Depression is a widespread mental disorder globally affecting about 280 million people, corresponding to 5 % of the population, and is one of the leading causes of disability worldwide. It is characterised by persistent sadness and a lack of interest or pleasure in activities that were previously rewarding or enjoyable(1). The prevalence of depression increases significantly with age, reaching one-third of the older population(Reference Cai, Jin and Liu2,Reference Hu, Zhao and Wu3) .
Sex is a significant risk factor for depressive symptoms, especially in older people(Reference Lin and Wang4). Women are more likely to develop depression than men, with the disadvantage appearing in adulthood and worsening after the age of 60 years (5 % of adults, including 4 % of men and 6 % of women, and 5·7 % of adults aged 60 years and over worldwide)(1). In Italy, 10 out of 100 people over the age of 65 years suffer from depressive symptoms and the prevalence tends to increase with advancing age, from 8·3 % in the 65–74 age group to about 13·6 % after the age of 85 years (12·8 % in women compared with 5·7 % in men in the 65–85+ years age group)(5).
The aetiology of depression is likely to include a contribution from biological (e.g. genetics, microbiome and inflammation), psychological (e.g. negative self-concept, rejection sensitivity or negative emotionality), social determinants (e.g. age, socio-demographic or socio-economic status, social support, education, employment status, living arrangements, marital status, etc.) and environmental factors(Reference Remes, Mendes and Templeton6). Among the latter, diet is a modifiable risk factor that may play a role in the development of depression and is amenable to intervention.
In general, a dietary pattern defined as healthy, that is, characterised by a high intake of fruit and vegetables, wholegrain cereals, fish, olive oil, low-fat dairy products and antioxidants, and a low consumption of foods of animal origin, has been associated with a low risk of depression(Reference Rahe, Unrath and Berger7,Reference Chan, Chan and Woo8) . Among the healthy patterns, there is growing evidence that the Mediterranean diet (MD) (characterised by a high intake of fruits, nuts, vegetables, pulses, cereals, olive oil, and fish, a low intake of meat and dairy products, and a moderate intake of alcohol) is the dietary strategy that provides an ideal balance of nutrients, antioxidants and other beneficial molecules that can promote mental health in the whole population(Reference Sofi, Abbate and Gensini9,Reference Mazza, Ferro and Pujia10) .
In particular, adherence to the MD has been found to be associated with a lower likelihood of developing depressive symptoms at any stage of life, from adolescence to adulthood(Reference Sánchez-Villegas, Delgado-Rodríguez and Alonso11,Reference Rienks, Dobson and Mishra12) . However, to the best of our knowledge, only a few studies have focused on the older population(Reference Hodge, Almeida and English13–Reference Mamalaki, Ntanasi and Hatzimanolis15), and little is known about the differences between men and women in the association of adherence to the MD and its individual dietary components with depressive symptoms in a Mediterranean population.
Based on previous considerations, in the current study, we hypothesised that (i) high adherence to the MD would be associated with low depressive symptoms in community-dwelling people aged 65 years and older, and that (ii) the association between the MD and its dietary components would differ between men and women. We aimed to test these hypotheses by examining the cross-sectional associations of the MD and its components with depressive symptoms in a community-dwelling population aged 65 years and older and to assess sex differences in these associations. The results of this study will allow more targeted advice and guidelines to be given to doctors and health professionals on how to prevent the development of depressive symptoms through dietary intervention.
Methods
Study design, setting and participants
Cross-sectional data from the Nutrition, Gut Microbiota and Brain Aging (NutBrain) project, a population-based cohort study of community-dwelling older adults aged 65 years and older living in the Lombardy region (Italy), were used. The data collection period was between October 2019 and January 2023, during which participants underwent a detailed assessment that included the collection of validated questionnaire data and anthropometric measurements(Reference Prinelli, Jesuthasan and Severgnini16), as described in detail below. All examinations were performed by trained and certified study technicians according to harmonised protocols. A total of 807 participants were recruited; excluding those who did not complete the screening data collection (n 9), 798 subjects were included in the present analysis.
Data collection
Depressive symptoms
Depressive symptoms were assessed using the twenty-item Centre for Epidemiological Studies Depression Scale (CES-D), which focuses on the frequency of the subject’s feelings and behaviours in the week before the interview(Reference Radloff17). Response options ranged from 0 to 3 for each item related: 0 = rarely or never (less than one d), 1 = sometimes or a few times (1–2 d), 2 = moderately or most of the time (3–4 d), and 3 = most or almost all of the time (5–7 d). The scoring of positive items (4, 8, 12 and 16) was reversed. The possible score ranged from 0 to 60, with higher scores indicating greater depressive symptoms. We dichotomised the score to classify individuals with depressive symptoms, by using the standard cut-off point of a CES-D score of 16 or more(Reference Weissman, Sholomskas and Pottenger18).
Dietary exposure and adherence to Mediterranean diet score
Information on dietary behaviours in the previous year was collected using a 102-item semi-quantitative food frequency questionnaire (SFFQ), administered by a certified dietician. The questionnaire was derived from the validated Willett questionnaire used in the Nurses’ Health Study(Reference Willet19), which had previously been adapted and used in another Italian study, the Bollate Eye Study(Reference Prinelli, Yannakoulia and Anastasiou20,Reference Leite and Nicolosi21) , but it has not been validated in the Italian language. The questionnaire was divided into different categories (bread/cereals/tubers, eggs/meat/processed meat, pulses, fish, dairy products/cheeses, vegetables, fruit, sweets, oils/condiments/sauces, beverages/coffee, alcohol, sugars and miscellaneous). Respondents were asked to indicate how often they consumed a portion of the given food, using a nine-point frequency scale. The options ranged from never/rarely (less than once a month) to 4–5 times a day, while the other consumption frequencies were 1–3 times/month, 1–2 times/week, 3–4 times/week, 1 time/d and 2–3 times/d. In order to make the standard portion sizes easier to understand, colour images(Reference Passannanti and Prati22) were presented to illustrate the portion size of each food, according to the reference intake levels of nutrients and energy for the Italian population (LARN)(23). To measure the adherence to the MD, we used the scale proposed by Trichopoulou et al. (Reference Trichopoulou, Costacou and Bamia24). Nine food groups were used to calculate the dietary index, namely vegetables, fruits and nuts, cereals, legumes, dairy products, fish and seafood, meat, alcohol, and the ratio of MUFA:SFA. For dietary components thought to be beneficial (i.e. vegetables, fruits and nuts, cereals, legumes, fish and seafood, and a high MUFA:SFA ratio), we scored participants who consumed less than the population median as ‘0’ and people who consumed at or above the population median as ‘1’. For dietary components thought to be less beneficial (i.e. dairy products and meat products), consumption below the population median was scored as ‘1’, whereas consumption level at or above the population median was scored as ‘0’. Alcohol consumption of 5–25 g of ethanol per d for women and 10–50 g of ethanol per d for men was scored as ‘1’, indicating moderate intake, and consumption outside of these ranges was scored as ‘0’. The scores for all nine components were then summed and the total score ranged from 0 to 9, with higher scores indicating greater adherence. The continuous score was then categorised as 0 = low (0–3), 1 = moderate (4–5) and 2 = high (6–9).
Other covariates
For the present analysis, we considered the following variables: age (continuous), socio-economic status (low, moderate and high) calculated by combining information on education (categorised as high school or higher, middle school, primary school or less), occupation (managerial, intermediate professional and manual), living arrangement (living alone v. not living alone), waist circumference (in cm), number of daily drugs used (as a proxy for co-morbidities), smoking habits (classified as never and former or current smoker) and self-perceived health status (fair v. good). Physical activity was assessed using the International Physical Activity Questionnaire (IPAQ)(Reference Craig, Marshall and Sjostrom25), and according to the international guidelines(Reference Haskell, Lee and Pate26), participants were classified as inactive if they did no physical activity (score = 0), minimally active if they did only one activity such as, for example, slow walking (score = 1), moderately active if they did more than one light or moderate activity such as, for example, slow swimming, dancing, or cycling (score = 2), and vigorously active if they did vigorous activities such as hiking, skiing, fast swimming, running, etc. (score = 3). Daily energy intake was derived using the Italian Food Composition Databases for Epidemiological studies in Italy (http://www.bda-ieo.it/) and MetaDieta software (Meteda s.r.l., San Benedetto del Tronto).
Statistical analysis
Characteristics of the study participants were described using mean and standard deviation for continuous variables and frequency (%) for categorical variables. The dependent variable was CES-D ≥ 16, whereas the independent variables were Mediterranean diet score (MDS) and its dietary components. The association between the dependent and independent variables was examined using binary logistic regression, controlling for several covariates selected on the basis of previous theoretical knowledge; OR with 95 % CI were estimated. MDS was analysed as both a continuous and a categorical variable. To evaluate the effect of each of the nine MDS components, we included them all simultaneously in a separate model. Analyses were stratified by sex and were performed using IBM SPSS Statistics for Windows version 25.0 (IBM Corp.) and Stata version 15.0 (StataCorp LP). A two-sided P-value < 0·05 was considered statistically significant.
Sample size calculation
To estimate the minimum sample size for this analysis, we considered the expected prevalence of depressive symptoms based on data from the Italian general population of the same age, which ranged from 8·3 % in the 65–74 age group to about 13·6 % after the age of 85 years. Given an expected prevalence of depressive symptoms of about 20 %, a desired precision of 3 % and a CI of 95 %, a sample size of at least 683 people was required (Sergeant, ESG, 2018. Epitools epidemiological calculator. Ausvet. Available at: http://epitools.ausvet.com.au).
Results
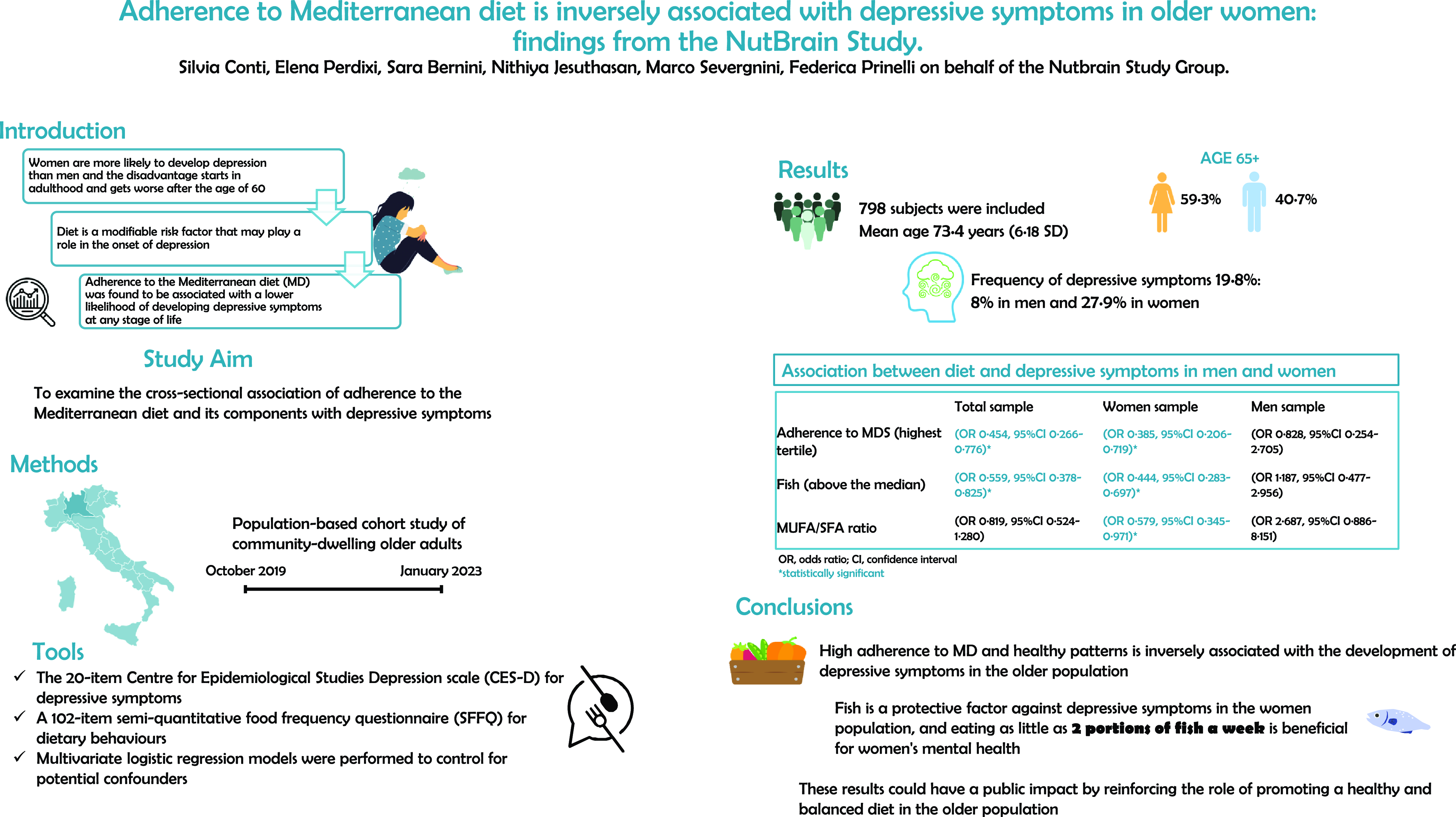
A total of 325 men (40·7 %) and 473 women (59·3 %) completed the assessment. Table 1 shows the characteristics of the participants separately for men and women by depressive symptomatology. The majority of the sample was aged 65–74 years (59·5 %) (mean age 73·4 years, 6·18 sd), married (67·3 %), and not living alone (76·1 %), and half of the sample had a low socio-economic status. The prevalence of depressive symptoms was 19·8 % (n 158), 8 % (n 26) in men and 27·9 % (n 132) in women. Among men, there were no statistically significant differences between the characteristics of those with depressive symptoms and those without, except for the number of daily medications (P-value = 0·042), which was greater in the former group.
Table 1. Characteristics of the study participants by depressive symptoms in men and women (n 798)
MUFA: Monounsaturated fatty acids; SFA: saturated fatty acids
Among women, the group of people with depressive symptoms was more likely to be unmarried (P-value = 0·031), to live alone (P-value = 0·033), to use more drugs (P-value = 0·010), to have the worst self-perceived health status (P-value = 0·002), had lower adherence to MDS (P-value < 0·001), consumed less fish (P-value < 0·001) and vegetables (P-value = 0·019), and had a lower less MUFA:SFA ratio (P-value = 0·056).
Compared with men, women had lower intakes of cereals, meat, and alcohol and higher intakes of vegetables and MUFA:SFA ratio (P-value < 0·05) (online Supplementary Table 1). There was a statistically significant difference in the adherence to the MDS between men and women, with men having higher adherence than women (high 32·9 % v. 24·5 %, P-value = 0·029, Fig. 1).
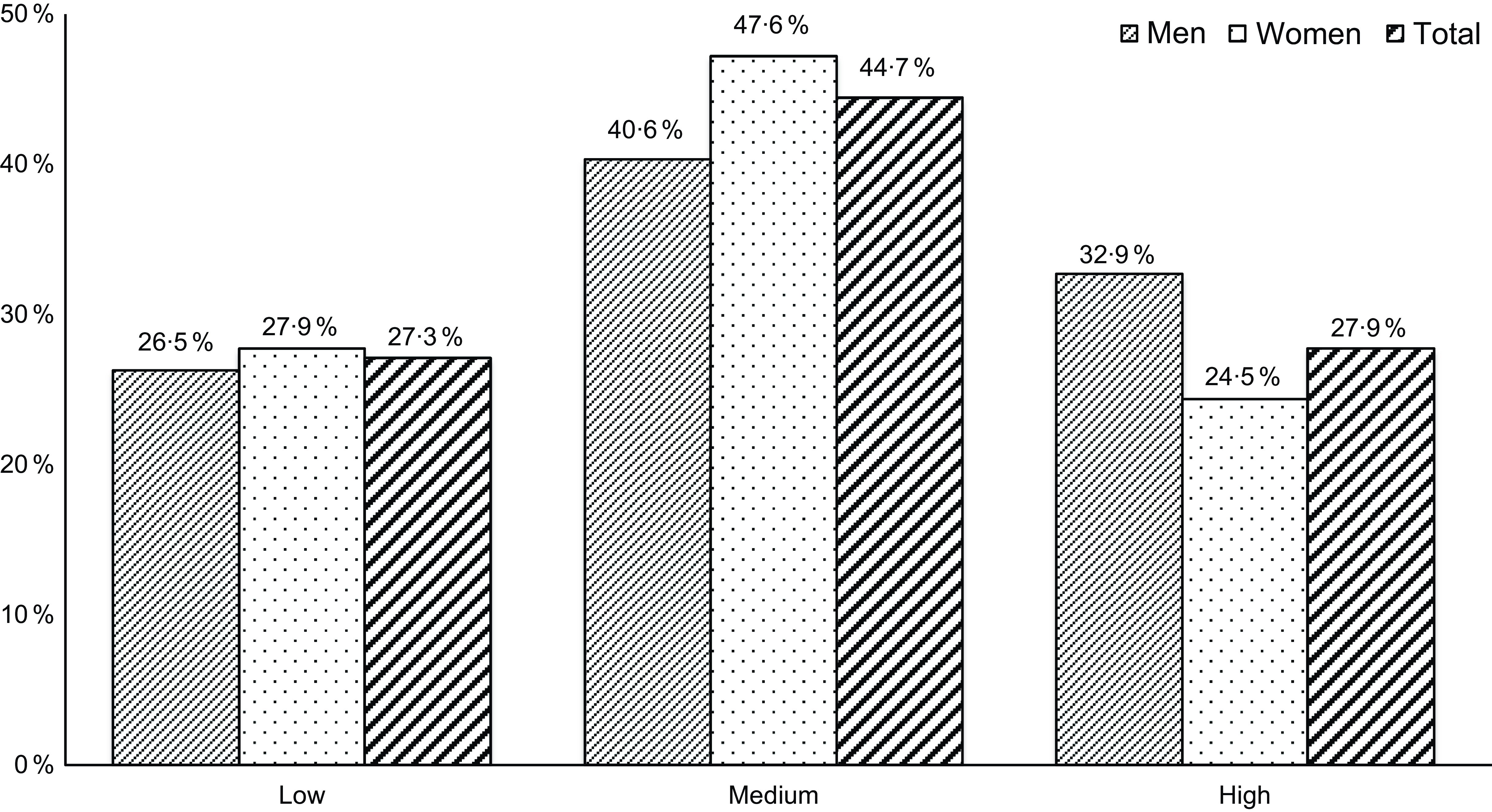
Fig. 1. Adherence to the MDS in men and women. MDS, Mediterranean diet score.
Multivariable logistic regression (Table 2) shows that high adherence to the MDS (highest tertile) significantly reduced the odds of having depressive symptoms by 54·6 % (OR 0·454, 95 % CI 0·266, 0·776) in the whole sample, independent of covariates. When we stratified the analysis by sex, we found an inverse association between high adherence to MDS and depressive symptoms in women (OR 0·385, 95 % CI 0·206, 0·719) but not in men (OR 0·828, 95 % CI 0·254, 2·705). Looking at the continuous variable, we observed that each 1-point increase in MDS was associated with a significant reduction in the odds of having depressive symptoms of 16·4 % (OR 0·836, 95 % CI 0·746, 0·938) in the whole sample and of 18·3 % (OR 0·817, 95 % CI 0·716, 0·932) in women; again, no effect was observed in men. When we examined the association of MDS dietary components with depressive symptoms, we found an inverse significant association with fish consumption above the median in the whole sample (OR 0·559, 95 % CI 0·378, 0·825). In the sex-stratified analysis, this effect was observed only in women (OR 0·444, 95 % CI 0·283, 0·697). There was also an inverse association between depressive symptoms and fruit and nut consumption in men (OR 0·182, 95 % CI 0·062, 0·533) and MUFA:SFA ratio in women (OR 0·579, 95 % CI 0·345, 0·971).
Table 2. Logistic regression for the association of MDS and its dietary components with depressive symptoms by sex
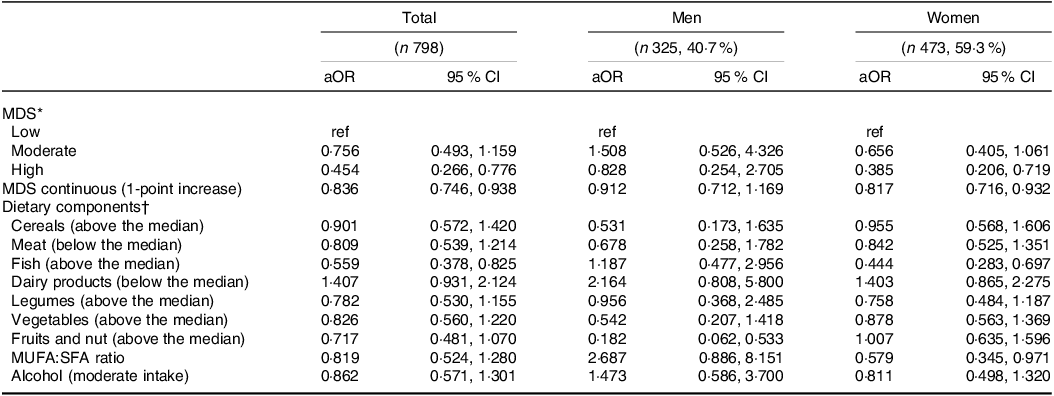
MDS, Mediterranean diet score; MUFA, Monounsaturated fatty acids; SFA, saturated fatty acids.
* Model included age, sex, socio-economic status, living arrangement, waist circumference, number of daily drugs, smoking habits and self-perceived health status, kcal. Sex-stratified model included age, socio-economic status, living arrangement, waist circumference, number of daily drugs, smoking habits and self-perceived health status, kcal.
† Model included age, sex, socio-economic status, living arrangement, waist circumference, number of daily drugs, smoking habits, and self-perceived health status, kcal, and the nine MDS components simultaneously. Sex-stratified model included age, socio-economic status, living arrangement, waist circumference, number of daily drugs, smoking habits, and self-perceived health status, kcal and the nine MDS components simultaneously.
In multivariable analysis, there was a trend for each additional gram of fish per d to reduce the odds of depression by 1.2 % in the whole sample (OR 0·988, 95 % CI 0·980, 0·996). When stratified by sex, this effect was observed only in women (OR 0·984, 95 % CI 0·975, 0·994) (Table 3). Regarding dietary recommendations, consumption of ≥ 3 servings/week of fresh fish (such as sole, trout, cod, hake, sea bream and sea bass) was associated with lower odds of depressive symptoms in the whole sample (OR 0·376, 95 % CI 0·171, 0·826). Women who consumed 2–3 servings/week and ≥ 3 servings/week of fresh fish had 43·4 % (OR 0·566, 95 % CI 0·344, 0·931) and 70 % (OR 0·300, 95 % CI 0·121, 0·743) lower odds of depressive symptoms than those who consumed < 2 servings/week, respectively. There was no association with canned tuna. Among men, fish consumption was not associated with the odds of depression. When we examined the effect of different types of fish, simultaneously included in the model, we found that an increased intake in grams per d of crustaceans or shellfish such as prawns, scampi, mussels and clams reduced the odds of depression in the whole sample (OR 0·962, 95 % CI 0·933, 0·993) and in women only (OR 0·954, 95 % CI 0·919, 0·989).
Table 3. Logistic regression for the association of fish intake with depressive symptoms by sex
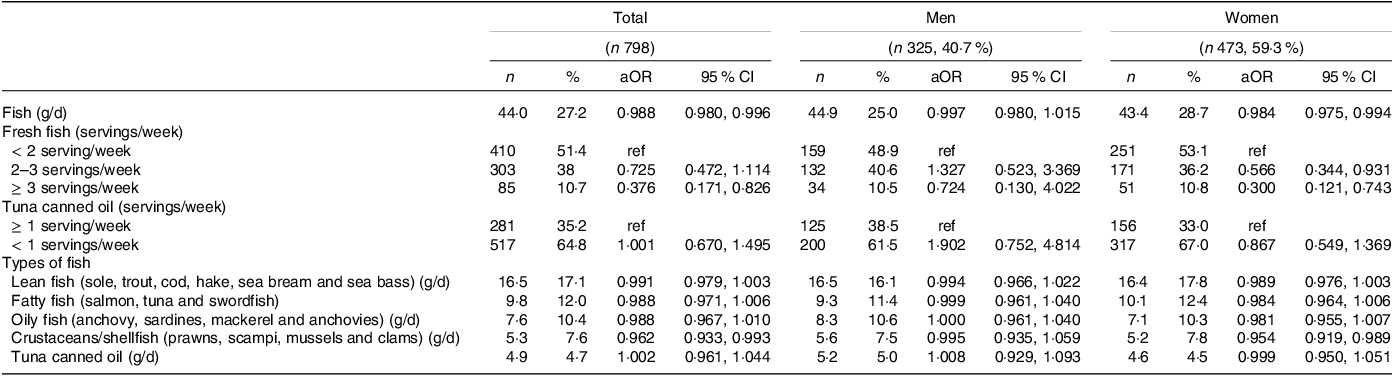
Standard serving: 150 g of fresh fish and 50 g of tuna canned oil.
Models included age, sex, socio-economic status, living arrangement, waist circumference, number of daily drugs, smoking habits and self-perceived health status, kcal.
Sex-stratified model included age, socio-economic status, living arrangement, waist circumference, number of daily drugs, smoking habits and self-perceived health status, kcal.
Discussion
The aim of our study was to investigate the relationship between adherence to the MD, which is the most widespread dietary model in Italy, and depressive symptoms in an Italian population of men and women aged 65 years and over. Our results show that adherence to the highest tertile of the MD is inversely associated with depressive symptoms and, in particular, this is only evident in women when the analysis is stratified by sex.
We found that the prevalence of depressive symptoms was 19·8 %, with a mean age of 73 years. Women made up the majority of the sample and had more depressive symptoms (27·9 %) than men (8 %). According to the Italian ‘Passi D’Argento’ surveillance study (2021–2022)(5) of 18 845 older people aged ≥ 65 years, 9·5 % of the Italian population have depressive symptoms (5·7 % men and 12·8 %). A possible reason for this discrepancy may be that the ‘Passi D’Argento’ study used a structured clinical interview that allowed diagnosis according to the Diagnostic and Statistical Manual of Mental Disorders-IV edition (DSM-IV) criteria for clinical mental disorders(Reference Kroenke, Spitzer and Williams27). Instead, we used an instrument designed to measure depressive symptoms in the general population, which is not intended to be diagnostic, but rather for epidemiological purposes. Our data on the prevalence of depression are more comparable, in terms of the instruments used, with two recent reviews and meta-analyses of observational studies, cross-sectional and longitudinal, investigating depressive symptoms in the general population, which reported that about one-third of the older population suffers from depression(Reference Cai, Jin and Liu2,Reference Hu, Zhao and Wu3) . In our sample, women with depressive symptoms were more likely to be unmarried, to live alone, to take more medications and to have poorer self-perceived health status than women without depressive symptoms, in line with previous literature(Reference Rienks, Dobson and Mishra12,Reference Lee and Hong28,Reference Aung, Moolphate and Koyanagi29) .
To date, only a few studies have examined MD in relation to depressive symptoms in an older population. For example, Skarupski et al. (Reference Skarupski, Tangney and Li14) analysed a cohort of 3502 individuals aged 65+ years (59 % African American) who had no evidence of depression at the baseline and showed that MD was associated with a reduced number of new depressive symptoms over a mean of 7·2 years. Hodge et al. (Reference Hodge, Almeida and English13) (n 8660, aged 50–69 years) showed that MD was associated with less psychological distress, with the OR in the highest scoring group relative to the lowest scoring group being 0·72 (95 % CI = 0·54, 0·95). A very recent study carried out in Greece (Mamalaki et al.)(Reference Mamalaki, Ntanasi and Hatzimanolis15), including 879 participants aged 65 years and older without depression at baseline, showed that each unit increase in MD score was associated with a 6·2 % decrease in the risk of depression. Although Skarupsi et al. (Reference Skarupski, Tangney and Li14) and the studies by Hodge(Reference Hodge, Almeida and English13) and Mamalaki(Reference Mamalaki, Ntanasi and Hatzimanolis15) reported an inverse association between high adherence to MD and depressive symptoms in older people, they did not consider stratification by sex. Indeed, stratification revealed important differences in the association of MD and its components with depressive symptoms between women and men in our sample.
Our findings are supported by the study by Hart et al. (n 4082 subjects aged 55+ years), although performed in a younger population, who reported that a healthy MD-like dietary pattern characterised by frequent intake of vegetables, fruit and fish was associated with lower depressive symptoms in women but not in men(Reference Hart, Milte and Torres30).
Similarly, when we analysed the association between the individual dietary components of the MD and depressive symptoms, we found that consumption of fresh fish and the MUFA:PUFA ratio were associated with reduced odds of having depressive symptoms in women.
According to the Italian dietary guidelines and recommendations(31), we found that women who consumed 2–3 servings/week and ≥ 3 servings/week of fresh fish had a lower odds of depressive symptoms than those who consumed < 2 servings/week. Interestingly, when we analysed the different types of fish, we found this strong association mainly for crustaceans/shellfish (prawns, scampi, mussels and clams) and not for canned tuna. Our results support previous studies(Reference Tanskanen, Hibbeln and Tuomilehto32–Reference Colangelo, He and Whooley36) performed in younger cohorts, many of which underline the protective effect of fish only in women as seen in our sample, for example, Tanskanen et al. (2001)(Reference Tanskanen, Hibbeln and Tuomilehto32), Timonen et al. (2004)(Reference Timonen, Horrobin and Jokelainen33), Jacka et al. (2013)(Reference Jacka, Pasco and Williams35) and Colangelo et al. (2009)(Reference Colangelo, He and Whooley36) showed a relatively strong and independent association between fish consumption and depressive symptoms, but the strength of the association was particularly striking in women(Reference Tanskanen, Hibbeln and Tuomilehto32,Reference Timonen, Horrobin and Jokelainen33,Reference Jacka, Pasco and Williams35,Reference Colangelo, He and Whooley36) . In addition, an Australian longitudinal study reported that women who ate fish ≥ 2 times per week at baseline had a 25 % lower risk of depression, as assessed by the Composite International Diagnostic Interview during follow-up than those who ate fish less frequently. In contrast, baseline fish consumption was not associated with the risk of depression in men(Reference Smith, Sanderson and McNaughton34). A meta-analysis of ten prospective cohort studies involving 109 764 individuals showed that the pooled adjusted relative risk (RR) of depression for the highest v. lowest category of fish consumption was 0·89 (95 % CI 0·80, 0·99); in sex-stratified analysis, this inverse association was more evident only in women (RR 0·89, 95 % CI 0·75, 1·00)(Reference Yang, Kim and Je37).
From a biological point of view, modulation of many pathways involved in inflammation, the hypothalamic–pituitary–adrenal (HPA) axis, obesity, epigenetics, oxidative stress, mitochondrial dysfunction, gut microbiota, tryptophan kynurenine metabolism, neurogenesis and brain-derived neurotrophic factor (BDNF) have been identified as pathways through which components of a MD could plausibly affect mental health(Reference Marx, Lane and Hockey38). For example, the already known link between microbiota and diet, with the changes in the former caused by the latter, could also promote/prevent the onset of depressive symptoms via the gut–brain axis. Data from animal models support that diet-related changes in the gut microbiota can contribute to behavioural changes that mimic symptoms of common mental disorders such as anxiety and depression. For example, a high-fat, western-style diet resulted in an increased Firmicutes/Bacteroidetes ratio and decreased exploratory behaviour, increased anxiety-like behaviour, and decreased memory in rodent models(Reference Marx, Lane and Hockey38).
Looking at the MD components, we observed that the protective effect is provided by fish and the MUFA:SFA ratio, which could increase the fluidity of the brain membranes, facilitating the transduction of neurotransmitters and promoting better mental health(Reference Fernandes, Mutch and Leri39). However, this effect was only observed in women and not in men. The reasons for these sex differences in fish consumption need to be clarified(Reference Smith, Sanderson and McNaughton34), but it can be speculated that this may be due to the effects of vitamin D, deficiency of which has been associated with poorer mental health, depression and psychotic disorders, and this is particularly evident in women(Reference Boulkrane, Fedotova and Kolodyaznaya40), possibly affecting the structure and function of the human brain(Reference Saji Parel, Krishna and Gupta41). It can also be hypothesised that the presence of depression in women may be due to the effect of hormones. In addition, n-3 fatty acids may alter the phospholipid composition of brain cell membranes or cause changes in membrane microstructure and the function of membrane-associated proteins(Reference Li, Dai and Ekperi42). However, even though n-3 fatty acids are thought to be the beneficial component of fish, the results of randomized controlled trial (RCT) investigating the association between n-3 supplements and depressive symptoms remain unclear(Reference Appleton, Rogers and Ness43). The same ‘good’ fatty acids, such as MUFA and PUFA, appear to have an inverse, although weak, association with the risk of depression(Reference Sánchez-Villegas, Verberne and De Irala44), although the protective role of these fats may also depend on other factors, such as diet quality and the social environment(Reference Grosso, Galvano and Marventano45). Further experimental studies are needed to confirm these hypotheses and to clarify the underlying biological mechanisms.
Limitations and strengths
One of the limitations of this study is the impossibility of directly identifying the relationship between dietary exposure and depressive symptoms: the NutBrain Study is an observational cross-sectional study, so reverse causality bias cannot be ruled out. In addition, the people who took part in the study were volunteers and therefore self-selected. On the other hand, the entire eligible population of each municipality was recruited by letter with the involvement of the authorities, which ensured that the sample was quite representative of older people living independently in the community. The tools used also have limitations: one of the most important is that the scale used to investigate the presence of depressive symptoms is only a rough proxy for depressive symptoms and cannot replace the clinical diagnosis of major depression. All questionnaires used during the visit were self-reported, which introduces recall bias, although the presence of the interviewer may have reduced misunderstanding of the questions. A specific limitation of the semi-quantitative FFQ is that it is very difficult to recall diet in relation to the previous year, and therefore the reported information is often very general or, unintentionally, related only to the current season. Finally, although we controlled for several covariates, we cannot completely rule out the possibility of residual confounding due to unmeasured factors.
Finally, because the cross-sectional design uses prevalent cases of disease, the OR can also be considered a prevalence OR (POR). Prevalence OR are good estimators of OR when the mean duration of disease is the same in exposed and unexposed subjects (i.e. exposure is not a prognostic factor) and when the disease is unaffected by exposure(Reference Miettinen46,Reference Morrison47) .
In terms of strengths, the study population is very well characterised and, as a population-based cohort study rather than a clinical setting, is quite representative of the community-dwelling older people. The CES-D scale is a validated tool that assesses different aspects of a person’s depressive domain and mood, and it is the most commonly used tool in large-scale epidemiological studies because it is rapid, potentially self-reporting and suitable for our purpose, which was not to diagnose depression but only to detect the depressive symptomatology in the population. In addition, all the questionnaires we used were administered by a trained staff, who helped the subjects to recall and reconstruct the different information requested.
Conclusions
In conclusion, this study highlights the important role of a healthy diet in preventing and promoting mental health in old age. Following the MD dietary model and eating at least two portions of fish per week are beneficial against depressive symptoms, especially in older women. These findings have public health implications, as they support the role of promoting a healthy, balanced diet in the older population. Further longitudinal and experimental studies are needed to better understand the underlying biological mechanisms.
Acknowledgements
The authors would like to thank all the participants and their families for taking part in the NutBrain Study. A special thank is extended to the municipalities of Bollate, Baranzate and Segrate for their cooperation and practical support. The authors also would like to gratefully thank the researchers, physicians, technicians, nurses and the administrative staff for their efforts, contributions, and support to the project. °NutBrain Study Group: Fulvio Adorni, Sara Bernini, Silvia Conti, Maria Lea Correa Leite, Alfredo Costa, Matteo Cotta Ramusino, Nithiya Jesuthasan, Alfonso Mastropietro, Massimo Musicco, Orietta Pansarasa, Federica Prinelli, Elena Perdixi, Anna Pichiecchio, Giovanna Rizzo, Evelin Scarian, Marco Severgnini, Elena Sinforiani.
This work was supported by the Ministero della Salute (Bando di Ricerca Finalizzata Giovani Ricercatori 2016, GR − 2016-02361730). The study funders had no involvement in the design and conduct of the study, collection, management, analysis and interpretation of data; preparation, review or approval of the manuscript; and the decision to submit the manuscript for publication.
Silvia Conti: conceptualisation, formal analysis, investigation, methodology and writing – original draft. Sara Bernini: conceptualisation, funding acquisition, investigation, methodology, and writing – review and editing. Marco Severgnini: conceptualization, funding acquisition, investigation, methodology, and writing - review and editing. Elena Perdixi: conceptualisation, investigation, methodology, and writing – review and editing. Nithiya Jesuthasan: conceptualization, investigation, methodology, and writing – review and editing. Federica Prinelli: conceptualisation, data curation, formal analysis, funding acquisition, investigation, methodology, project administration, supervision, and writing – review and editing.
There are no conflicts of interest.
All authors approved the final version to be published and agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was conducted according to the guidelines laid down in the Declaration of Helsinki, and all procedures involving human subjects were approved by the Medical Ethics Committee of Pavia, Italy (protocol n. 20180036036, 7/5/2019 and further amendments). Written informed consent was obtained from all subjects. Data were handled and stored according to the European Union General Data Protection Regulation (EU GDPR) 2016/679, and data transmission was protected by encryption/decryption and password protection. The study protocol was registered on the ClinicalTrials.gov platform (trial registration number NCT04461951, registration date 7 July 2020).
Supplementary material
For supplementary material/s referred to in this article, please visit https://doi.org/10.1017/S0007114524000461