CVD is a major cause of death worldwide( Reference Alwan, Armstrong and Cowan 1 ). Therefore, understanding the pathophysiology of CVD and developing strategies to minimise its appearance in the population have become of paramount importance( Reference Basciano, Federico and Adeli 2 ). In fact, some conferences had attempted to examine and propose strategies for identifying high-risk cardiovascular patients who require primary prevention( Reference Smith, Greenland and Grundy 3 – Reference Grundy, Garber and Goldberg 5 ).
The elevated incidence of CVD is believed to be partially due to the modern lifestyle( Reference Basciano, Federico and Adeli 2 ). Diet has changed on a global scale, and the emphasis on fructose consumption has increased in recent decades. In addition, high fructose consumption has been correlated to the onset of cardiometabolic alterations in the world’s population( Reference Bantle, Swanson and Thomas 6 ). Chronic fructose ingestion has been used as a model of CVD( Reference Medeiros, Gaique and Bento-Bernardes 7 ), and this model can be characterised by increased serum insulin and TAG levels, as well as vascular disorders( Reference Nade, Kawale and Todmal 8 – Reference Mahmoud, El-Nagar and El-Bassossy 10 ).
Because of the elevated prevalence of CVD, some researchers are dedicated to the evaluation and prevention of cardiovascular risk factors in different ethnic groups, and it has been observed that metabolic alterations are emerging earlier in life( Reference Park, Falconer and Viner 11 ). In this context, non-pharmacological treatment might be considered an important intervention for avoiding the appearance and progression of CVD because it usually has a low cost( 12 ). Considering the above information, understanding the mechanisms underlying how non-pharmacological treatment might benefit a population that shows subclinical alterations and is at a constant risk of developing CVD might be crucial to decreasing the incidence of such diseases.
It is worth noting that the endothelium has been considered crucial to the regulation of vascular homoeostasis( Reference Vita and Keaney 13 ); therefore, endothelial cell dysfunction could precede the development of atherosclerotic and morphological changes. In addition, endothelial changes contribute to the development of late clinical complications( Reference Ross 14 ), including arterial remodelling and abnormal angiogenesis( Reference Muiesan, Salvetti and Paini 15 – Reference Iiyama, Nagano and Yo 17 ).
l-Arginine, a semi-essential amino acid, is the precursor of nitric oxide (NO) production via endothelial nitric oxide synthase (eNOS). NO activates smooth muscle soluble guanylate cyclase and thereby increases the cyclic GMP (cGMP) and decreases the intracellular Ca2+ concentrations, culminating in the relaxation of smooth muscle cells and the inhibition of platelet aggregation( Reference Moncada and Higgs 18 ). Because arginine is a substrate for eNOS and NO is crucial for endothelial health, a number of early experiments using animal models and humans investigated the potential efficacy of arginine supplementation in reversing endothelial dysfunction in pathological states( Reference Cooke, Andon and Girerd 19 – Reference Bode-Boger, Boger and Galland 24 ). A research group showed that acute intravenous( Reference Cooke, Andon and Girerd 19 ) and chronic oral arginine ingestion( Reference Cooke, Singer and Tsao 20 ) in rabbits reverses the vascular damage caused by a hypercholesterolaemic diet. In humans, the results are controversial because some studies have shown an improvement in the endothelial function of individuals with existing endothelial damage, primarily in the elderly population( Reference Bode-Boger, Muke and Surdacki 21 , Reference Creager, Gallagher and Girerd 22 ), but in healthy patients oral arginine supplementation at the same dose does not improve endothelial function( Reference Gornik and Creager 23 , Reference Bode-Boger, Boger and Galland 24 ). Thus, whether arginine supplementation would modulate metabolic variables and vasodilation in healthy subjects who present with metabolic alterations but do not have the established disease remains unknown.
Regular aerobic exercise is widely recognised as a non-pharmacological approach to reduce CVD morbidity and mortality( Reference Thompson, Buchner and Pina 25 , Reference Fletcher, Balady and Blair 26 ). In recent years, several researchers have studied the mechanisms that explain the beneficial health effects of regular exercise and have shown that exercise decreases TAG, increases HDL cholesterol( Reference Mann, Beedie and Jimenez 27 ), lowers blood pressure( Reference Whelton, Chin and Xin 28 ), improves glucose metabolism and insulin sensitivity( Reference Thomas, Elliott and Naughton 29 ) and reduces body weight and inflammatory markers( Reference Szostak and Laurant 30 ). These risk factor improvements can explain nearly 59 % of the reduction in CVD( Reference Mora, Cook and Buring 31 ). However, other mechanisms, such as improving endothelial function and NO bioavailability( Reference Joyner and Green 32 ) and enhancing vagal tone( Reference Beere, Glagov and Zarins 33 ), might be involved in the negative correlation between chronic physical activity and CVD incidence.
Some mechanisms, such as decreases in the total peripheral resistance( Reference Forjaz, Tinucci and Ortega 34 ) and sympathetic nervous activity( Reference Kulics, Collins and DiCarlo 35 ) and increases in the bioavailability of NO( Reference Murias, Dey and Campos 36 ), could underlie the effect of arginine and training on CVD, but whether aerobic training and arginine supplementation have similar or additive effects in preventing CVD onset in individuals who are continuously exposed to a CVD-causing agent remains unknown. Thus, the aims of this study were to compare the effects of aerobic training and chronic arginine supplementation and investigate whether they exert similar or potentiating effects on metabolic variables and vascular reactivity in rats at high risk for developing the metabolic syndrome.
Methods
Experimental design
The handling and experimental protocols were approved by the Ethics Committee for the Care and Use of Laboratory Animals of Fluminense Federal University (Protocol 466/2013) and complied with the ethical guidelines of the Brazilian Society of Laboratory Animal Science. The experiments were performed on 2-month-old male Wistar rats (n 7/group; 329·9 (sem 8·9) g), and the experimental model has been previously described by our group( Reference Medeiros, Gaique and Bento-Bernardes 7 ). The animals were maintained under controlled temperature conditions (24±1°C) on a 12-h light cycle (lights on at 07.00 hours).
The animals were initially randomly allocated into two groups: a control (C) group that received water and commercial chow (Nuvilab Cr-1®, Nuvital Nutrientes S/A; Table 1) ad libitum for 2 weeks and a fructose group that received an overload of 10 % D-fructose (Sigma-Aldrich) in drinking water ad libitum and commercial chow for 2 weeks (Fig.1). After 2 weeks, seven animals from each group were euthanised, and the remaining animals from the C group (n 7) were maintained under the same previously described conditions for an additional 8 weeks. The rats from the F group were divided into four sub-groups, all of which continued receiving D-fructose in their drinking water for an additional 8 weeks. The fructose+arginine (FA) group received 880 mg/kg per d of arginine (Sigma-Aldrich) by orogastric gavage. The fructose+training (FT) group was subjected to aerobic exercise training, and the fructose+training+arginine (FTA) group was subjected to a combination of aerobic training with arginine supplementation. The arginine dose was calculated according to the formula for dose translation based on the body surface area( Reference Reagan-Shaw, Nihal and Ahmad 37 ), which was considered to equal 10 g for an adult person( Reference Siani, Pagano and Iacone 38 ). In this context, it is important to note that this amino acid is naturally found in human nutrition, specifically in edible mushrooms( Reference Sun, Liu and Fan 39 ) and in various meats, such as beef, pork and poultry( Reference Varnam and Sutherland 40 ). The energy intake was assessed twice weekly and calculated by considering that 1·0 g of chow corresponds to 14·06 kJ (3·36 kcal) and 1·0 g of fructose corresponds to 16·7 kJ (4·0 kcal).

Fig. 1 Experimental protocol.
Table 1 Nutritional information of the Nuvilab Cr-1 commercial chowFootnote *
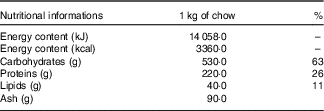
* Ingredients: ground whole maize, soya bean meal, wheat bran, calcium carbonate, dicalcium phosphate, sodium chloride, vitamins A, D3, E, K2, B1, B6 and B12, niacin, calcium pantothenate, folic acid, biotin, chloride choline, iron sulphate, manganese monoxide, zinc oxide, calcium sulphate, sodium selenite, cobalt sulphate, lysine, methionine and butylatedhydroxytoluene. The nutritional information and ingredient composition were obtained from the chow label forNuvilab Cr-1 (Nuvital Nutrientes S/A).
Aerobic exercise protocol
Before initiating the experimental protocol, all animals were subjected to an adaptation period on a treadmill (Inbrasport®) for 4 weeks (5 min/d with weekly increases of 0·3–1·0 km/h). All of the animals underwent a maximal exercise test (MET) on a treadmill with an 11 % inclination, an initial speed of 1·0 km/h and increases in velocity of 0·1 km/h every 2 min.The MET was performed before the experiment, 2 weeks after the initiation of the experiment and at the end of the experiment to determine the maximum running speed( Reference Medeiros, Gaique and Bento-Bernardes 7 ).
The groups assigned to aerobic training were subjected to a moderate-intensity exercise training regimen (50–75 % maximal running speed) with a 0–7 % inclination on a treadmill 4 d/week during the last 8 weeks of the experimental protocol( Reference Medeiros, Gaique and Bento-Bernardes 7 ). All of the animals allocated to the sedentary groups were subjected to 5 min in the treadmill at low intensity (0·3 km/h) once a week to maintain their adaptation to the treadmill.
Biochemical analysis
Glycaemia (Accu-Chek glucometer; Roche Diagnostics) and serum TAG (enzymatic kit; Labtest) were measured in blood collected from the tail after 5 h of fasting, during which the animals received only tap water ad libitum. Subsequently, the animals were euthanised, and blood was collected by cardiac puncture from the right atrium, centrifuged for 15 min at 1700 g and 4°C to separate the serum and stored at −80°C for future analysis.
The serum insulin was measured by ELISA (Cayman Chemical Company)( Reference Paula, Souza and Cabanelas 41 ) at the end of the protocol. The serum NO levels were measured for the assessment of the nitrite content using an ozone-based chemiluminescence assay with an NO analyzer (Sievers Model 280 Nitric Oxide Analyzer; GE Healthcare), as previously described( Reference Gomes, Casella-Filho and Chagas 42 ).
Body composition
After euthanasia, the eviscerated carcasses were frozen at −20°C for posterior analyses. The body fat and protein content were determined using the carcass technique( Reference Souza, Cordeiro and Oliveira 43 ). In brief, the eviscerated carcasses were weighed, autoclaved and homogenised in distilled water (1:1). Aliquots of the homogenate were used to measure the protein and fat contents. The homogenate was used to gravimetrically determine the fat mass. The samples were hydrolysed in a shaking water bath at 70°C for 2 h with 30 % KOH in ethanol. After the addition of 9 m sulphuric acid, the total lipids were extracted through three successive washes with petroleum diethyl ether. The samples were dried at room temperature until a constant weight was obtained. The protein from the homogenates was extracted at 37°C for 1 h using 0·6 N KOH. The samples were then centrifuged at 800 g and room temperature for 10 min, and the supernatant was collected to measure the protein concentration using a Pierce™ BCA Protein Assay Kit (Thermo Fisher Scientific). The results are expressed as grams of fat or protein per 100 g of carcass.
Vascular reactivity
The vascular reactivity was assessed as previously described( Reference Medeiros, Gaique and Bento-Bernardes 7 , Reference da Motta, Kummerle and Marostica 44 ). In brief, the thoracic aorta was dissected after 2 and 10 weeks of the experimental protocol and prepared for isometric tension recording. The aortas were placed in freshly prepared Krebs solution containing 119·0 mm NaCl, 14·9 mm NaHCO3, 11·5 mm glucose, 4·7 mm KCl, 4·2 mm KH2PO4, 1·2 mm MgSO4 and 1·6 mm CaCl2. The aortas were cleaned, separated from adherent tissue and cut into rings with a diameter of approximately 3 mm. Each ring was suspended between two wire hooks, mounted in 4-ml organ chambers with Krebs solution at 37°C and pH 7·4 and continuously aerated with a mixture of 95 % O2 and 5 % CO2 under a resting tension of 1·0 g.
The isometric tension of the tissues was recorded using a force-displacement transducer (Ugo Basile) controlled by software (Data Capsule Digital Recorder 17400; Ugo Basile). After a 1-h stabilisation period, the intact endothelium aortic rings were pre-contracted with phenylephrine (1 mm), and the cumulative concentration–response curves of the aortic rings to acetylcholine (10−9–10−4 m) were obtained. The relaxation curves are expressed as percent changes from the contraction induced by phenylephrine. The cumulative concentration–response curves to phenylephrine (10−9–10−4 m) were also determined( Reference da Motta, Kummerle and Marostica 44 ).
Quantitative histopathology
At the moment of euthanasia, which was performed immediately after blood collection, samples of the thoracic aorta were immersed in 4 % phosphate-buffered formalin (pH 7·2) for 48 h. After fixation, the samples underwent routine histology processing and were embedded in Paraplast® plus (#P3558; Sigma-Aldrich). Quantitative analyses were performed using morphometric tools( Reference Medeiros, Gaique and Bento-Bernardes 7 ).
The aortic rings were sectioned at 3-μm thickness and stained with haematoxylin–eosin. Four non-consecutive digital images were acquired per animal using the Evos XL Core system (Thermo Fisher Scientific) and were analysed using Image-Pro® Plus version 4.5 (Media Cybernetics). The intima-media thickness (IMT) was estimated by taking two manual measurements at positions 0° and 90° for each aorta ring with the line feature. The lumen diameter was assessed from four digital images by measuring the smallest and largest luminal diameters using the line feature. The above-mentioned parameters were used to assess the IMT:lumen ratio.
Statistical analyses
The appropriate sample size of seven animals per group was calculated using the vasodilation of the thoracic aorta under acetylcholine stimulation as the primary outcome and assuming an average difference of 5 % (effect size) and an equal sd of 5 %. Both values were obtained from preliminary experiments by adjusting the power of the statistical test to 0·8 and the α error to 0·05.
The data normality was verified using the Shapiro–Wilk test. The experimental groups were compared by one-way ANOVA followed by Student’s–Newman–Keuls post hoc test, when appropriate. Nonparametric variables were logarithmically transformed before analysis. The results are expressed as the means with their standard errors of the mean, and differences were considered significant if P<0·05.
Results
As observed in Table 2, fructose overload and aerobic training and/or arginine treatment did not change the body mass gain. The analysis of the body composition through a study of the carcass content showed no change in protein content after 2 weeks of the protocol. However, the protein content was improved after 8 weeks of training compared with that of the untrained groups (C, F and FA groups), which shows that training, but not arginine, improves the body protein content. However, fructose overload, even when administered for a short period of 2 weeks, increased the body fat content, and both arginine and aerobic training reversed this adverse effect.
Table 2 Weight and body composition (Mean values with their standard errors)

C group, control group; F group, fructose group; FA group, fructose+arginine group.
The body fat and body protein were assessed using the carcass method (see the ‘Methods’ section). Statistical analysis (one-way ANOVA and post hoc Student’s–Newman–Keuls test): * P<0·05 v. the C group; † P<0·05 v. the F group; ‡ P<0·05 v. the FA group; § P<0·05 v. the same group 2 weeks later.
As demonstrated through the MET (Table 3), neither fructose overload nor arginine supplementation altered the exercise capacity, although the trained groups (FT and FTA) showed an improvement in the velocity achieved on the MET (P<0·001). A period of 2 weeks of fructose overload increased the serum insulin (29 %; P=0·046), and this effect was sustained until the 10th week of the experiment (144 %; P=0·014). Aerobic training and arginine supplementation protected the serum insulin from increasing after fructose overload. Glycaemia was not altered by fructose intake or training. At the end of the protocol, fructose consumption increased the serum TAG by 91 % (P<0·001), and only training inhibited this increase.
Table 3 Training and metabolic parameters (Mean values with their standard errors)

C group, control group; F group, fructose group; FA group, fructose+arginine group.
Statistical analysis (one-way ANOVA and post hoc Student’s–Newman–Keuls test): * P<0·05 v. the C group, † P<0·05 v. the F group, ‡ P<0·05 v. the FA group; § P<0·05 v. the same group 2 weeks later.
A period of 2 weeks of fructose overload had no effect on the vascular reactivity response to acetylcholine (P=0·39) or phenylephrine (P=0·09). However, 10 weeks of fructose supplementation impaired the endothelium-dependent relaxation response induced by acetylcholine (Fig. 2(a)) (EC50=36·37×10−7 (sem 9·61×10−7) m; P<0·0; 71·27 %) compared with the C group (EC50=3·13×10−7 (sem 1·9×10−7) m), and arginine (EC50=0·93×10−7 (sem 0·4×10−7) m), training (EC50=11·44×10−7 (sem 5·1×10−7) m) and training plus arginine (EC50=1·89×10−7 (sem 0·68×10−7) m) were capable of preventing this endothelial alteration because the values were similar to those of the C group, despite the constant intake of fructose. The analysis of the vasoconstrictory response (Fig. 2(b)) revealed that the F group (EC50=2·43×10−7 (sem 0·65×10−7) m) showed a hyperreactivity contractile response (P<0·01) to phenylephrine compared with the C group (EC50=13·6×10−7 (sem 2·12×10−7) m). This phenomenon was prevented by arginine (EC50=4·56×10−7 (sem 0·78×10−7) m), training (EC50=7·99×10−7 (sem 1·5×10−7) m) and training plus arginine (EC50=9·22×10−7 (sem 2·39×10−7) m).

Fig. 2 Vascular reactivity response to acetylcholine (a) and to phenylephrine (b) after 10 weeks of fructose overload. Values are means with their standard errors represented by vertical bars. C group, control group; F group, fructose group; FA group, fructose+arginine group; FT group, fructose+training group; FTA group, fructose+training+arginine group; CE50, effective concentration needed to promote 50 % of the maximum contractile effect. * P<0·05 v. the C group; † P<0·05 v. the F group.
Fructose intake did not decrease the NO bioavailability (480·6 (sem 42·97) nm) (Fig. 3) compared with that detected in the C group (445·3 (sem 36·27) nm). The treatment with arginine or training increased the NO serum levels (P<0·00; FA=838·8 (sem 118·0) nm; FT=991·5 (sem 180·7) nm), and no potentiating effects were observed with the combination of the exercise and arginine treatments (FTA=1275·0 (sem 205·6) nm; P<0·001).

Fig. 3 Nitric oxide (NO) after 10 weeks of fructose overload. Values are means with their standard errors represented by vertical bars. C, control group; F group, fructose group; FA group, fructose+arginine group; FT group, fructose+training group; FTA group, fructose+training+arginine group. * P<0·05 v. the C group; † P<0·05 v. the F group.
Thoracic aorta morphometry (Table 3 and Fig. 4) showed no significant changes in the IMT (P=0·13), lumen diameter (P=0·15) or IMT:lumen ratio (P=0·07) among the experimental groups (Table 4).

Fig. 4 Photomicrographs obtained from the thoracic aorta of control rats (a) or rats that received fructose in their drinking water (b) for 10 weeks. After 2 weeks of fructose drinking, some animals were subjected to aerobic exercise training (c), arginine supplementation (d) or the combination of both non-pharmacological treatments (e). Note that the aorta in (b) is morphologically similar to the aorta of the control group. The treatments alone or in combination did not alter the aorta morphology.
Table 4 Thoracic aorta morphometryFootnote * (Mean values with their standard errors)
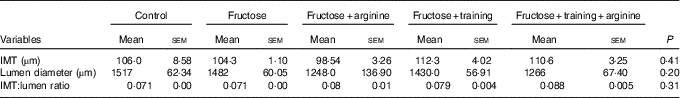
IMT, intima-media thickness.
* Statistical analysis: one-way ANOVA and post hoc Student’s–Newman–Keuls test.
Discussion
The data presented confirm that short-term fructose overload leads to metabolic changes and to vascular damage in the longer term, as previously demonstrated by our group( Reference Medeiros, Gaique and Bento-Bernardes 7 ), and these effects might culminate in the onset of CVD. The novelty of the present work is that arginine or training, either by themselves or in combination, are able to avoid the progression of CVD in its earlier stages. Aerobic training, but not arginine, is able to improve the protein content and prevent an increase in the TAG content, and both treatments, namely arginine supplementation and/or aerobic training, reversed the increases in serum insulin and adipose tissue and increased the serum NO concentration. In addition, both treatments were able to prevent the endothelial dysfunction, but no additive effects were observed.
Fructose and metabolic alterations
The association between an increase in fructose ingestion and the appearance of metabolic alterations has been previously demonstrated. Morris et al.( Reference Morris, Araujo and Pohlman 45 ) showed that animals that received 10 % fructose in water during either the day or night for 6 weeks exhibited an increase in fat mass, which corroborates the data obtained in the present study and confirms that fructose overload, even without altering the body mass, is capable of leading to alterations in body composition.
Adipose tissue is responsible for increasing the level of adipokines, which play an important role in energy homoeostasis, insulin sensitivity, immune response and vascular disease. In this context, a high ingestion of fructose is correlated with the appearance of CVD in animals( Reference Yoo, Ahn and Park 46 ) and humans( Reference Jin, Welsh and Le 47 ) by increasing insulin resistance, TAG, oxidative stress and impairing vascular function( Reference Medeiros, Gaique and Bento-Bernardes 7 ), which is in agreement with the data obtained in the present study. In addition, one described mechanism for impaired endothelial function in patients with the metabolic syndrome is the decrease in NO bioavailability and fatty acid metabolism in adipose tissue, muscle and liver in health and disease( Reference Wilkes, Hevener and Olefsky 48 ). In this perspective, a high-fructose diet has been widely used in previous studies as an animal metabolic model( Reference Ibrahim, Amin and Ibrahim 49 – Reference Kim, Okubo and Juneja 51 ), and non-pharmacological strategies for blunting the fructose-induced alterations have received increased interest.
Training protocol
The presented training protocol was effective, as demonstrated by the finding that the trained rats exhibited an increased maximal velocity capacity at the end of the 8 weeks of the training protocol. In addition, the present data show that training was able to improve the body protein content, which agrees with the literature( Reference Medeiros, Gaique and Bento-Bernardes 7 ). A previous study has shown that 8 weeks of aerobic exercise on a treadmill can prevent muscle atrophy in rats with heart failure( Reference Cunha, Bechara and Bacurau 52 ). Aerobic exercise has been recognised as an efficient preventive and therapeutic strategy for CVD because of its ability to reduce CVD risk factors( Reference Powers, Lennon and Quindry 53 , Reference Emter, McCune and Sparagna 54 )and improve both exercise tolerance and quality of life( Reference Roveda, Middlekauff and Rondon 55 ).
In contrast, arginine supplementation separately did not show an improvement in the exercise performance in this study, showed by the maintenance of the maximal running speed at the end of protocol by the fructose+arginine group. Alvares et al. showed that 500 mg of isolated acute arginine supplementation does not result in alterations in exercise performance when supplemented before the physical activity( Reference Alvares, Conte-Junior and Silva 56 ), and Doutreleau et al. showed that 6 weeks of isolated chronic arginine supplementation is unable to alter the exercise capacity of heart failure patients( Reference Doutreleau, Mettauer and Piquard 57 ).
Energetic ingestion and body fat mass
The presented data show that the animals that received fructose, even under an isoenergetic diet, showed the same body mass gain as the C group but an increased body fat content, and this increase was reversed by isolated aerobic training and by isolated arginine supplementation, but these treatments did not exert a synergic effect. Although divergences with respect to fructose-stimulated leptin have been detected( Reference De Angelis, Senador and Mostarda 58 , Reference Suga, Hirano and Kageyama 59 ), many studies have shown that a high fructose intake is not able to change the circulating leptin concentration( Reference Suga, Hirano and Kageyama 59 , Reference Madani, Louchami and Sener 60 ), which can explain the same energetic ingestion. However, an increase in visceral fat has been associated with the serum insulin level( Reference Bogdanski, Suliburska and Grabanska 61 – Reference Miczke, Suliburska and Pupek-Musialik 63 ) because an increase in insulin sensibility leads to improved glucose uptake and, consequently, to increased fat deposition( Reference Matravadia, Zabielski and Chabowski 64 ).
Exercise practice leads to changes in body composition, and it has been elucidated that regular exercise decreases the amount of abdominal and subcutaneous fat in overweight and obese individuals, independent of the diet type( Reference Grundy, Blackburn and Higgins 65 ). It is important to emphasise that an excess of abdominal fat is a complicating factor of metabolic disorders, regardless of weight loss; thus, chronic exercise can reduce the risk of onset of, or reverse, metabolic disorders( Reference Lakka and Laaksonen 66 ).
In addition, NO, which is increased by exercise, might stimulate the oxidation of energy substrates (including fatty acids and glucose) in adipocytes, liver, skeletal muscle, heart and the whole body( Reference Fu, Haynes and Kohli 67 ). According to previous studies, a decrease in fat can be achieved through an increase in the expression of PPARγ coactivator-1α, which is a regulator of oxidative phosphorylation and mitochondrial biogenesis( Reference Wu, Puigserver and Andersson 68 , Reference Lehman, Barger and Kovacs 69 ).
Metabolic parameters
The analysis of serum insulin revealed that fructose ingestion leads to insulin resistance with no glucose alteration, as previously described( Reference Medeiros, Gaique and Bento-Bernardes 7 ). Both aerobic training and arginine supplementation were separately able to normalise serum insulin, but the combined treatment did not exert synergistic effects. Arginine is an amino acid involved in a number of physiological processes, and it is considered one of the most powerful secretagogues of the growth hormone( Reference Chromiak and Antonio 70 ). It has also been described as a powerful stimulator of insulin secretion that acts directly on pancreatic β-cells( Reference Ishiyama, Ravier and Henquin 71 ). In addition, it is largely accepted that regular aerobic exercise enhances insulin sensitivity. A meta-analysis showed that structured exercise training has a positive effect on HbA1c level in adults with type 2 diabetes( Reference Umpierre, Ribeiro and Kramer 72 ). In addition, regular exercise improves insulin sensitivity, and this improvement might persist for 72 h or longer after the last training bout, even in type 2 diabetes individuals( Reference Way, Hackett and Baker 73 ).
The role of arginine in insulin resistance remains under investigation, and the published data are inconsistent( Reference Bogdanski, Suliburska and Grabanska 61 , Reference Kawano, Nomura and Nisikado 74 ). More recent studies have shown the beneficial effects of arginine supplementation. For example, Miczke et al.( Reference Miczke, Suliburska and Pupek-Musialik 63 )showed that 20 g/kg per d of arginine for 40 d can improve insulin resistance in rats fed a high-fat diet, and similar results have been obtained in humans( Reference Paolisso, Tagliamonte and Marfella 75 ).
However, the training regimen alone could prevent hypertriglyceridaemia, and its combination with arginine supplementation exerted no additional effects. Chronic aerobic exercise also provides positive adaptations to the lipid profile, such as increased HDL levels and decreased levels of LDL, TAG and VLDL compared with those sedentary individuals, regardless of sex, biotype and diet. It is likely that this effect of exercise on the lipid profile occurs because of an increase in the consumption of fatty acids by muscle tissue and the activity of the lipoprotein lipase enzyme of the muscle( Reference Frayn, Arner and Yki-Jarvinen 76 ), a mechanism that is not stimulated by arginine supplementation.
Vascular function
The presented data show that arginine supplementation and aerobic training separately have the same effect on reversing vascular dysfunction, but their combination does not exert a synergic effect. Arginine, as it is largely known, is a natural substrate for nitric oxide synthesis (NOS) that, as a result of the action of eNOS and the presence of many cofactors, such as NADPH oxidase, migrates to the adjacent smooth muscle and stimulates the guanylate cyclase, resulting in an increased conversion of GTP into cGMP, which decreases the intracellular calcium concentrations and promotes the relaxation of vascular smooth muscle( Reference Andrew and Mayer 77 ). However, the available results for the chronic effect of arginine are conflicting. The Nitric Oxide in Peripheral Arterial Insufficiency study, which included 133 patients administered 6 months of supplementation, observed no effects on vascular reactivity( Reference Wilson, Harada and Nair 78 ). In contrast, in 2009, Bai et al. ( Reference Bai, Sun and Yang 79 ) published a meta-analysis of twelve studies and concluded that short-term oral l-arginine is effective at improving endothelial function.
The aerobic exercise training also increases NO production, and a 4-week training period resulted in increases in NOS in the skeletal muscle arterioles of rats and in the vasodilatory response to acetylcholine but not in the response to sodium nitroprusside, an endothelium-independent vasodilator, suggesting an improvement in NO-dependent endothelial function( Reference Sun, Huang and Koller 80 ). However, a potentiating effect of the training and arginine supplementation treatments on endothelial function was not observed, which indicates that the training and arginine groups exhibited the same effects as the group subjected to both treatments concomitantly. These results are likely due to the ceiling effect because both treatments separately improved the similar maximum magnitude of NO and decreased oxidative stress( Reference Medeiros, Gaique and Bento-Bernardes 7 , Reference Carvalho, Diniz and Haidar 81 ). In addition, only limited data are available for the combined treatment. Hambrecht et al. demonstrated that in patients with chronic heart failure long-term arginine supplementation and training with a submaximal handgrip exercise presented a synergistic effect on blood flow and radial artery diameter( Reference Hambrecht, Hilbrich and Erbs 82 ). It is important to note that this type of exercise mobilises a small muscle group and can thus show only local responses. However, to the best of our knowledge, the effect of arginine supplementation and the systemic effect of exercise have not been previously studied. It is important to emphasise that in healthy individuals the NOS enzyme activity will likely be at its maximum; thus, the enzymes exposed to l-arginine do not show improvements in activity. In individuals with a metabolic pathological condition, the NOS enzyme cannot reach its highest activity because of an increase in the intracellular concentration of NOS, resulting in decreased NO formation compared with that found under physiological conditions( Reference Tsikas, Boger and Sandmann 83 ).
In addition, the arginine paradox is largely discussed in the literature with controversial results. Wilson et al. ( Reference Wilson, Harada and Nair 78 ) demonstrated that arginine fails to improve endothelial function( Reference Wilson, Harada and Nair 78 ). In their work, arginine supplementation decreased both the NO levels and endothelium-dependent vasodilation in individuals with peripheral arterial disease. In contrast, arginine supplementation improved the impaired endothelium-dependent vasodilation in hypercholesterolaemic animals with endothelial dysfunction( Reference Girerd, Hirsch and Cooke 84 ). Previous studies have demonstrated that reactive O2 species are associated with an impaired arginine-NO pathway in individuals with metabolic alterations( Reference Garcia, Rocha and Silva 85 – Reference Furukawa, Fujita and Shimabukuro 88 ). Considering the increased systemic and tissue-specific oxidative status observed in this experimental model( Reference Medeiros, Gaique and Bento-Bernardes 7 ), this oxidative stress can adversely affect the action of NO and, consequently, vascular function. In contrast, arginine and aerobic training could avoid the oxidative profile caused by high fructose ingestion and could thus prevent vascular dysfunction. However, other mechanisms should be considered.
Conclusion
Thus, aerobic training is able to prevent an increase in TAG and improve the protein mass content, and both the aerobic training and arginine supplementation treatments have similar effects on preventing increases in fat content, serum insulin and serum NO, as well as endothelial dysfunction, but do not show any additive effects.
Acknowledgements
The authors would like to thank Gustavo Mataruna da Silva for technical assistance. Also, we would like to thank the people who work at the Laboratory of Exercise Sciences.
This work was supported by CAPES (Coordination for the Improvement of Higher Education Personnel; grant no. 473816/2014-8), FAPERJ (State of Rio de Janeiro Agency for Research Support; grant no. 110·310/2014) and CNPq (National Council of Scientific and Technological Development; grant no. 487·584/2013-9).
Conception and design of the experiments: R. F. M., K. J. O. and A. C. L. N.; performed the clinical experiments: R. F. M., T. G. G., T. B.-B., R. K and T. M. B. G; performed the analyses: R. F. M., N. A. V. M., F. C. B., C. F.-S. and K. J. O.; interpreted the results: R. F. M., K. J. O. and A. C. L. N.; drafted the article: R. F. M. and A. C. L. N.; edited and revised the article critically for important intellectual content and approved final version: all authors.
None of the authors has any conflicts of interest to declare.










