-κB dimers for translocation to the nucleus, where they can bind to the promoter regions of genes involved in the inflammatory response. Quercetin appears to exert in vitro anti-inflammatory effects via inhibition of IκB phosphorylation(Reference Nair, Mahajan and Reynolds6). Binding sites for NF-κB proteins have been found in the promoter regions of the genes that code for both TNF-α and IL-6, and studies have shown that IL-6 mRNA production does not occur in the absence of activated NF-κB(Reference Azzolina37, Reference Marquardt and Walker38). Despite its known anti-inflammatory properties, the present study did not find a quercetin-related effect on circulating levels of IL-6 and TNF-α. It should be noted that, although the mean plasma TNF-α and IL-6 levels of subjects in the present study were slightly elevated compared with those reported for lean females in other studies(Reference Bastard, Jardel and Bruckert35, Reference Rexrode, Pradhan and Manson36), they fell within the normal ranges for adult females(Reference Marques-Deak, Cizza and Eskandari39). The lack of quercetin-related effect on inflammation reported in the present study could be because these subjects were already within normal limits with regard to the outcome measures. Future studies comparing the effects of quercetin supplementation on inflammatory status in normal, overweight and obese subjects would be valuable.
In the present study, quercetin supplementation had no measurable effect on NKCA, GOBA or phagocytosis. A moderate time effect was noted for GOBA, but this could be due to seasonal effects on innate immune function. The post-supplementation blood draw was in November, 12 weeks after the August pre-supplementation blood draw. Similar circannual rhythms in immune function have been observed in previous studies performed by our laboratory(Reference Nieman, Henson and Gusewitch40, Reference Nieman, Nehlsen-Cannarella and Henson41). Nonetheless, the pattern of change over time was not significantly different between treatment groups for any measure of immune function. These findings are consistent with studies previously conducted in our laboratory investigating the effects of short-term quercetin supplementation on similar measures of innate immune function in athletes(Reference Henson, Nieman and Davis23, Reference Nieman, Henson and Gross24), but they are contrary to the results of quercetin studies utilising rodent models and in vitro study designs(Reference Akbay, Basaran and Undeger16, Reference Yu, Lai and Yang18, Reference Exon, Magnuson and South19). A study by Exon et al. showed that rats fed quercetin dihydrate for 7 weeks had significantly elevated NKCA compared with controls(Reference Exon, Magnuson and South19). Both NKCA and the phagocytic activity of peritoneal macrophages were higher in BALB/c mice that were treated with quercetin for 3 weeks following injection with WEHI-3 leukaemia cells compared with mice that did not receive quercetin in a recent study by Yu et al. (Reference Yu, Lai and Yang18). Akbay et al. found that quercetin glycosides increased the intracellular killing activity of human neutrophils in vitro (Reference Akbay, Basaran and Undeger16). However, these human and animal studies may not be comparable for a number of reasons. For example, the Exon et al. study used a larger dosage of quercetin (100 mg/kg) compared with the present study, which supplemented with average daily doses of 7 and 14 mg quercetin/kg for Q-500 and Q-1000(Reference Exon, Magnuson and South19). The dosages used in the Yu et al. study were smaller (2 and 4 mg/kg), but the relevance of their results is confounded by the lack of a quercetin-treated group not injected with WEHI-3 cells(Reference Yu, Lai and Yang18). Finally, data from animal studies attributing an immunomodulatory effect to quercetin supplementation may not be applicable to humans because of possible species-dependent variations in phase II metabolism(Reference Graf, Ameho and Dolnikowski42).
Aglycone quercetin supplementation may lack an anti-inflammatory or immunomodulatory effect in humans due to either its reduced bioavailability or its metabolic transformation in vivo. Quercetin is found in fruits and vegetables in a water-soluble form in which the quercetin molecule is conjugated to a sugar(Reference Manach, Williamson and Morand2). The in vitro study conducted by Akbay et al. showed that neutrophil intracellular killing activity was increased by incubation with quercetin in such a glycosylated form(Reference Akbay, Basaran and Undeger16). However, when quercetin is consumed, the sugar is cleaved off in the small intestine, allowing the lipid-soluble aglycone form of quercetin to passively diffuse into the cells lining the intestinal wall(Reference Nemeth and Piskula43). In the liver, aglycone quercetin is either methylated, sulfated or glucuronidated. Quercetin predominately circulates in the bloodstream in these conjugate forms, which differ not only from aglycone quercetin, but also from the glycosylated forms found in plant foods. Relatively few studies have examined the bioactivity of quercetin conjugates(Reference Williamson, Barron and Shimoi44) and further work in this area is indicated.
While supplementation with aglycone quercetin appears to have no effect on NKCA or granulocyte cell function in human subjects, quercetin may exhibit an immunostimulatory effect when ingested in other forms or in combination with other flavonoids. A recent human study found that 4 weeks of supplementation with a fermented food rich in quercetin and other flavonoids significantly increased activation of NK cell cytotoxicity in response to IL-2 stimulation, but did not influence basal (non-stimulated) NKCA(Reference Schoen, Schulz and Schweikart45). Supplementation with polyphenol-rich cereal fractions has been shown to increase basal NKCA in prematurely ageing mice, in addition to augmenting macrophage phagocytosis and reactive oxygen species production(Reference Álvarez, Alvarado and Puerto46). Our laboratory recently found that a 2-week supplementation with a combination of quercetin, epigallocatechin 3-gallate, isoquercetin and n-3 PUFA increased GOBA and decreased post-exercise plasma concentration of IL-6(Reference Nieman, Henson and Maxwell31). Future research will examine the combined supplements' potential effects on NKCA and phagocytosis. It may also be helpful to investigate whether IL-2-stimulated NK cell lytic activity in human subjects is affected by quercetin supplementation, alone or in combination with other flavonoids.
In the present study, we found that total NK cell lytic activity was related inversely to BMI and body fat percentage, and positively with self-reported fitness level. These findings are consistent with previous studies linking NK cell function with physical fitness and healthy lifestyles(Reference Nieman, Buckley and Henson47, Reference Li, Morimoto and Nakadai48). A cross-sectional comparison between marathon runners and sedentary controls found that the marathoners had significantly greater NKCA than the controls, and that percentage body fat was negatively correlated with NKCA for all subjects combined(Reference Nieman, Buckley and Henson47). In addition to physical exercise, other lifestyle factors such as not smoking and eating a balanced diet have been associated with elevated numbers of NK cells and enhanced NKCA, possibly due to an increased percentage of NK cells expressing perforin, granulysin, and granzymes A and B in subjects with good health practices(Reference Li, Morimoto and Nakadai48). This suggests that optimisation of NK cell function can be achieved through the maintenance of a healthy lifestyle.
In summary, 12-week aglycone quercetin supplementation in doses of 500 and 1000 mg/d had no effect on leucocyte subset counts, plasma IL-6 or TNF-α concentration, NKCA, GOBA or granulocyte phagocytosis relative to placebo. A growing body of evidence indicates that a mixed flavonoid approach to modifying innate immunity is more effective than supplementation with a pure flavonoid such as aglycone quercetin. In athletes, a quercetin supplement combined with epigallocatechin 3-gallate, isoquercetin, and n-3 PUFA significantly reduced post-exercise inflammation and augmented neutrophil function(Reference Nieman, Henson and Maxwell31). Future research will determine if the immunomodulatory effects of quercetin can be enhanced through the addition of other flavonoids (for example, epigallocatechin 3-gallate) or food components.
Acknowledgements
The present study was supported by grants from Coca-Cola and Quercegen Pharma. S. A. H., D. A. H. and D. C. N. designed the research and wrote the paper; D. C. N. analysed the data and had primary responsibility for the final content. All authors conducted research and read and approved the final manuscript. The authors would like to thank Krista Kennerly, Ashley Williams, Kendra Maxwell, Patrick O'Reilly, Jackie Hellwege, Ada Bamford Young and Katelyn Graeme for their invaluable assistance with this project.
S. A. H., D, A. H., M, D. A. and F. J. declare no conflicts of interest. D. C. N. holds a position on the science advisory board for Quercegen Pharma.











