Endothelial dysfunction is considered the first reversible stage in the initiation of atherosclerotic lesions(Reference Ikeda, Mochizuki and Nara1). Endothelium, as a metabolically active organ, balances vascular tone, vascular growth and coagulation function and thereby regulates vascular homoeostasis(Reference Lüscher and Barton2,Reference Kinlay, Libby and Ganz3) . The balance between endothelial-derived vasodilatory factors such as endothelial nitric oxide (eNO), prostacyclin, bradykinin, and hyperpolarising factor and vasoconstrictory factors such as endothelin-1 (ET-1), angiotensin II, and thromboxane A determines endothelium-dependent vasodilation(Reference Chhabra4,Reference Strijdom and Lochner5) . Of these factors, eNO is the strongest endogenous vasodilator in the body that additionally inhibits vascular inflammation, platelet adherence and aggregation/proliferation of smooth muscle cells(Reference Davignon and Ganz6). Damage to the endothelium impairs vascular homoeostasis and initiates a number of processes that progressed endothelial dysfunction to atherosclerosis and eventually CVD(Reference Davignon and Ganz6). Therefore, from a pathophysiological viewpoint, improvement of vascular disorders and restoration of vascular homoeostasis should be the main focus of therapeutic strategies to prevent and manage CVD.
Epidemiological studies supported the inverse relationship between consumption of dairy products and CVD risk or mortality(Reference Abbott, Curb and Rodriguez7,Reference Guo, Astrup and Lovegrove8) . Dairy products contain valuable bioactive compounds which may mediate particular biological actions beyond nutrient supply, such as anti-hypertensive effects(Reference FitzGerald, Murray and Walsh9). However, it remains to fully elucidate the compounds underlying these effects, milk-derived proteins and bioactive peptides may be responsible for the observed activities(Reference Fekete, Givens and Lovegrove10). Amongst these proteins, milk-derived whey protein (WP), as a biologically active protein which may counteract cardiomatabolic disorders such as hypertension (HTN), diabetes mellitus, dyslipidemia, obesity and oxidative stress, has attracted a great deal of attention(Reference Pal and Radavelli-Bagatini11–Reference Baer, Stote and Paul13). As evidence shows, WP exerts the angiotensin-converting enzyme (ACE) inhibitory behaviours and thereby modulates blood pressure and vascular reactivity(Reference Abubakar, Saito and Kitazawa14,Reference FitzGerald and Meisel15) . Indeed, ACE inhibitors block the renin–angiotensin system which has principal role in the regulation of blood pressure(Reference Te Riet, van Esch and Roks16). In spite of ACE inhibitory and the apparent anti-hypertensive effects of WP(Reference Fekete, Giromini and Chatzidiakou17,Reference Kawase, Hashimoto and Hosoda18) , questions still remain in elucidation of the vascular effects of WP.
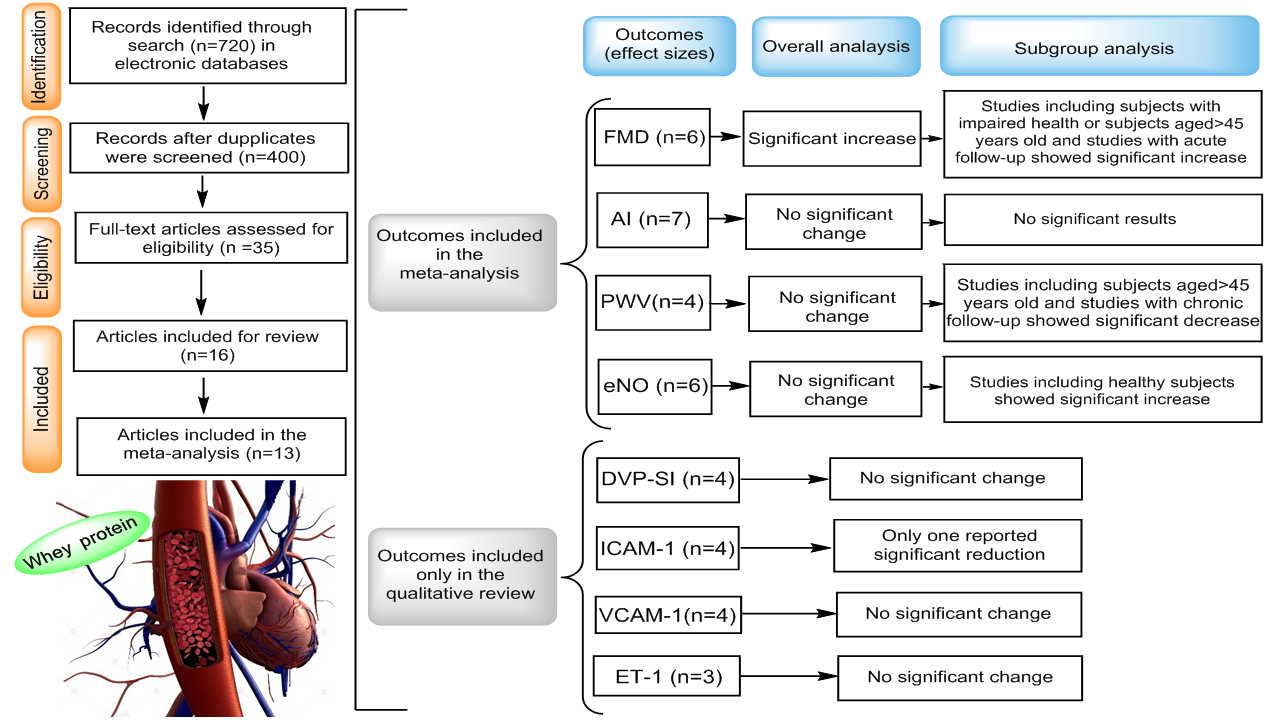
A recent meta-analysis reported that WP intake was significantly associated with improvement in some cardiovascular risk factors, including body weight, blood pressure and lipid profile(Reference Wirunsawanya, Upala and Jaruvongvanich19). However, the systematic review and meta-analysis by Wirunsawanya et al. did not include vascular parameters such as endothelial function, arterial stiffness and circulatory biomarkers of vascular function as study outcomes which are key factors in development and progression of CVD(Reference Wirunsawanya, Upala and Jaruvongvanich19). To the best of our knowledge, there is no complete overview of the evidence of the associations between WP consumption and markers of vascular function. Therefore, the objectives of this study are to systematically review and analyse all available randomised controlled trials (RCT) investigating the relationship between WP consumption and vascular function measurements and to identify existing research gaps. Common non-invasive methods to evaluate vascular function can be categorised as measures of endothelial function and arterial stiffness. The main techniques used to quantify endothelial function include flow-mediated dilation (FMD), laser Doppler imaging and peripheral artery tonometry. Arterial stiffness also is assessed by pulse wave velocity (PWV), augmentation index (AI) and digital volume pulse-stiffness index (DVP-SI)(Reference Vizzardi, Gavazzoni and Della Pina20).
Methods
This systematic review and meta-analysis was conducted according to the guidelines of the preferred reporting items for systematic reviews and meta-analysis (PRISMA) statement. The review protocol has been registered at PROSPERO database of Systematic Reviews (registration number: CRD42020180068).
Eligibility criteria
Studies were considered eligible if they fulfilled following criteria: (1) original studies published in English language and (2) randomised, controlled trials investigating the effect of WP on vascular function in humans. Reviews, letters, case reports or case series, abstracts as well as studies in which WP was combined with other supplements were excluded.
Search strategy and study selection
The study was designed in accordance with the PICOS criteria: the population was human model; the intervention was a treatment with WP; the comparator was no treatment, a placebo or standard treatment; the outcomes were vascular responses to WP; the study design was clinical trial. A systematic search was carried out in PubMed, SCOPUS and Web of science electronic databases for clinical trials investigating the relationship between WP and vascular function published before 30 July 2020. Key search terms were ‘whey’ OR ‘whey proteins’ OR ‘whey protein’ AND ‘arterial stiffness’ OR ‘vascular function’ OR ‘endothelial function’ OR ‘flow-mediated dilation’ OR ‘peak wave velocity’ OR ‘augmentation index’ OR ‘carotid thickness’ OR ‘intima-media thickness’ OR ‘carotid plaque’ OR ‘nitrate-mediated dilation’ OR ‘IMT’ OR ‘endothelial’ OR ‘endothelium’ OR ‘blood flow’ OR ‘vascular resistance’ OR ‘arterial stiffness’ OR ‘FMD’ OR ‘PWV’ OR ‘AI’ OR ‘inflammation’ OR ‘inflammatory’ OR ‘adhesion molecules’ OR ‘intercellular adhesion molecule-1’ OR ‘ICAM-1’ OR ‘vascular adhesion molecule-1’ OR ‘VCAM-1’ OR ‘nitric oxide’ OR ‘endothelin-1’ OR ‘ET-1’. Initially, the titles and abstracts of all identified studies were screened according to the selection criteria and then retrieved and assessed full-text versions of potentially relevant articles for eligibility criteria. Any discrepancies between reviewers were resolved through discussion to reach a consensus. The following data were extracted from included studies: author/date/country, study design, characteristics of participants, type and dosage of WP, duration of the intervention, vascular parameters and other studied outcomes.
Study quality and risk of bias within the studies
The risk of bias for each study was assessed using the Cochrane collaboration tool which encompasses domains of selection bias, performance bias, detection bias, attrition bias, reporting bias and other bias. The quality of evidence for each outcome was classified as low risk, high risk or unclear risk of bias in each study. The overall risk of bias was assigned, with each study being rated as high (high risk of bias for one or more key domains), low (low risk of bias for all key domains) or unclear (low or unclear risk of bias for all key domains). Any disagreement regarding the risk of bias was resolved by discussion.
Statistical analysis
Focusing on main vascular outcomes, a meta-analysis was performed to evaluate the strength of the association of vascular outcomes with WP supplementation. Any outcomes that were examined by at least four studies were included in the meta-analysis. For trials that examined the changes in outcomes at multiple time points, only the last measurement was included in the meta-analysis. For each included studies, the pooled effects for FMD (%), AI (%) and PWV (m/s), and eNO (μmol/l) were determined as weighted mean differences (WMD; final values – baseline values) and 95 % CI using the random-effects model. If not provided by the author, standard deviation was calculated using the following formula: sd = sem × square root of the sample size. Also, data were extracted from the figures, if outcomes were not expressed in the main text or tables. Forest plots were produced to graphically present the means along with corresponding 95 % CI for each study. To quantify statistical inconsistency of measurements across studies, the heterogeneity index (I 2 ) was used and I 2 > 50 % or P< 0·01 were considered to represent substantial heterogeneity. In addition, subgroup analyses according to health condition, participants’ age and intervention duration were used to explore possible sources of heterogeneity. Publication bias was determined by Begg’s and Egger’s regression test. All analyses were conducted using Comprehensive Meta Analysis V2, and a P< 0·05 indicated statistical significance.
Results
Figure 1 presents the flow diagram of study selection process. A total of 720 records were identified from the database search, of which 326 duplicate papers were removed, 365 of the remaining 400 records were excluded on the basis of title and abstract. Finally, thirty-five full-text articles were screened for eligibility, and sixteen studies were identified as being RCT of WP on vascular function and included in the systematic review. Moreover, only thirteen RCT provided sufficient data to be included in the meta-analysis. Remaining three studies were excluded from quantitative assessment due to insufficient data. We tried to contact the authors of the trials with insufficient data by email to access full data on related outcomes, but no response was received from authors.
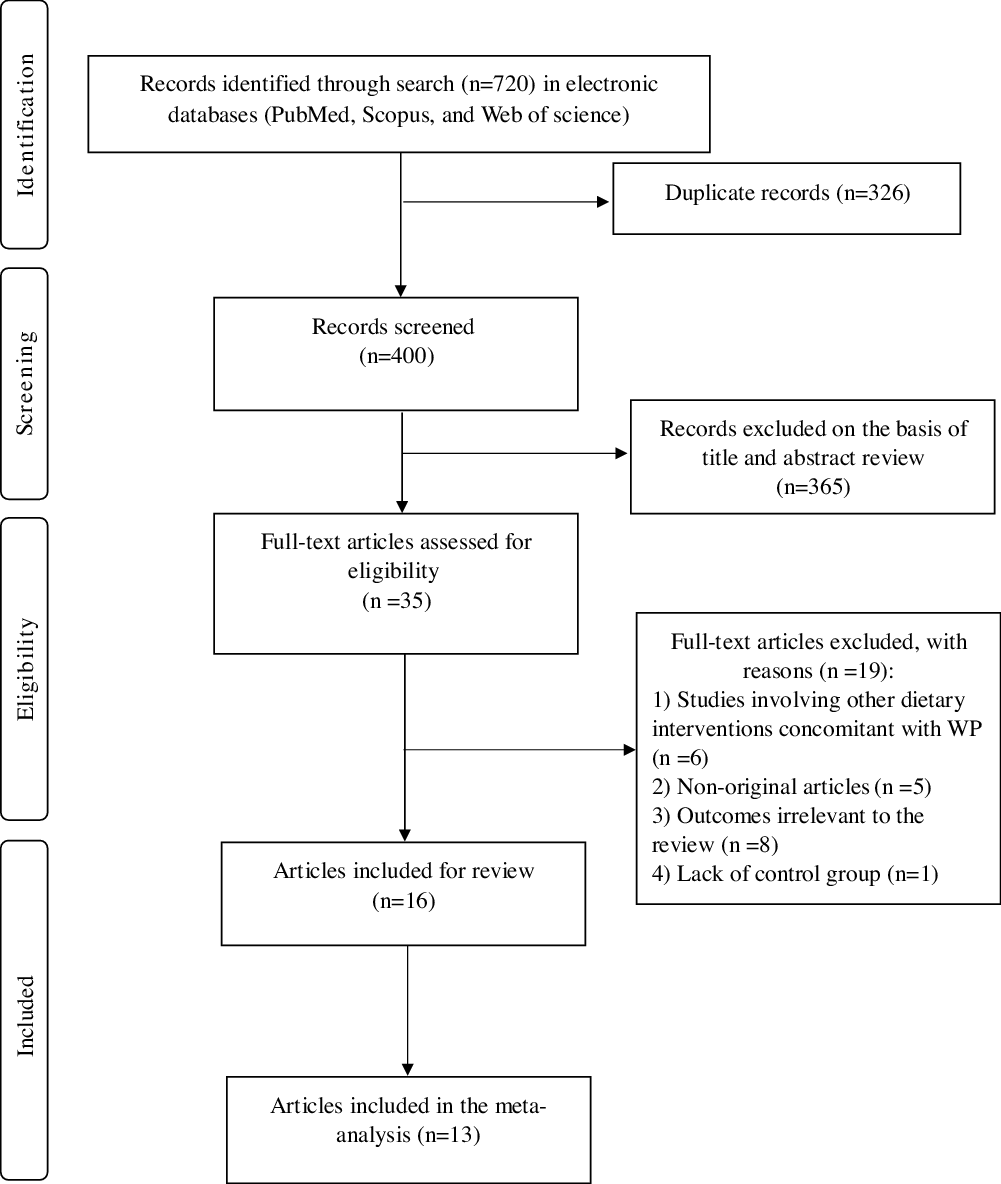
Fig. 1. Flow diagram of the literature search and study selection process. WP, whey protein.
Study characteristics
Tables 1 and 2 detailed the main characteristics of the sixteen studies of WP interventions. Seven of the included studies involved acute follow-up and eight evaluated the effect of chronic WP on study outcomes. In addition, one study had reported both acute and chronic effects of WP on different outcomes(Reference Ballard, Bruno and Seip21). All included studies were RCT, of which nine had a crossover design. The sample size of the acute studies ranged from nine to twenty-five subjects, while in the chronic studies, the number of participants that completed each study varied from 20 to 173. Based on BMI values, six studies included obese population(Reference Pal and Ellis22–Reference McDonald, Mah and Chitchumroonchokchai27), seven included overweight(Reference Fekete, Giromini and Chatzidiakou17,Reference Arnberg, Larnkjaer and Michaelsen28–Reference Petyaev, Dovgalevsky and Klochkov33) and other three studies included normal-weight participants(Reference Ballard, Bruno and Seip21,Reference Rontoyanni, Werner and Sanders34,Reference Yang, Wang and Tong35) . Most of included RCT were carried out in the individuals who had at least one cardiovascular risk factor, including overweight, obesity and HTN. FMD responses to WP were assessed in eight RCT, of which four were carried out on pre-HTN/mild HTN(Reference Fekete, Giromini and Chatzidiakou17,Reference Fekete, Giromini and Chatzidiakou30,Reference Petyaev, Dovgalevsky and Klochkov33,Reference Yang, Wang and Tong35) , two on healthy volunteers(Reference Ballard, Bruno and Seip21,Reference Oliveira, Volino-Souza and Cordeiro32) , one on obese individuals(Reference McDonald, Mah and Chitchumroonchokchai27) and one on subjects with impaired FMD(Reference Ballard, Kupchak and Volk29). Also, ten RCT evaluated the effect of WP on arterial stiffness(Reference Fekete, Giromini and Chatzidiakou17,Reference Pal and Ellis22–Reference Jeong, Biruete and Tomayko26,Reference Arnberg, Larnkjaer and Michaelsen28,Reference Fekete, Giromini and Chatzidiakou30,Reference Lefferts, Augustine and Spartano31,Reference Rontoyanni, Werner and Sanders34) , of which four were conducted on overweight/obese individuals(Reference Pal and Ellis22,Reference Pal and Ellis23,Reference Mariotti, Valette and Lopez25,Reference Arnberg, Larnkjaer and Michaelsen28) , three on pre-HTN/mild HTN(Reference Fekete, Giromini and Chatzidiakou17,Reference Figueroa, Wong and Kinsey24,Reference Fekete, Giromini and Chatzidiakou30) , one on haemodialysis patients(Reference Jeong, Biruete and Tomayko26) and two on healthy volunteers(Reference Lefferts, Augustine and Spartano31,Reference Rontoyanni, Werner and Sanders34) . Effects of WP on the plasma levels of , intercellular adhesion molecule-1 (ICAM-1) and vascular adhesion molecule-1 (VCAM-1) were evaluated in three studies of which two were conducted on healthy(Reference Ballard, Bruno and Seip21,Reference Mariotti, Valette and Lopez25) and one on HTN/mild HTN individuals(Reference Fekete, Giromini and Chatzidiakou17). There were six studies that examined the impacts of WP on eNO. Three studies were conducted on pre-HTN/mild HTN(Reference Fekete, Giromini and Chatzidiakou17,Reference Petyaev, Dovgalevsky and Klochkov33,Reference Yang, Wang and Tong35) , one on healthy subjects(Reference Ballard, Bruno and Seip21), one on obese individuals(Reference McDonald, Mah and Chitchumroonchokchai27) and one on subjects with impaired FMD(Reference Ballard, Kupchak and Volk29). In addition, three studies evaluated the effects of WP on plasma levels of ET-1(Reference McDonald, Mah and Chitchumroonchokchai27,Reference Ballard, Kupchak and Volk29,Reference Yang, Wang and Tong35) of which one study conducted on obese subjects(Reference McDonald, Mah and Chitchumroonchokchai27), one on patients with impaired FMD(Reference Ballard, Kupchak and Volk29) and one on pre-HTN/mild HTN individuals(Reference Yang, Wang and Tong35).
Table 1. Overview of the characteristics and main findings of the acute RCT included in the systematic review
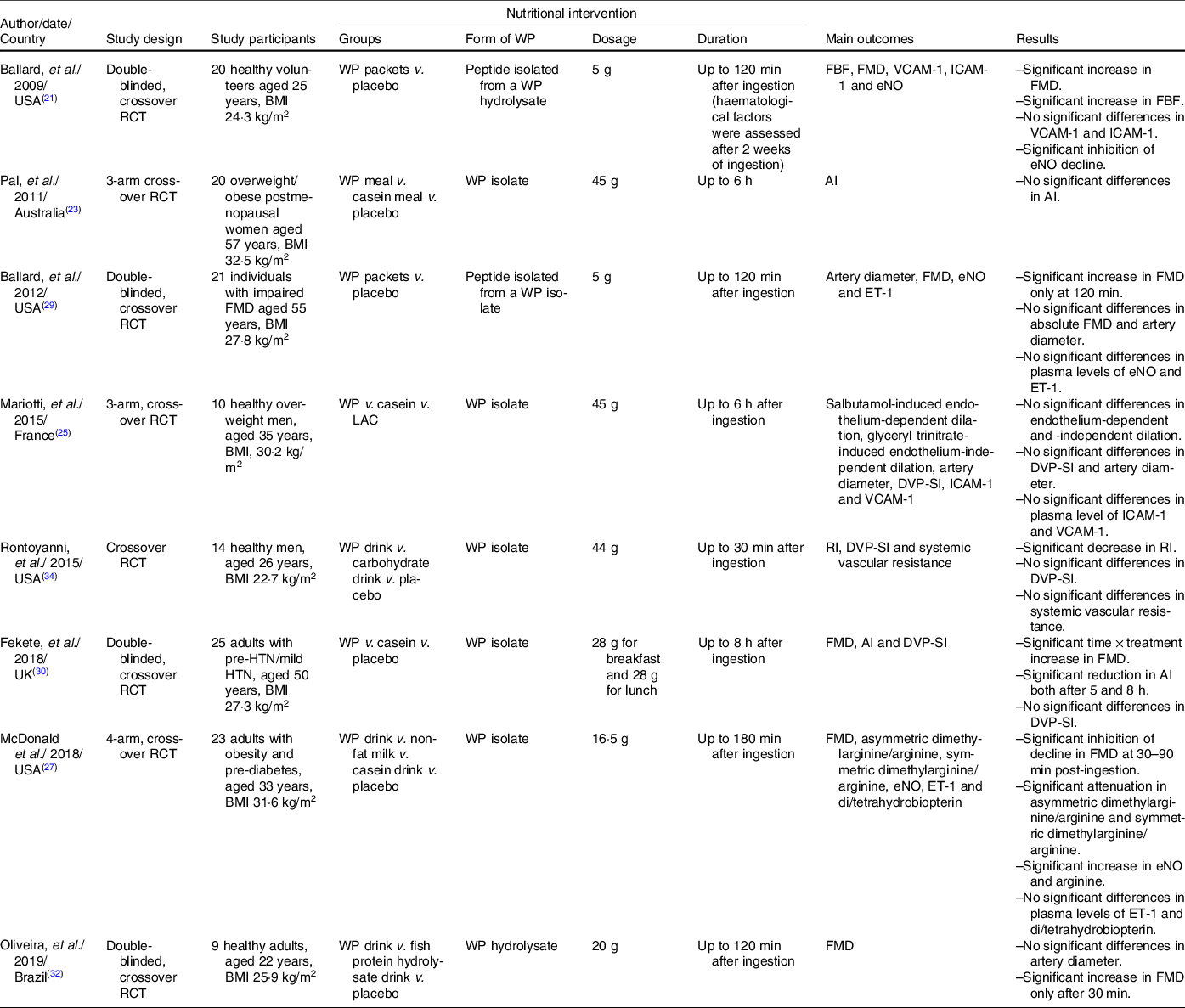
RCT, randomised controlled trial; WP, whey protein; FBF, forearm blood flow; FMD, flow-mediated dilatation; VCAM-1, vascular adhesion molecule-1; ICAM-1, intercellular adhesion molecule-1; eNO, endothelial nitric oxide; AI, augmentation index; ET-1, endothelin-1; LAC, α-lactalbumin-enriched whey protein; DVP-SI, digital volume pulse-stiffness index; RI, reflection index; HTN, hypertension.
Table 2. Overview of the characteristics and main findings of the chronic RCT included in the systematic review
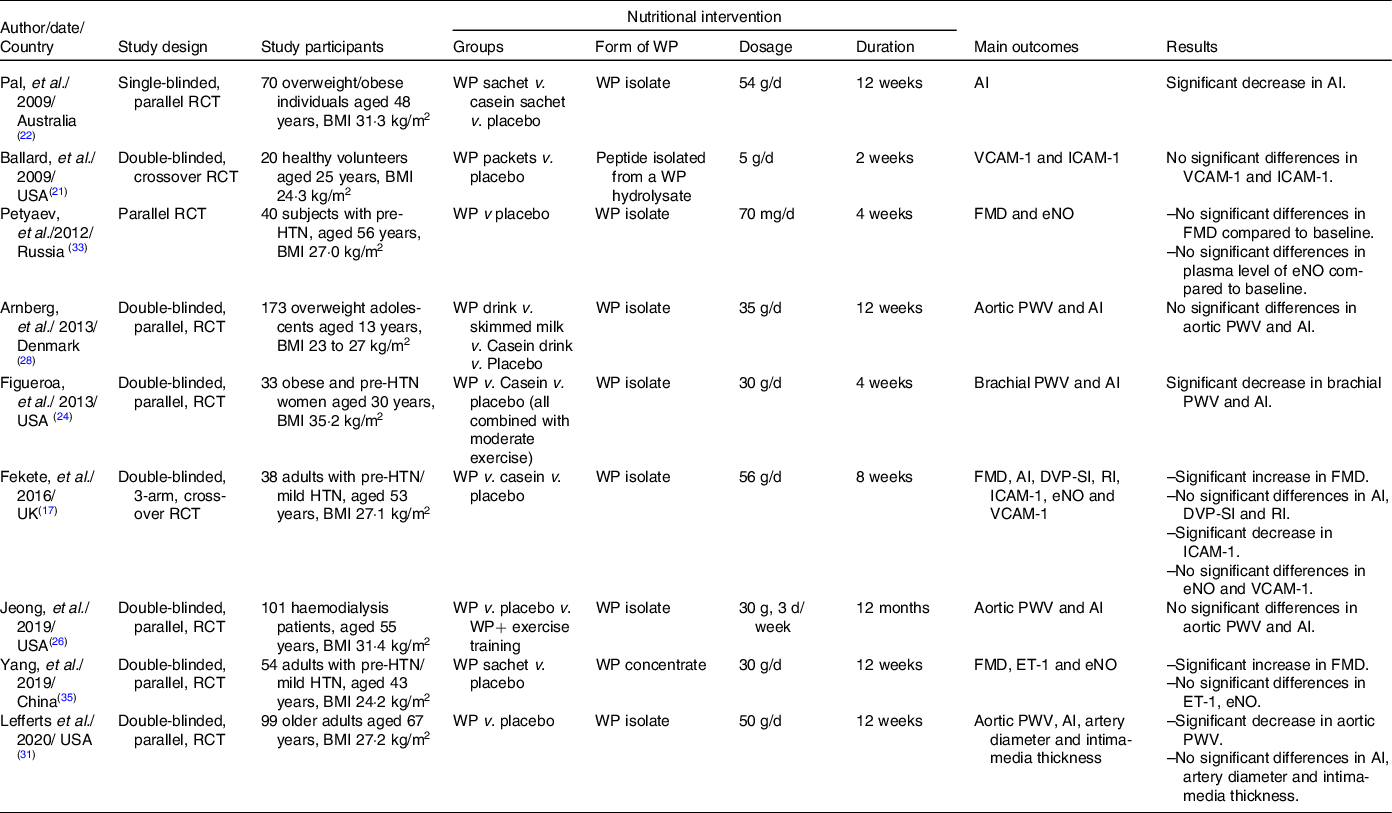
RCT, randomised controlled trial; WP, whey protein; VCAM-1, vascular adhesion molecule-1; ICAM-1, intercellular adhesion molecule-1; FMD, flow-mediated dilatation; eNO, endothelial nitric oxide; PWV, pulse wave velocity; AI, augmentation index; HTN, hypertension; DVP-SI, digital volume pulse-stiffness index; RI, reflection index; ET-1, endothelin-1.
Thirteen of the included studies used isolate type of WP to determine the effect of WP on vascular endothelial function. Remaining RCT also utilised a peptide isolated from a WP isolate (n 2), WP concentrate (n 1) and WP hydrolysate (n 1). Chronic studies used various dosages of WP from 0·7 to 56 g/d during a time frame ranging from 2 weeks to 12 months. In the acute studies, participants had consumed 5–45 g of WP during 0·5 to 8 h. These sixteen studies originated from the USA (n 7), UK (n 2) and Australia (n 2), and single studies from Denmark, France, China, Russia and Brazil. Finally, of sixteen eligible documents that were described in qualitative review, thirteen provided sufficient data on some outcomes and were included in the meta-analysis.
Studies of WP’ association with vascular function: a qualitative review
Acute studies
Main results obtained in acute studies of WP intervention have been summarised in Table 1. These studies mainly used FMD(Reference Ballard, Bruno and Seip21,Reference McDonald, Mah and Chitchumroonchokchai27,Reference Ballard, Kupchak and Volk29,Reference Fekete, Giromini and Chatzidiakou30,Reference Oliveira, Volino-Souza and Cordeiro32) , DVP-SI(Reference Mariotti, Valette and Lopez25,Reference Fekete, Giromini and Chatzidiakou30,Reference Rontoyanni, Werner and Sanders34) , AI(Reference Pal and Ellis23,Reference Fekete, Giromini and Chatzidiakou30) , digital volume pulse-reflection index (DVP-RI)(Reference Mariotti, Valette and Lopez25,Reference Rontoyanni, Werner and Sanders34) , adhesion molecules(Reference Ballard, Bruno and Seip21,Reference Mariotti, Valette and Lopez25) , eNO(Reference Ballard, Bruno and Seip21,Reference McDonald, Mah and Chitchumroonchokchai27,Reference Ballard, Kupchak and Volk29) and ET-1(Reference McDonald, Mah and Chitchumroonchokchai27,Reference Ballard, Kupchak and Volk29) to assess vascular function. Ballard et al. reported that the consumption of 5 g/d of a WP-derived peptide (NOP-47) mixed in water significantly increased FMD of healthy volunteers at 30 (8·87 %), 60 (9·94 %) and 90 (9·02%) min post-ingestion compared with the same time points following consumption of placebo(Reference Ballard, Bruno and Seip21). NOP-47 also markedly inhibited plasma eNO reduction at 120 min compared with placebo. However, no significant effects of NOP-47 on ICAM-1 and VCAM-1 were obtained(Reference Ballard, Bruno and Seip21). In addition, the authors examined eNO-independent vasodilation using venous occlusion strain gauge plethysmography from 20 to 120 min post-NOP-47 ingestion and observed that NOP-47 significantly increased forearm blood flow (29·9%) at 120 min(Reference Ballard, Bruno and Seip21). In another study, the same authors found that acute ingestion of 5 g of NOP-47 in the individuals with impaired FMD significantly enhanced FMD at 30 (4·6%) and 120 (5·1%) min post-intervention compared with the baseline, although, between-group changes in FMD reached to significance only at 120 min. Also, compared with placebo, absolute FMD responses, brachial artery diameter and plasma levels of eNO and ET-1 during the NOP-47 ingestion did not change significantly(Reference Ballard, Kupchak and Volk29). In the overweight or obese postmenopausal women, Pal et al. did not observe significant changes in AI (the indicator of arterial stiffness) after 6 h of the ingestion of a breakfast meal in conjunction with 45 g WP isolate(Reference Pal and Ellis23). Mariotti et al. did not find significant changes in the vascular endothelial-dependent reactivity (based on the salbutamol-induced decrease in DVP-RI), endothelial-independent vascular reactivity (based on the glyceryl trinitrate-induced decrease in DVP-RI), DVP-SI and plasma levels of ICAM-1 and VCAM-1 in overweight men during 6 h after oral consumption of 45 g WP compared with the control group(Reference Mariotti, Valette and Lopez25). Similarly, in healthy men, drinks containing 44 g WP did not make significant changes in DVP-SI, and systemic vascular resistance up to 30 min post-ingestion compared with isoenergetic drinks containing carbohydrate or water; however, it significantly prevented an increase in DVP-RI(Reference Rontoyanni, Werner and Sanders34).
In another study by Fekete et al., adults with pre-HTN or mild HTN ingested a high-fat, isoenergetic breakfast and lunch with either WP (28 g for breakfast and 28 g for lunch), casein or maltodextrin and then they were examined for vascular function at time 0, 180, 300 and 420 min after meals(Reference Fekete, Giromini and Chatzidiakou30). The researchers found a significant increase in FMD (0·60 %) at 5 h after breakfast meal in conjunction with WP compared with maltodextrin. In addition, AI reduced after WP consumption compared with maltodextrin both after 5 (18·2 %) and 8 (30·9 %) hours; however, the changes in DVP-SI were not significant between groups(Reference Fekete, Giromini and Chatzidiakou30). In a study by McDonald et al,. the adults with obesity and pre-diabetes were randomised to WP isolate (16·5 g), non-fat milk, sodium caseinate and placebo in which all groups consumed glucose in addition to dietary interventions to induce hyperglycemia-induced impairments in endothelial function and then % FMD was assessed at 30 min intervals for 180 min post-prandially(Reference McDonald, Mah and Chitchumroonchokchai27). The authors observed that compared with the placebo, both WP and milk significantly inhibited the declines in FMD at 120–150 min, while in the placebo group, %FMD significantly decreased up to 2 % post-prandially. Moreover, WP significantly increased post-prandial eNO, with no significant changes in plasma levels of ET-1(Reference McDonald, Mah and Chitchumroonchokchai27). Oliveira et al. examined the acute effect of WP on vascular function in healthy adults up to 120 min post-ingestion and reported a significant increase in FMD (9·02 %) only at 30 min after consumption. No significant differences were observed for brachial artery diameter(Reference Oliveira, Volino-Souza and Cordeiro32).
Chronic studies
Outcomes of the included chronic RCT are detailed in Table 2. Chronic studies mainly used FMD(Reference Fekete, Giromini and Chatzidiakou17,Reference Ballard, Bruno and Seip21,Reference Petyaev, Dovgalevsky and Klochkov33,Reference Yang, Wang and Tong35) , arterial stiffness parameters(Reference Fekete, Giromini and Chatzidiakou17,Reference Pal and Ellis22,Reference Figueroa, Wong and Kinsey24,Reference Jeong, Biruete and Tomayko26,Reference Arnberg, Larnkjaer and Michaelsen28,Reference Lefferts, Augustine and Spartano31) , adhesion molecules(Reference Fekete, Giromini and Chatzidiakou17,Reference Ballard, Bruno and Seip21) , eNO(Reference Fekete, Giromini and Chatzidiakou17,Reference Ballard, Bruno and Seip21,Reference Petyaev, Dovgalevsky and Klochkov33,Reference Yang, Wang and Tong35) and ET-1(Reference Ballard, Bruno and Seip21,Reference Yang, Wang and Tong35) to measure the impact of WP on vascular function. Pal et al. found a significant reduction in arterial stiffness measured by AI (14 %) in overweight/obese individuals who consumed 54 g/d WP isolate mixed with 250 ml water for 12 weeks(Reference Pal and Ellis22). In contrast, the consumption of 35 g/d WP for 12 weeks did not make significant changes in arterial stiffness obtained from AI measurement in overweight adolescents(Reference Arnberg, Larnkjaer and Michaelsen28). In another study, the intake of 30 g/d WP over 4 weeks significantly decreased arterial stiffness measured by aortic AI (8·2 %) and brachial–ankle PWV (57 cm/s) in obese and pre-HTN women(Reference Figueroa, Wong and Kinsey24). Fekete et al. found a significant increase in FMD (1·31 %) of adults with pre-HTN or mild HTN following 8 weeks of 56 g/d WP supplementation(Reference Fekete, Giromini and Chatzidiakou17). However, the differences in DVP-SI, AI (measures of arterial stiffness) and DVP-RI (measure of vascular tone) were not statistically significant(Reference Fekete, Giromini and Chatzidiakou17). The authors also reported a significant reduction in ICAM-1 level with no significant changes in eNO and VCAM-1 concentrations compared with the controls(Reference Fekete, Giromini and Chatzidiakou17). Ballard et al. in another study showed that the administration of 5 g/d of NOP-47 for 2 weeks had no significant effects on serum levels of ICAM-1 and VCAM-1 in healthy subjects(Reference Ballard, Bruno and Seip21). In another study by Petyaev, et al. in which subjects with pre-HTN consumed 70 mg/d WP isolate for 4 weeks, no significant changes were detected in FMD or plasma level of eNO(Reference Petyaev, Dovgalevsky and Klochkov33). In a recent study by Jeong et al., intradialytic WP supplementation (30 g, three d/week) did not make significant changes in arterial stiffness which was measured by AI and PWV after 6 and 12 months of supplementation in haemodialysis patients(Reference Jeong, Biruete and Tomayko26). Yang et al. showed that the consumption of 30 g/d of WP for 12 weeks led to a significant increase in FMD (5·2 %) in adults with pre-HTN/mild HTN compared with the controls(Reference Yang, Wang and Tong35). In the overweight and obese individuals, also the FMD showed a significantly higher increase in the WP group than in the controls (7·3 %). In contrast, differences in FMD change were not significant in the normal-weight individuals. The changes in the plasma levels of ET-1 and eNO were not significant, as well(Reference Yang, Wang and Tong35). In another study, the consumption of 50 g/d of WP isolate for 12 weeks in elderly participants made a significant decrease in the aortic stiffness, assessed via gold standard carotid-femoral PWV (4 %), without significant changes in AI and pulse pressure(Reference Lefferts, Augustine and Spartano31).
Meta-analysis
Effect of WP on FMD and arterial stiffness measures
Of included trials, 6 (4 acute studies and 2 chronic study) with 153 individuals provided adequate data on FMD (Table 3). The pooled estimate showed a significant improvement in FMD after WP intake (Fig. 2; WMD: 1·09 %, 95 % CI: 0·17, 2·01, P= 0·01). There was a high degree of heterogeneity between the studies (I 2 index 99·85 %, P= 0·000). Subgroup analysis was conducted to find the causes of high heterogeneity. This analysis showed no detectable effect of WP on FMD in individuals aged less than 45 years or healthy individuals (P= 0·33), whereas the subjects aged ≥ 45 years old or individuals with impaired health showed a significant increase in FMD (WMD: 1·41, 95 % CI: 1·05, 1·78, P= 0·000). Subgroup analysis based on the study duration, showed no significant effect on FMD following chronic supplementation with WP (P= 0·12), while acute ingestion of WP significantly increased FMD (WMD: 1·59, 95 % CI: 1·45, 1·72, P= 0·000). A sensitivity analysis conducted to test the effect of individual study on the overall results and indicated that one study on adults with pre-HTN/mild HTN had the largest influence on FMD(Reference Fekete, Giromini and Chatzidiakou30); as after exclusion of this study from analysis, the pooled
Table 3. The effects of WP supplementation on vascular function in the participants of included studies
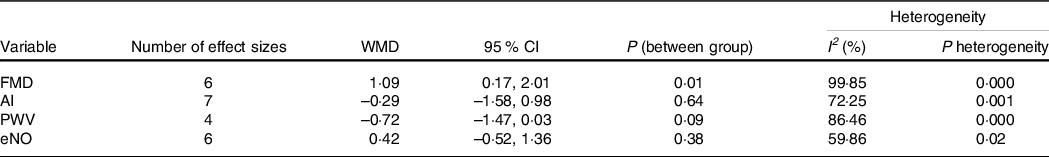
WP, whey protein; WMD, weighted mean difference; FMD, flow-mediated dilation; AI, augmentation index; PWV, pulse wave velocity; eNO, endothelial nitric oxide.
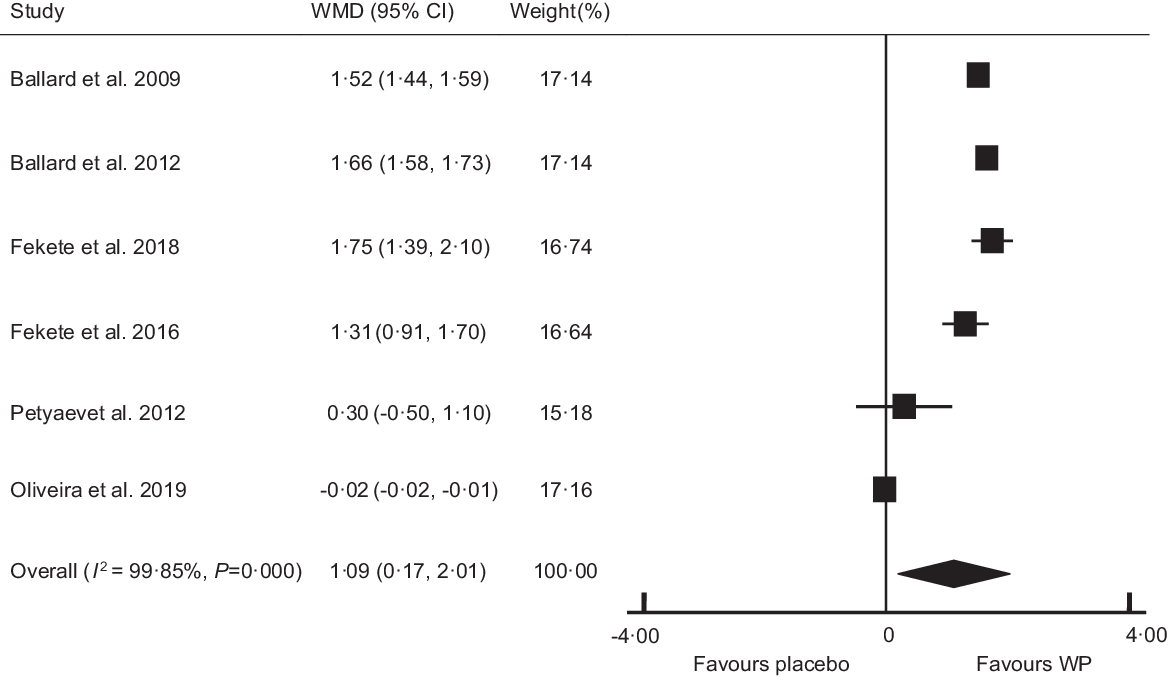
Fig. 2. Forest plot of the effect of whey protein on flow-mediated dilation. WMD, weighted mean difference; WP, whey protein.
WMD became insignificant (WMD: 0·96 %, 95 % CI: −0·03, 1·97, P= 0·06) compared with that from the main analysis. Begg’s (P= 0·45) and Egger’s test (P= 0·10) did not confirm the presence of publication bias.
Of included studies, 7 (6 chronic studies and 1 acute study) with 534 participants provided adequate data on AI (Table 3) and revealed a non-significant decrease in AI following WP consumption (Fig. 3; WMD: −1·29 %, 95 % CI: −1·58, 0·98, P= 0·64). The I 2 index (72·25 %) and Cochrane Q test (P= 0·001) revealed a high inter-trial heterogeneity. This finding did not change after subgroup analyses based on health condition, participants’ age and intervention duration (Table 4). The pooled WMD from sensitivity analysis was similar to that from the main analysis. The results from Begg’s (P= 0·54) and Egger’s test (P= 0·31) did not confirm the likely of publication bias across studies.
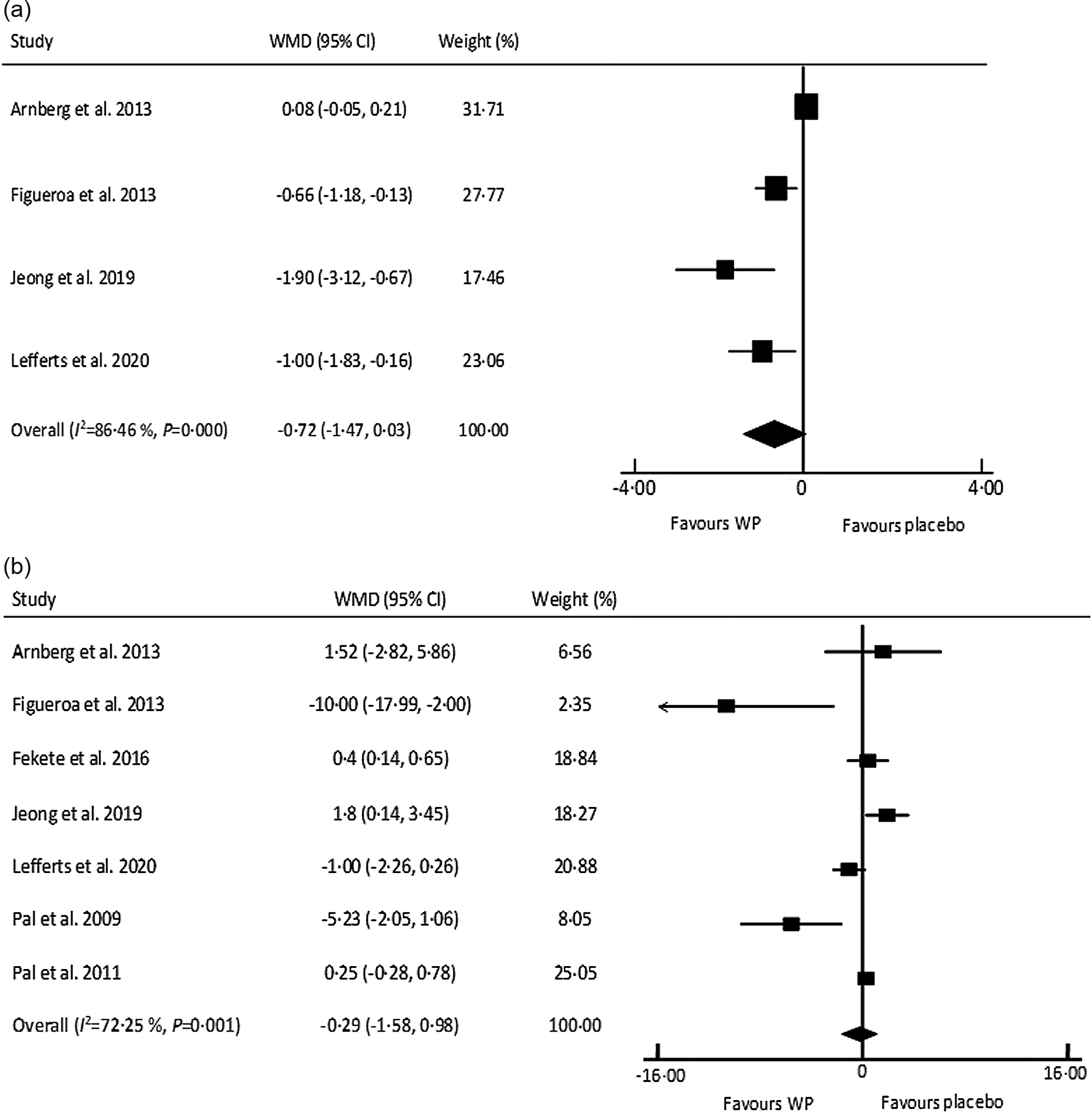
Fig. 3. Forest plot of the effect of whey protein on arterial stiffness measures: (a) pulse wave velocity and (b) augmentation index. WMD, weighted mean difference; WP, whey protein.
Table 4. Subgroup analyses for the effects of WP supplementation on vascular function in the participants of included RCT
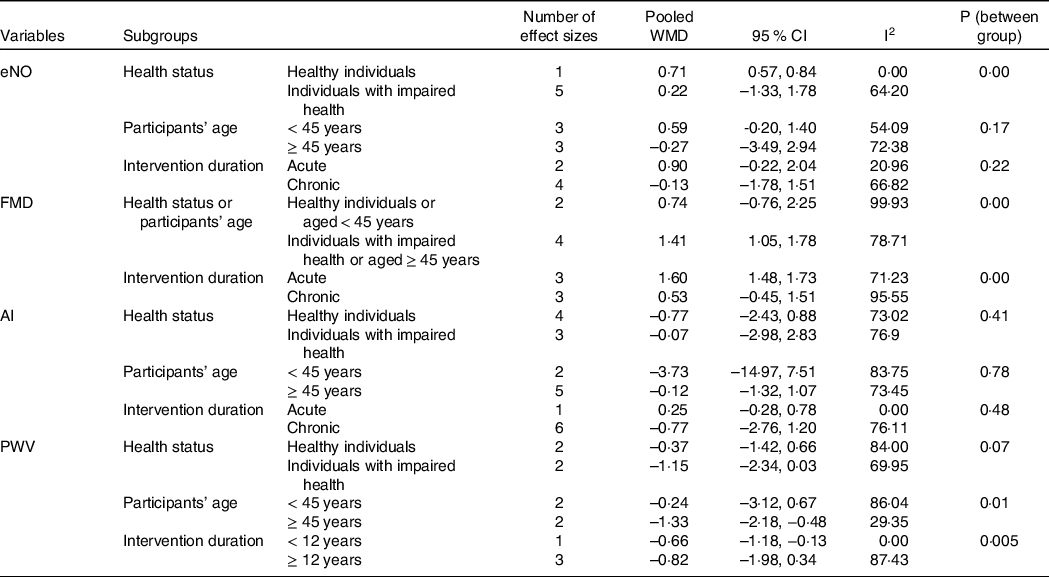
WP, whey protein; RCT, randomised controlled trial; WMD, weighted mean difference..
The pooled analysis of 4 studies, with 406 participants showed that WP supplementation did not affect PWV significantly, while there was a trend towards a reduction in PWV (Fig. 3; WMD: –0·72 m/s, 95 % CI: −1·47, 0·03, P= 0·06). Considering I 2 index (86·46 %) and Cochrane Q test (P= 0·000), a high inter-trial heterogeneity was detected. The subgroup analyses indicated a significant decrease in this marker in the studies with participant’s aged ≥ 45 years and duration of ≥ 12 weeks (Table 4). Moreover, a sensitivity analysis indicated that one study on overweight adolescents had the most effect on PWV(Reference Arnberg, Larnkjaer and Michaelsen28); as the pooled WMD was shifted to the significance after exclusion of this study from analysis (WMD: –1·00 m/s, 95 % CI: −1·61, 0·39, P= 0·001)(Reference Arnberg, Larnkjaer and Michaelsen28). In contrary to Begg’s test (P= 0·30), Egger’s test (P= 0·003) showed evidence of publication bias.
Effect of WP on circulating biomarkers of vascular function
The only circulating biomarker that met the meta-analysis criteria was eNO. There were five RCT with six effect sizes regarding the effect of WP on eNO. The pooled analysis of these trials involving 173 participants showed no significant effects on plasma levels of eNO following WP supplementation (Fig. 4; WMD: 0·42 μmol/l, 95 % CI: −0·52, 1·36, P= 0·38). The I 2 index (59·86 %) and Cochrane Q test (P= 0·02) revealed a moderate inter-trial heterogeneity. As the Table 4 shows, subgroup analysis indicated a significant increase in eNO level in healthy individuals, but not in subjects with impaired health. However, subgroup analyses based on participants’ age or study duration indicated no significant effect on FMD after WP consumption. A sensitivity analysis showed that one chronic study on middle-aged pre-HTN subjects had the largest influence on eNO levels(Reference Petyaev, Dovgalevsky and Klochkov33); as after exclusion of this study from analysis, the pooled WMD reached the significance level (WMD: 0·64 μmol/l, 95 % CI: 0·01, 1·27, P= 0·04) compared with that from the main analysis. Based on Begg’s (P= 0·70) and Egger’s tests (P= 0·54), there was no evidence of publication bias.
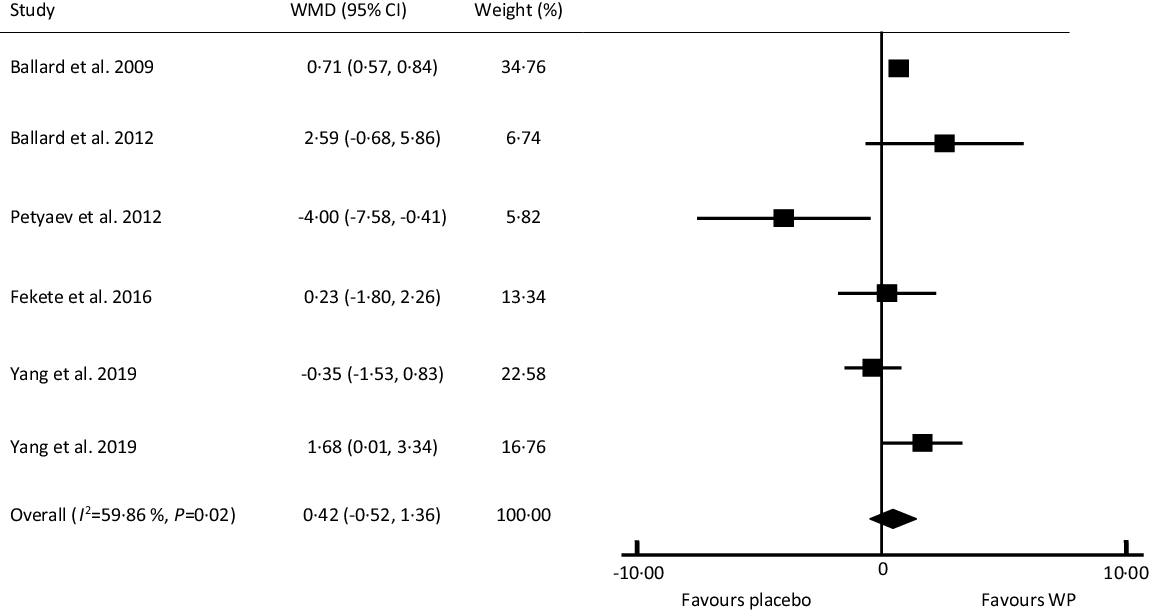
Fig. 4. Forest plot of the effect of whey protein on plasma level of endothelial nitric oxide. WMD, weighted mean difference; WP, whey protein.
Assessment of the quality and risk of bias
Figure 5 shows the risk of bias in each individual studies. Although, all of sixteen RCT were randomised trial, only six of the sixteen RCT provided adequate information about the method of randomisation and three studies explained the methods of allocation concealment. Blinding of participants and researchers also did not conduct by six RCT or was unclear in six studies. Six RCT did not blind the outcome assessors and seven did not specify if they were blind to the trial groups. Regarding attrition bias, three did not explain the completeness of outcome data such as attrition and exclusions from the analysis. Fifth criterion was the only item that all RCT had low risk of bias. For the last criterion, five studies were at high risk of bias because of no consideration of baseline values for weigh and/or dietary habits or the variations in weight and/or nutritional habits during the chronic intervention in the statistical analyses. Two studies conducted by Fekete et al. could be considered as a high-quality trial, because of being all key domains at low risk of bias. Based on judgements of risk of bias for each item, ten RCT were at high risk of bias out of which six studies were at risk of performance and detection bias. Remaining four studies judged to be unclear risk of bias.
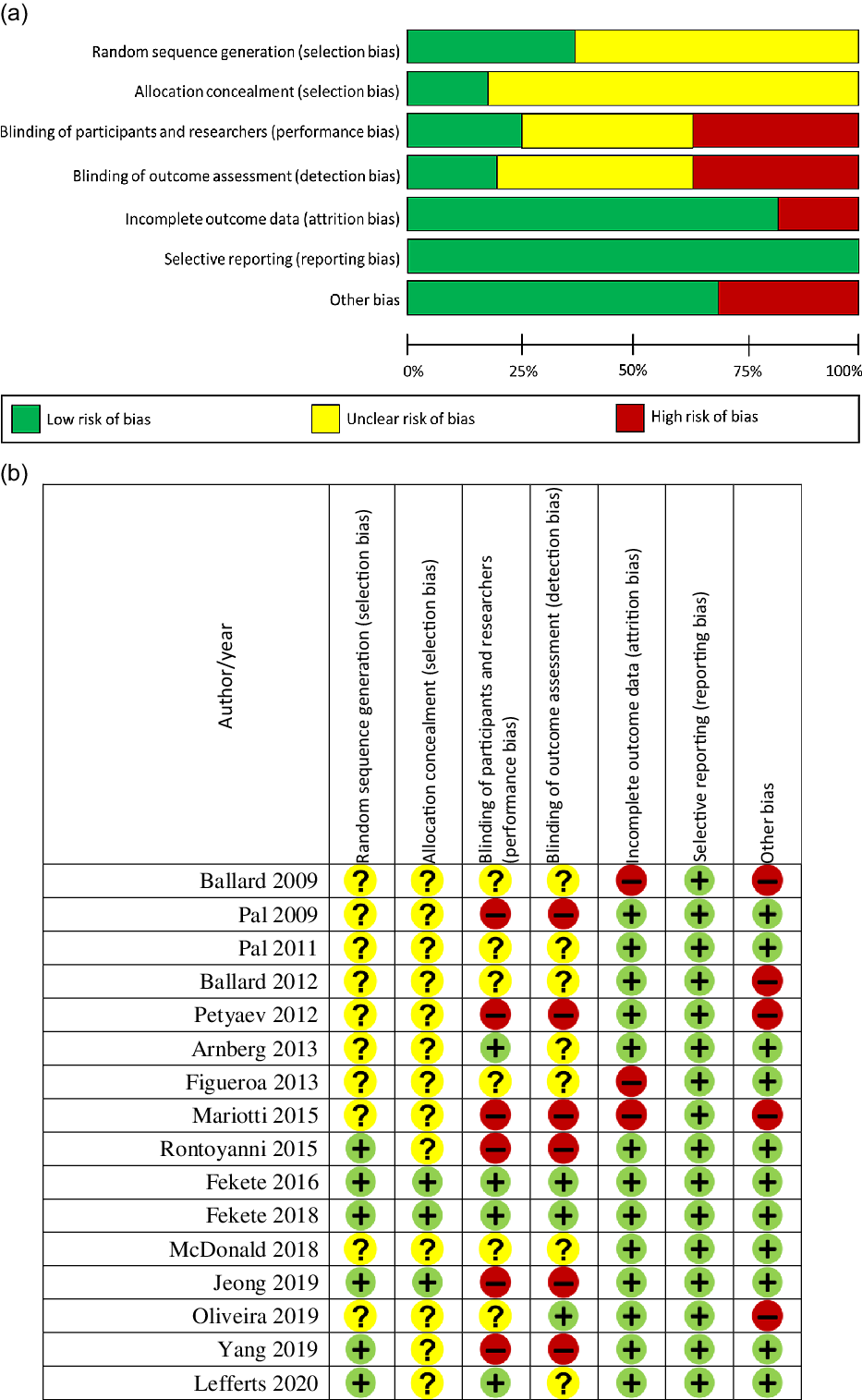
Fig. 5. Risk of bias graph (a) and summary (b) of the included studies. + shows a low risk of bias, – shows a high risk, and ? shows an unclear risk. WMD, weighted mean difference; WP, whey protein.
Discussion
To the best of our knowledge, the present systematic review and meta-analysis is the first to assess the effects of WP on vascular function and provides a comprehensive synthesis of results from available RCT. In most included studies, endothelial function was measured by FMD responses which is the gold standard measure of endothelial function and is capable of detecting small effects of interventions(Reference Korkmaz and Onalan36). The key finding of this meta-analysis was the significant beneficial effect of WP on FMD. This finding could be of clinical value, because each 1 % increase in FMD, independently of confounding variables may decrease the risk of cardiovascular events up to 10–13 %(Reference Inaba, Chen and Bergmann37–Reference Matsuzawa, Kwon and Lennon39). In addition, further subgroup analyses revealed a remarkable improvement in FMD only in studies including individuals with impaired health or individuals aged ≥ 45 years old and in studies with acute follow-up. Although due to the limited number of trials available for subgrouping, such analyses should be interpreted conservatively. Our sensitivity analysis on FMD was also in line with subgroup analyses, where the exclusion of one study investigating acute effects of WP on adults with pre-HTN/mild HTN and aged 50 years significantly altered the results(Reference Fekete, Giromini and Chatzidiakou30). Accordingly, the individual’s health status and age as well as the study duration may play important roles in determining the FMD response to WP. A lack of a significant effect of WP in young individuals may be due to a ‘ceiling effect’ in young adults with no space to ameliorate the function of a healthy vascular endothelium. This finding might indicate that WP was effective in restoring age-related declines in endothelial function in the middle-aged and older adults who are at high risk of developing CVD(Reference Hunter, Dhindsa and Cunningham40–Reference Craighead, Freeberg and Seals42).
WP has high content of arginine, which is the precursor for eNO synthesis; therefore, increasing eNO production was speculated to be possible mechanism of WP to explain the observed improvement in the vascular function(Reference Ballard, Bruno and Seip21,Reference Da Silva, Bigo and Barbier43) . Nevertheless, we did not observe significant effects of WP on eNO concentrations. Therefore, this suggests that WP may employ eNO-independent mechanisms to augment vasodilation. Although, noteworthy is that eNO predominantly acts in an autocrine and paracrine manner, suggesting that the serum levels of eNO may not thoroughly reflect its bioactivity(Reference Ballard, Kupchak and Volk29). On the other hand, some WP-derived peptides such as α- and β-lactorphins exhibit opioid activities and may employ an analgesic impact on the nervous system(Reference Pihlanto-Leppälä44–Reference Meisel and Fitzgerald46). The interaction of WP-derived peptides with opioid receptors presented in endothelial cells may stimulate the release of eNO into the underlying vascular tissues to dilate vessels in an autocrine or paracrine way, without affecting plasma levels of eNO(Reference Pihlanto-Leppälä44–Reference Amiya, Watanabe and Komuro47). An extract isolated from a WP hydrolysate, namely NOP-47 also may improve FMD through eNO-dependent and -independent pathways and needed to be more studied.
Growing evidence also shows that the ACE inhibitory activities as well as the release of other endothelial-derived vasoactive substances such as prostanoids and endothelial hyperpolarising factor are potential candidates for vasodilatory properties of WP. Intestinal digestion of WP has shown to generate bioactive peptides such as isoleucine-proline-alanine tripeptides which are subsequently passed to the circulatory system intact to exert physiological functions(Reference Foltz, Meynen and Bianco48). These WP-derived tripeptides show ACE inhibitory activities which not only reduce the production of the vasoconstrictor angiotensin II but also impede degradation of the vasodilatory molecule bradykinin and boost the release of endothelial-derived vasoactive substances and thereby decrease wave reflection magnitude(Reference Abubakar, Saito and Kitazawa14,Reference Ballard, Bruno and Seip21,Reference Faggiotto and Paoletti49,Reference Yoshizawa, Maeda and Miyaki50) . Particularly, stimulation of prostanoids synthesis following WP consumption, as showed in several studies(Reference Rosaneli, Bighetti and Antonio51–Reference Tavares, Monteiro and Possenti53), may mediate endothelial-dependent vasodilation generated in response to WP. Nevertheless, the exact mechanisms by which WP may affect FDM responses are yet to be elucidated and needed to be more investigated.
Our meta-analysis demonstrated no significant decreases in AI or PWV. Further subgroup analysis showed that the individuals aged over 45 years would obtain the maximum benefit from WP consumption with respect to PWV reduction, but not AI. In this regard, sensitivity analysis also showed that the elimination of the only study on the adolescents(Reference Arnberg, Larnkjaer and Michaelsen28) reached PWV to the significance level. Moreover, subgroup analysis based on study duration indicated that the interventions with longer periods are needed to occur significant changes in the PWV, as the gold standard index of arterial stiffness. Also, four studies used DVP-SI method to measure arterial stiffness, of which the number of studies providing adequate data on DVP-SI was less than that of our meta-analysis criteria. Therefore, we qualitatively reviewed the available RCT in which none of four studies detected significant changes in DVP-SI, after WP consumption(Reference Fekete, Giromini and Chatzidiakou17,Reference Mariotti, Valette and Lopez25,Reference Fekete, Giromini and Chatzidiakou30,Reference Rontoyanni, Werner and Sanders34) . Also, of two available studies investigating the effect of WP on reflection index, as a measure of vascular tone, only one reported significant decrease in comparison with controls(Reference Rontoyanni, Werner and Sanders34). It is worth mentioning that none of included studies considered average intake of WP supplied by foods, suggesting that the wide ranges of consumption of WP sources such as dairy products by participants may dilute the ‘true’ response of WP, thus lowering the definite effectiveness within meta-analyses.
This systematic review has also demonstrated that available RCT have mainly focused on studying arterial stiffness measures, FMD and eNO level; however, the evidence on the impact of WP on ET-1 and vascular inflammatory biomarkers such as ICAM-1 and VCAM-1 is scarce at present and requires more study. In this regard, three RCT assessed the effect of WP on ET-1, none of which detected significant changes in the plasma level of ET-1(Reference McDonald, Mah and Chitchumroonchokchai27,Reference Ballard, Kupchak and Volk29,Reference Yang, Wang and Tong35) . We found four human studies investigating the effect of WP consumption on serum levels of ICAM-1 and VCAM-1; however, the eligible studies were less than that to be included in the meta-analysis. Based on qualitative review of these studies, only one of four RCT reported significant decrease in ICAM-1 after WP consumption compared with controls(Reference Fekete, Giromini and Chatzidiakou17). In line with our results, other trials investigating anti-inflammatory effects of dairy products also did not report significant changes in adhesion molecules(Reference Nestel, Pally and MacIntosh54,Reference Nestel, Mellett and Pally55) .
Overall, it seems that the exposure to WP results in the shift of properties of the endothelium towards a vasodilatory phenotype, while to occur remarkable variations in arterial stiffness indices, long-term interventions addressing older adults are required. Further studies with a focus on the possible mechanisms that orchestrate the communication between the WP and the vascular system are warranted.
Knowledge gaps and future directions
In spite of methodological strength and comprehensive overview of the available research, caution should be taken in interpreting the observed results, because high heterogeneity between studies was observed in terms of study design, study quality, assessment methodology, characteristics of subjects, the type and dose of WP, and the duration of the intervention. Given that most studies included participants without known CVD, the impaired parameters of vascular function that were seen in the patients with CVD were absent and the changes in some vascular parameters to WP were not significant. Therefore, future studies evaluating the effect of WP on vascular responses in subjects with vascular dysfunction are needed to allow more definitive conclusions to be obtained. Also, it seems that the short-term interventions are unlikely to induce remarkable changes in arterial stiffness in participants without vascular dysfunction. In addition, the effect size observed in vascular function in the acute studies may be affected by test meal which slow down gastric emptying and delay the effect of bioactive components. Therefore, acute studies should assay vascular response to WP only at fasting state. Meanwhile, future research is recommended to consider the intake of dairy products, as the dietary sources that contributes most to the WP intake. Future research is highly recommended to address potential components of WP (individual WP, peptide fractions or amino acids) that mediate the improvements in FMD observed in the present meta-analysis.
Conclusion
The present systematic review and meta-analysis suggests a beneficial role for WP in the endothelial function, but not for arterial stiffness, and serum level of eNO. Moreover, qualitative review of studies investigating the effect of WP on adhesion molecules and ET-1 did not show definitive impact in these factors. Our study suggested that individuals with impaired health or aged over than 45 years old may be more likely to benefit from WP than other populations. Moreover, in contrary to FMD which is likely improved with acute consumption of WP, the effects on PWV may require longer interventions to take place. Taking these findings into account, the period of exposure to the dietary agent and participants’ characteristics may play determinant roles in the effectiveness of WP on vascular outcomes. More research that is adequately powered, addresses current knowledge gaps and evaluates dose and time responses across different populations should be conducted to further enhance our understanding of the efficacy of WP on vascular function and to elucidate the mechanisms underlying the observed effects.
Acknowledgements
The authors express their sincere gratitude to Dr Sepideh Soltani (Department of Nutrition, School of Public Health, Shahid Sadoughi University of Medical Sciences, Yazd, Iran) for her valuable guidance.
This research received no specific grant from any funding agency, commercial or not-for-profit sectors.
F. H-S. contributed to the study conception, design and data collection, and interpretation and drafting the manuscript. E. S. Z. contributed to the data collection and drafting the manuscript. A. T-E participated in revising the paper critically and approving the version of the manuscript being submitted.
The authors declare no conflicts of interest.