The intestine is not only important for digestion and absorption of dietary nutrients, but also plays a key role in defence against harmful bacteria-derived endogenous and exogenous agents( Reference Blikslager, Moeser and Gookin 1 ). Many factors such as infection and inflammation can cause intestinal damage and dysfunction( Reference Blikslager, Moeser and Gookin 1 – Reference Liu, Huang and Hou 3 ). Recently, research has shown that the intestine requires a high amount of energy to maintain its health and function; thus, energy deficits in intestinal mucosa are closely related to various degrees of injury in the intestine( Reference Wang, Liu and Chen 4 ). Amino acids can serve as a central fuel source for intestinal mucosa( Reference Wu 5 ). Accumulating evidence suggests that amino acids play a critical role in intestinal integrity, function and health in animals and humans( Reference Wang, Qiao and Li 6 ). Thus, nutritional regulation (especially dietary addition of amino acids) targeting intestinal energy metabolism may hold great promise for intestinal disease prevention and improvement of animal and human health.
Asparagine (Asn) is a non-essential amino acid. As has been stated in a recent review, Asn, aspartate, glutamine, glutamate, arginine, citrulline, ornithine and proline are interconvertible via complex interorgan metabolism in most mammals( Reference Wu, Bazer and Davis 7 ). Asn can be converted to aspartate via deamination, and glutamate can subsequently be generated from α-ketoglutarate and aspartate by a transamination reaction( Reference Rhoads, Argenzio and Chen 8 ). Emerging evidence has demonstrated that glutamine, aspartate and glutamate are the major sources of ATP in mammalian enterocytes( Reference Wu 5 ). In addition, Asn contributes to mounting an effective immune response in normal subjects, but can also contribute to abnormal lymphoblastic growth in leukaemia patients( Reference Li, Yin and Li 9 ). Newburg et al. ( Reference Newburg, Frankel and Fillios 10 ) reported that deletion of Asn from diet formulations led to significant growth deficits in weanling rats. Moreover, Asn stimulates ornithine decarboxylase and cell proliferation in all kinds of cells, including intestinal cells( Reference Rhoads, Argenzio and Chen 8 , Reference McCormack, Tague and Gragoe 11 ). However, to our knowledge, only a few studies have focused on the protective effect of Asn in the intestine.
Recent studies have shown that AMP-activated protein kinase (AMPK), silent information regulator 1 (SIRT1) and PPARγ coactivator-1α (PGC1α) play key roles in the regulation of cellular energy metabolism( Reference Cantó and Auwerx 12 , Reference Chau, Gao and Yang 13 ). AMPK is a heterotrimeric serine/threonine kinase. AMPK activation can restore energy status in human cells with mitochondrial dysfunction( Reference Sanli, Steinberg and Singh 14 , Reference Wu and Wei 15 ). Several lines of evidence indicate that AMPK can increase SIRT1 activity by increasing cellular NAD+ levels, culminating in the modulation of downstream target activity( Reference Cantó, Gerhart-Hines and Feige 16 ). In addition, AMPK and SIRT1 have been found to have a direct impact on the activity of PGC-1α via phosphorylation and deacetylation, respectively( Reference Cantó and Auwerx 12 ). These processes can initiate catabolic pathways including fatty acid oxidation and glycolysis to produce ATP, while synchronously inhibiting anabolic processes including fatty acid synthesis and gluconeogenesis to utilise energy( Reference Kim, Lim and Youn 17 ).
Accordingly, we hypothesise that Asn could improve intestinal integrity by regulating energy status through the modulation of the AMPK signalling pathway. In the present study, we established an acute model of intestinal injury by injecting Escherichia coli lipopolysaccharide (LPS)( Reference Liu, Huang and Hou 3 , Reference Liu, Chen and Odle 18 ). Furthermore, we used a piglet model, which is an excellent animal model for studying the potential nutritional role of Asn in humans( Reference Puiman and Stoll 19 , Reference Merrifield, Lewis and Claus 20 ). The aim of the present study was to investigate whether Asn could attenuate negative changes caused by LPS challenge in the intestine, and to elaborate its molecular mechanisms.
Materials and methods
Animal care and experimental design
All the experimental procedures were approved by the Animal Care and Use Committee of Hubei Province, China. A total of twenty-four weaned, castrated barrows (Duroc × Large White × Landrace, 35 (sem 1) d old, 8·9 (sem 0·1) kg initial body weight (BW)) were randomly divided into four treatment groups (six replicate pens per treatment). Piglets were individually caged in a 1·80 × 1·10 m pen equipped with a feeder and a nipple drinker to allow ad libitum access to feed and water. All piglets were housed in an environmentally controlled room. The basal diet (Table 1) was formulated to meet NRC( 21 ) requirements for all nutrients.
Table 1 Ingredients and composition of the experimental diets (as-fed basis)
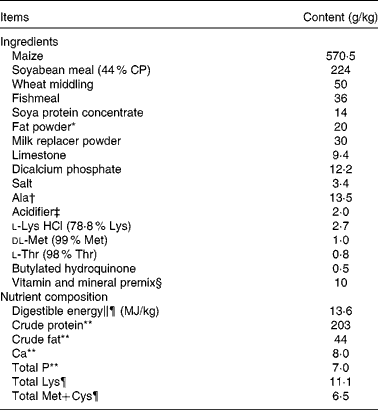
CP, crude protein.
* Rumen-stable fat powder, purchased from Berg+Schmidt.
† In the 0·5 % asparagine diet, 1·35 % alanine was replaced by 0·5 % asparagine, 0·68 % alanine and 0·17 % maize starch. In the 1·0 % asparagine diet, 1·35 % alanine was replaced by 1·0 % asparagine and 0·35 % maize starch. All diets were isonitrogenous.
‡ A compound acidifier including lactic acid and phosphoric acid, provided by Wuhan Fanhua Biotechnology Company.
§ Vitamin and mineral premix (defatted rice bran as the carrier) provided the following amounts per kg of complete diet: retinol acetate, 2700 μg; cholecalciferol, 62·5 μg; dl-α-tocopheryl acetate, 20 mg; menadione, 3 mg; vitamin B12, 18 μg; riboflavin, 4 mg; niacin, 40 mg; pantothenic acid, 15 mg; choline chloride, 400 mg; folic acid, 700 μg; thiamin, 1·5 mg; pyridoxine, 3 mg; biotin, 100 μg; Zn, 80 mg (ZnSO4.7H2O); Mn, 20 mg (MnSO4.5H2O); Fe, 83 mg (FeSO4.H2O); Cu, 25 mg (CuSO4.5H2O); I, 0·48 mg (KI); Se, 0·36 mg (Na2SeO3.5H2O).
∥ Based on diets containing maize starch.
¶ Calculated.
** Analysed.
The four treatment groups were as follows: (1) non-challenged control (CONTR) group (piglets fed a control diet and injected with 0·9 % NaCl solution); (2) LPS+0 % Asn treatment group (piglets fed the same control diet and injected with E. coli LPS (Escherichia coli serotype 055: B5; Sigma Chemical, Inc.)); (3) LPS+0·5 % Asn treatment group (piglets fed a 0·5 % Asn diet and injected with LPS); (4) LPS+1·0 % Asn treatment group (piglets fed a 1·0 % Asn diet and injected with LPS). The Asn doses (purity >99 %; Amino Acid Bio-Chemical Company Limited) were selected on the basis of our previous studies( Reference Li, Liu and Shi 22 ). Our previous investigations showed that before the administration of LPS challenge, dietary supplementation of 0·5 and 1·0 % Asn did not affect growth performance, total and differential leucocyte counts, and serum biochemical parameters of weanling pigs (X Wang, Y Liu, S Li, D Pi, H Zhu, Y Hou, H Shi and W Leng, unpublished results; see online Supplementary Tables S1–S3), indicating that the Asn level of the basal diet was adequate to maintain growth performance and physiological function in weanling pigs under normal physiological conditions. However, our previous studies also showed that after the administration of LPS challenge, dietary supplementation of 0·5 % Asn attenuated weight loss, and both 0·5 and 1·0 % Asn supplementation attenuated the changes in total and differential leucocyte counts and serum biochemical parameters induced by LPS challenge in weanling pigs( Reference Li, Liu and Shi 22 ), indicating the importance of exogenous Asn supply under pathological conditions. Thus, in the present experiment, we focused our investigation upon the effect of 0·5 and 1·0 % dietary Asn supplementation on intestinal variables in LPS-challenged piglets, but did not investigate the effect of Asn in non-LPS-challenged piglets. To obtain isonitrogenous diets, we added 1·35, 0·68 and 0 % alanine (purity >99 %; Amino Acid Bio-Chemical Company Limited) to the control, 0·5 % Asn and 1·0 % Asn diets, respectively. Feed consumption and BW were recorded on day 1 and day 19 before the administration of saline or LPS injection. After 19 d of feeding the control, 0·5 % Asn and 1·0 % Asn diets, the challenged groups were treated with an intraperitoneal injection of LPS at a dose of 100 μg/kg BW, and the non-challenged group was treated with the same volume of 0·9 % NaCl solution. The LPS dose was chosen in accordance with our previous experiments( Reference Liu, Huang and Hou 3 , Reference Liu, Chen and Odle 18 ), in which this dose caused acute intestinal injury in weaned pigs. To avoid the potential effects of LPS-induced feed intake reduction on intestinal variables, all piglets were fed the same amount of feed per kg BW at 24 h following the administration of saline or LPS injection, causing no significant difference in feed intake (266, 258, 270 and 276 g, respectively) among the four treatment groups. According to the feed intake of LPS-challenged piglets, the amount of feed per kg BW was determined at 24 h after LPS challenge in our preliminary study. Piglets were supplied water ad libitum.
Blood and intestinal sample collection
At 24 h post-injection, blood samples were collected into uncoated vacuum tubes (Becton Dickinson Vacutainer System) and centrifuged (3500 g , 10 min, 4°C) to obtain serum samples. Serum was stored at − 80°C until analysis. After the collection of blood samples, piglets were humanely euthanised with pentobarbital, and sections were cut at the mid-jejunum (3 cm and 10 cm) and mid-ileum (3 cm and 10 cm), respectively( Reference Liu, Huang and Hou 3 ). The 3 cm sections were flushed, and then placed in 10 % neutral buffered formalin for the analysis of intestinal morphology( Reference Liu, Huang and Hou 3 ). The 10 cm sections were opened and the contents were flushed( Reference Liu, Huang and Hou 3 ). Then, mucosal samples were collected with a sterile glass slide, and immediately frozen in liquid N2 and stored at − 80°C for further analysis( Reference Liu, Huang and Hou 3 ). Previous experiments have shown that at 24 h post-injection, LPS induced changes in intestinal energy metabolism and intestinal damage( Reference Hou, Yao and Wang 23 ). Thus, the time point of 24 h after the administration of LPS or 0·9 % NaCl solution was selected for the experimental measurements.
Serum amino acid concentrations
A volume of 50 μl serum was deproteinised in 50 μl of 1·5 m-perchloric acid. After 2 min, the samples were neutralised with 25 μl of 2 m-potassium carbonate and 1·125 ml double-distilled water. Then, the samples were centrifuged at 10 000 g for 1 min, and the supernatant was used for amino acid analysis. Serum concentrations of Asn and associated amino acids were measured by HPLC methods involving pre-column derivatisation with o-phthaldialdehyde, as described previously( Reference Wu and Knabe 24 ).
Intestinal morphology
After fixation for 24 h, intestinal samples were dehydrated, embedded in paraffin, sectioned, and stained with haematoxylin and eosin( Reference Zhu, Liu and Xie 25 ). Villus height and crypt depth were measured according to the methods described in our previous study( Reference Zhu, Liu and Xie 25 ).
Intestinal mucosal protein, DNA and RNA contents
Frozen mucosal samples were homogenised in ice-cold NaCl solution at a 1:10 (w/v) ratio, followed by centrifugation at 2500 rpm for 10 min at 4°C to collect the supernatant. The supernatant was used for the measurement of protein, RNA and DNA contents. Intestinal mucosal protein content was measured according to the method of Lowry et al. ( Reference Lowry, Rosebrough and Farr 26 ). DNA content was measured by a fluorometric assay( Reference Labarca and Paigen 27 ). RNA content was measured by spectrophotometry with a modified Schmidt–Tannhauser method( Reference Munro, Fleck and Munro 28 ).
Intestinal mucosal disaccharidase activities
Disaccharidase activities in the supernatant of intestinal mucosa were determined according to the methods described by Liu et al. ( Reference Liu, Han and Huang 29 ) using glucose kits (#A082-1 for lactase, #A082-2 for sucrase and #A082-3 for maltase; Nanjing Jiancheng Bioengineering Institute). In brief, 10 μl double-distilled water, glucose standard solution (5·55 mmol/l) or test samples were added to a test-tube and incubated with 20 μl of respective substrate for 20 min at 37°C. Then, 10 μl of terminating agent and 1000 μl of chromogenic agent were added and incubated at 37°C for 15 min. Double-distilled water was used to set zero at 505 nm, followed by the reading of the optical density value of each tube. One unit (U) of enzyme activity was defined as 1 nmol substrate hydrolysed/min under assay conditions (37°C, pH 6·0).
Intestinal mucosal ATP, ADP and AMP concentrations
Frozen intestinal samples (0·10–0·20 g) were homogenised in 2 ml of pre-cooled 1·5 m-perchloric acid. The homogenates were centrifuged at 3000 g for 5 min at 4°C, and then the supernatants were collected. A volume of 1 ml supernatant was neutralised with 0·4 ml of 2 m-potassium carbonate, followed by centrifugation at 3000 g for 5 min at 4°C. The supernatant was stored at − 80°C until analysis. ATP, ADP and AMP concentrations were measured using HPLC, according to the method proposed by Hou et al. ( Reference Hou, Yao and Wang 23 ). Total adenine nucleotide and adenylate energy charge (AEC) levels were calculated by the following equations( Reference Hou, Yao and Wang 23 ):
Key enzyme activities of the tricarboxylic acid cycle in intestinal mucosa
The activities of key enzymes including citrate synthase (CS), isocitrate dehydrogenase (ICD) and α-ketoglutarate dehydrogenase complex (α-KGDHC) involved in the tricarboxylic acid cycle were assayed according to commercial enzyme assay kits (#45 126 for CS, #45 234 for ICD and #45 157 for α-KGDHC; Shanghai Yuanye Biotechnology Company). All variables were measured according to the manufacturer's guidelines. Briefly, 50 μl of standard solutions or diluted intestinal mucosal supernatants were added to a separately identified well of the microelisa stripplate. A solution of 100 μl horseradish peroxidase (HRP) conjugate reagent was added to each well, and then covered with an adhesive strip and incubated for 60 min at 37°C. After incubation, the plates were washed for five times with wash solutions. Subsequently, 50 μl of chromogen solution A and 50 μl of chromogen solution B were added, followed by incubation for 15 min at 37°C. Then, 50 μl of stop solution were added. Optical density was read at 450 nm using an ELISA plate reader (Model 550; Bio-Rad) within 15 min. The activities of the key enzymes in the tricarboxylic acid cycle were determined by comparing the optical density of intestinal samples with the standard curve. Results for CS and ICD activities were expressed as μIU/mg protein. One IU/mg protein was defined as 1 μmol substrate hydrolysed/min per mg protein under specified assay conditions.
mRNA abundance analysis by real-time PCR
Total RNA was extracted from intestinal mucosa using TRIzol reagent (#9108; TaKaRa Biotechnology (Dalian) Company Limited) following the manufacturer's instructions. RNA was spectrophotometrically quantified by determining absorbance at 260 nm, and integrity was assessed by agarose gel electrophoresis. Both genomic DNA removal and complementary DNA synthesis were performed using a PrimeScript RT reagent kit with a gDNA eraser (#RR047A; TaKaRa Biotechnology (Dalian) Company Limited) according to the protocol of the manufacturer. Real-time PCR analysis for gene expression was carried out on the Applied Biosystems 7500 Real-Time PCR System (Applied Biosystems, Life Technologies) using a SYBR® Premix Ex Taq™ (Tli RNase H Plus) qPCR kit (#RR420A; TaKaRa Biotechnology (Dalian) Company Limited), according to the manufacturer's guidelines. The PCR programme was as follows: 95°C for 30 s, followed by forty cycles of 95°C for 5 s and 60°C for 34 s. The primer pairs used are presented in Table 2. The sequences of the PCR primers were according to previous studies(
Reference Liu, Chen and Odle
18
,
Reference Oliver and Miles
30
,
Reference Weber, Trabue and Ziemer
31
). Quantitative PCR efficiencies of these primers used were close to 100 % in the present experiment. The PCR products of different primers were verified by agarose gel electrophoresis and sequencing. The expression of the target genes v. housekeeping gene (glyceraldehyde 3-phosphate dehydrogenase, GAPDH) was determined by the formula
![]() $$2^{ - \Delta \Delta C _{T}} $$
of Livak & Schmittgen(
Reference Livak and Schmittgen
32
). The results of the present study suggest that there was no difference in the expression of GAPDH among the tissues and treatments. The relative mRNA abundance of each target gene was normalised to the control group.
$$2^{ - \Delta \Delta C _{T}} $$
of Livak & Schmittgen(
Reference Livak and Schmittgen
32
). The results of the present study suggest that there was no difference in the expression of GAPDH among the tissues and treatments. The relative mRNA abundance of each target gene was normalised to the control group.
Table 2 Specific primer sequences used for real-time PCR
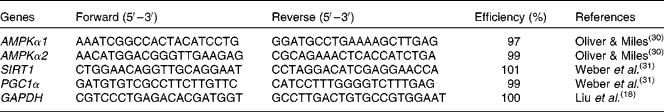
AMPKα1/α2, AMP-activated protein kinase-α1/α2; SIRT1, silent information regulator 1; PGC1α, PPARγ coactivator-1α; GAPDH, glyceraldehyde 3-phosphate dehydrogenase.
Protein abundance analysis by Western blot
Protein immunoblot analysis was carried out in accordance with the previously described method( Reference Liu, Chen and Odle 18 ). Briefly, intestinal samples (0·15–0·20 g) were homogenised in 1 ml of lysis buffer containing protease inhibitors, and centrifuged at 12 000 g for 15 min at 4°C to collect supernatants for Western blot and protein assay. The protein contents of the supernatants were determined using the bicinchoninic acid reagent( Reference Liu, Chen and Odle 18 , Reference Hou, Wang and Zhang 33 ). Equal amounts of intestinal mucosal proteins were loaded onto 10 % polyacrylamide gels, separated through SDS–PAGE, and then transferred to blotting membranes. Immunoblots were blocked with 3 % bovine serum albumin in TBS (Tris-HCl-buffered saline, including Tris-HCl, NaCl and KCl)/Tween-20 buffer for 60 min at room temperature. Then, the membranes were incubated overnight at 4°C with primary antibodies, followed by incubation with a secondary antibody for 120 min at room temperature. Specific primary antibodies included rabbit anti-phosphorylated AMPKα (pAMPKα, Thr172, 1:1000, #2535; Cell Signaling Technology, Inc.), rabbit anti-total AMPKα (tAMPKα, 1:1000, #2532; Cell Signaling Technology, Inc.) and mouse anti-β-actin (1:10 000, #A2228; Sigma-Aldrich, Inc.). Secondary antibodies included goat anti-rabbit IgG HRP (1:5000, #ANT020; Antgene Biotech) and goat anti-mouse IgG HRP (1:5000, #ANT019; Antgene Biotech). In our previous study, these antibodies were validated in weanling pigs( Reference Hou, Wang and Zhang 33 ). Blots were developed using an enhanced chemiluminescence Western blotting kit (Amersham Biosciences), and visualised using a Gene Genome Bioimaging System (Alpha Innotech). Bands were analysed by densitometry using GeneTools software (Syngene). pAMPKα was normalised to the total protein content of AMPKα.
Statistical analysis
Experimental data were analysed by variance specific for repeated measures using the mixed procedure of SAS (SAS Institute, Inc.), with treatments as the between-animal effect and gut segment (jejunum and ileum) as the within-animal effect according to the following model:
where αi is the effect of the treatment (i = CONTR, LPS+0 % Asn, LPS+0·5 % Asn and LPS+1·0 % Asn); ωj is the segment (jejunum and ileum); αω ij is the interaction between treatment and segment; μk~N(0,τ2) accounts for repeated measures made on the same individual, thereby rendering these observations correlated. The error term ɛijk~N(0,σ2) represents unexplained variation. The variance interaction between segment and diet was described as random by using
When a significant interaction between treatment and segment occurred, comparisons were made among the treatments in each segment (jejunum or ileum). LPS-challenged piglets (0 % Asn) were compared with CONTR piglets to determine the effect of LPS challenge. Linear and quadratic polynomial contrasts were used to determine the response to dietary Asn supplementation among the LPS-challenged piglets. Results are expressed as means with their pooled standard errors. P≤ 0·05 was considered as statistically significant, and 0·05 < P< 0·10 indicated a trend.
Results
Growth performance
During the entire 19 d feeding trial (pre-challenge), there were no differences in initial BW (9·0, 8·8, 9·0 and 8·8 kg, respectively) and final BW (18·0, 17·5, 18·3 and 18·7 kg, respectively), average daily gain (477, 459, 492 and 524 g, respectively), average daily feed intake (733, 741, 703 and 759 g, respectively) and feed:gain ratio (1·54, 1·64, 1·43 and 1·44, respectively) among the four treatment groups.
Serum amino acid concentrations
Compared with CONTR piglets, LPS-challenged (0 % Asn) piglets had decreased glutamate and glutamine concentrations (P< 0·05; Table 3). Among the LPS-challenged piglets, Asn supplementation increased the concentrations of aspartate (quadratic, P< 0·05), Asn (linear, P< 0·05; quadratic, P≤ 0·05), glutamine (linear, P< 0·001; quadratic, P< 0·05) and ornithine (quadratic, P< 0·05).
Table 3 Effects of asparagine (Asn) supplementation on serum amino acid concentrations in weaned piglets at 24 h after the administration of Escherichia coli lipopolysaccharide (LPS) challenge (Mean values with their pooled standard errors, n 6 (one piglet per pen))
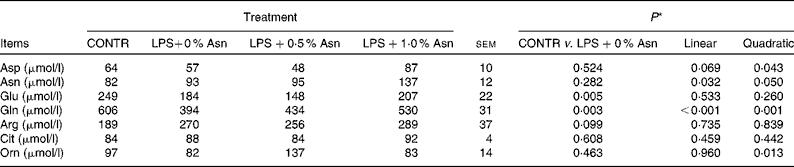
CONTR, non-challenged control group (piglets fed a control diet and injected with 0·9 % NaCl solution); Cit, citrulline; Orn, ornithine.
* LPS-challenged (0 % Asn) piglets were compared with CONTR piglets to determine the effects of LPS challenge. Linear and quadratic polynomial contrasts were used to determine the response to Asn supplementation among the LPS-challenged piglets.
Intestinal morphology
LPS challenge (0 % Asn) caused fever, diarrhoea, anorexia, shivering and inactivity within 1 h in all piglets (data not shown). Villus height and villus height:crypt depth ratio (VCR) in the jejunum were higher than those in the ileum (P< 0·05; Fig. 1). No significant treatment × segment interaction was observed for villus height. Overall, compared with CONTR piglets, LPS-challenged (0 % Asn) piglets tended to have decreased villus height (P =0·089). Among the LPS-challenged piglets, Asn supplementation increased villus height (linear, P< 0·001; quadratic, P< 0·05).

Fig. 1 Effects of asparagine (Asn) supplementation on (a) villus height, (b) crypt depth and (c) villus height:crypt depth ratio (VCR) in weaned piglets at 24 h after the administration of Escherichia coli lipopolysaccharide (LPS) challenge. Data were analysed as repeated measures with treatments (CONTR (![]() ), LPS+0 % Asn (
), LPS+0 % Asn (![]() ), LPS+0·5 % Asn (
), LPS+0·5 % Asn (![]() ) and LPS+1·0 % Asn (
) and LPS+1·0 % Asn (![]() )) as the between-animal effect and segment (jejunum and ileum) as the within-animal effect. LPS-challenged (0 % Asn) piglets were compared with CONTR piglets to determine the effects of LPS challenge. Linear (L) and quadratic (Q) polynomial contrasts were used to determine the response to Asn supplementation among the LPS-challenged piglets. Values are means (n 6; one piglet per pen), with their standard errors represented by vertical bars. Villus height (P< 0·001) and VCR (P= 0·016) in the jejunum were higher than those in the ileum. There were significant treatment × segment interactions observed for crypt depth (P= 0·046) and VCR (P= 0·044). There were no significant treatment × segment interactions observed for villus height (P= 0·118). (a) CONTR v. LPS+0 % Asn, P= 0·089; L, P< 0·001; Q, P= 0·001. (b) Jejunum: CONTR v. LPS+0 % Asn, P= 0·817; L, P= 0·002; Q, P= 0·006. Ileum: CONTR v. LPS+0 % Asn, P= 0·602; L, P= 0·515; Q, P= 0·750. (c) Jejunum: CONTR v. LPS+0 % Asn, P= 0·088; L, P< 0·001; Q, P< 0·001. Ileum: CONTR v. LPS+0 % Asn, P= 0·662; L, P= 0·011; Q, P= 0·036. CONTR, non-challenged control group (piglets fed a control diet and injected with 0·9 % NaCl solution).
)) as the between-animal effect and segment (jejunum and ileum) as the within-animal effect. LPS-challenged (0 % Asn) piglets were compared with CONTR piglets to determine the effects of LPS challenge. Linear (L) and quadratic (Q) polynomial contrasts were used to determine the response to Asn supplementation among the LPS-challenged piglets. Values are means (n 6; one piglet per pen), with their standard errors represented by vertical bars. Villus height (P< 0·001) and VCR (P= 0·016) in the jejunum were higher than those in the ileum. There were significant treatment × segment interactions observed for crypt depth (P= 0·046) and VCR (P= 0·044). There were no significant treatment × segment interactions observed for villus height (P= 0·118). (a) CONTR v. LPS+0 % Asn, P= 0·089; L, P< 0·001; Q, P= 0·001. (b) Jejunum: CONTR v. LPS+0 % Asn, P= 0·817; L, P= 0·002; Q, P= 0·006. Ileum: CONTR v. LPS+0 % Asn, P= 0·602; L, P= 0·515; Q, P= 0·750. (c) Jejunum: CONTR v. LPS+0 % Asn, P= 0·088; L, P< 0·001; Q, P< 0·001. Ileum: CONTR v. LPS+0 % Asn, P= 0·662; L, P= 0·011; Q, P= 0·036. CONTR, non-challenged control group (piglets fed a control diet and injected with 0·9 % NaCl solution).
Significant treatment × segment interactions were observed for crypt depth and VCR (P< 0·05). Compared with CONTR piglets, LPS-challenged (0 % Asn) piglets tended to have decreased VCR in the jejunum (P =0·088). Among the LPS-challenged piglets, Asn supplementation increased the VCR in the jejunum and ileum (linear, P< 0·05; quadratic, P< 0·05), and decreased crypt depth in the jejunum (linear, P< 0·05; quadratic, P< 0·05).
Protein, DNA and RNA contents
The RNA:DNA ratio in the ileum was higher than that in the jejunum (P< 0·05; Table 4). There were significant treatment × segment interactions observed for RNA:DNA and protein:DNA ratios (P< 0·05), and a trend for treatment × segment interactions was observed for mucosal protein content (P =0·069). Relative to CONTR piglets, LPS-challenged (0 % Asn) piglets had decreased mucosal protein content in the jejunum and ileum (P< 0·05), and RNA:DNA and protein:DNA ratios in the ileum (P< 0·05). Among the LPS-challenged piglets, Asn supplementation increased the RNA:DNA and protein:DNA ratios in the jejunum and ileum (linear, P< 0·05; quadratic, P≤ 0·05).
Table 4 Effects of asparagine (Asn) supplementation on intestinal mucosal protein, DNA and RNA contents in weaned piglets at 24 h after the administration of Escherichia coli lipopolysaccharide (LPS) challenge (Mean values with their pooled standard errors, n 6 (one piglet per pen))
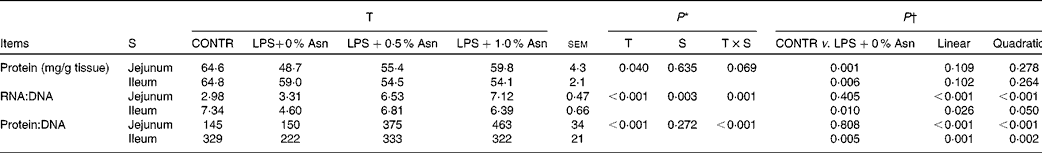
T, treatment; S, segment; CONTR, non-challenged control group (piglets fed a control diet and injected with 0·9 % NaCl solution).
* P values were obtained using treatment as the main effect and by analysing the data from the jejunum and ileum as repeated measures.
† LPS-challenged (0 % Asn) piglets were compared with CONTR piglets to determine the effects of LPS challenge. Linear and quadratic polynomial contrasts were used to determine the response to Asn supplementation among the LPS-challenged piglets.
Disaccharidase activities
Disaccharidase activities in the jejunum were higher than those in the ileum (P< 0·001; Table 5). No significant treatment × segment interaction was found for lactase, maltase and sucrase activities. Overall, compared with CONTR piglets, LPS-challenged (0 % Asn) piglets had decreased sucrase activity (P≤ 0·05). Among the LPS-challenged piglets, Asn supplementation increased lactase activity (linear, P< 0·05; quadratic, P< 0·05).
Table 5 Effects of asparagine (Asn) supplementation on intestinal disaccharidase activities in weaned piglets at 24 h after the administration of Escherichia coli lipopolysaccharide (LPS) challenge* (Mean values with their pooled standard errors, n 6 (one piglet per pen))
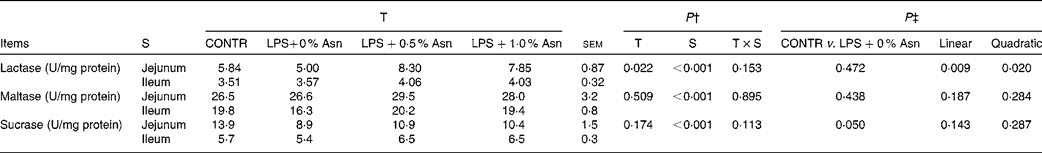
T, treatment; S, segment; CONTR, non-challenged control group (piglets fed a control diet and injected with 0·9 % NaCl solution).
* One enzyme activity unit (U) was defined as 1 nmol substrate hydrolysed/min under assay conditions (37°C, pH 6·0).
† P values were obtained using treatment as the main effect and by analysing the data from the jejunum and ileum as repeated measures.
‡ LPS-challenged (0 % Asn) piglets were compared with CONTR piglets to determine the effects of LPS challenge. Linear and quadratic polynomial contrasts were used to determine the response to Asn supplementation among the LPS-challenged piglets.
ATP, ADP and AMP concentrations in intestinal mucosa
AMP concentrations, AMP:ATP ratios and total adenine nucleotide concentrations in the jejunum were lower than those in the ileum, and AEC levels in the jejunum were higher than those in the ileum (P< 0·05; Table 6). Significant treatment × segment interactions were observed for ATP concentrations, AMP:ATP ratios and AEC levels (P< 0·05). Compared with CONTR piglets, LPS-challenged (0 % Asn) piglets had decreased ATP concentrations and AEC levels, and increased AMP:ATP ratios in the ileum (P< 0·05). Among the LPS-challenged piglets, Asn supplementation increased ATP concentrations in the ileum (linear, P< 0·05) and AEC levels in the jejunum (quadratic, P< 0·05) and ileum (linear, P< 0·05), but decreased AMP:ATP ratios in the jejunum (linear, P< 0·05; quadratic, P< 0·05) and ileum (linear, P< 0·05; quadratic, P< 0·05).
Table 6 Effects of asparagine (Asn) supplementation on intestinal adenylate purines in weaned piglets at 24 h after the administration of Escherichia coli lipopolysaccharide (LPS) challenge (Mean values with their pooled standard errors, n 6 (one piglet per pen))

T, treatment; S, segment; CONTR, non-challenged control group (piglets fed a control diet and injected with 0·9 % NaCl solution); TAN, total adenine nucleotide; AEC, adenylate energy charge.
* P values were obtained using treatment as the main effect and by analysing the data from the jejunum and ileum as repeated measures.
† LPS-challenged (0 % Asn) piglets were compared with CONTR piglets to determine the effects of LPS challenge. Linear and quadratic polynomial contrasts were used to determine the response to Asn supplementation among the LPS-challenged piglets.
‡ TAN = ATP+ADP+AMP.
§ AEC = (ATP+0·5ADP)/(ATP+ADP+AMP).
Key enzyme activities of the tricarboxylic acid cycle
The activities of CS and ICD in the jejunum were higher than those in the ileum (P< 0·001), and α-KGDHC activity in the jejunum was lower than that in the ileum (P< 0·05) (Table 7). No significant treatment × segment interaction was found for the activities of CS and α-KGDHC. Overall, compared with CONTR piglets, LPS-challenged (0 % Asn) piglets had decreased CS and α-KGDHC activities (P< 0·05). Among the LPS-challenged piglets, Asn supplementation increased the activities of CS and α-KGDHC (quadratic, P< 0·05).
Table 7 Effects of asparagine (Asn) supplementation on intestinal activities of key enzymes in the tricarboxylic acid cycle in weaned piglets at 24 h after the administration of Escherichia coli lipopolysaccharide (LPS) challenge* (Mean values with their pooled standard errors, n 6 (one piglet per pen))
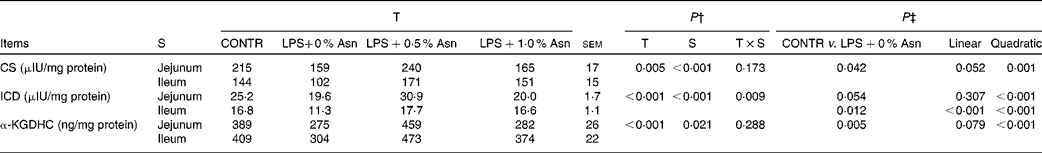
T, treatment; S, segment; CONTR, non-challenged control group (piglets fed a control diet and injected with 0·9 % NaCl solution); CS, citrate synthase; ICD, isocitrate dehydrogenase; α-KGDHC, α-ketoglutarate dehydrogenase complex.
* International unit (IU) was defined as 1 μmol substrate hydrolysed/min under specified assay conditions.
† P values were obtained using treatment as the main effect and by analysing the data from the jejunum and ileum as repeated measures.
‡ LPS-challenged (0 % Asn) piglets were compared with CONTR piglets to determine the effects of LPS challenge. Linear and quadratic polynomial contrasts were used to determine the response to Asn supplementation among the LPS-challenged piglets.
A significant treatment × segment interaction was observed for ICD activity (P< 0·05). Compared with CONTR piglets, LPS-challenged (0 % Asn) piglets had decreased ileal ICD activity (P< 0·05). Among the LPS-challenged piglets, Asn supplementation increased jejunal (quadratic, P< 0·001) and ileal (linear, P< 0·001; quadratic, P< 0·001) ICD activity.
mRNA expression of AMP-activated protein kinase α1, AMP-activated protein kinase α2, silent information regulator 1 and PPARγ coactivator-1α
The mRNA abundance of AMPKα1 and AMPKα2 in the jejunum was lower than that in the ileum (P< 0·05; Table 8). There was a significant treatment × segment interaction observed for the mRNA abundance of AMPKα2 (P< 0·05), and a trend for the treatment × segment interaction observed for the mRNA abundance of AMPKα1 (P =0·084). Compared with CONTR piglets, LPS-challenged (0 % Asn) piglets had increased mRNA abundance of ileal AMPKα1 and AMPKα2 (P< 0·05). Among the LPS-challenged piglets, Asn supplementation decreased the mRNA abundance of jejunal and ileal AMPKα1, and ileal AMPKα2 (linear, P< 0·05; quadratic, P< 0·05).
Table 8 Effects of asparagine (Asn) supplementation on the mRNA expression of intestinal AMP-activated protein kinase (AMPK)α1, AMPKα2, silent information regulator 1 (SIRT1) and PPARγ coactivator-1α (PGC1α) in weaned piglets at 24 h after the administration of Escherichia coli lipopolysaccharide (LPS) challenge* (Mean values with their pooled standard errors, n 6 (one piglet per pen))

T, treatment; S, segment; CONTR, non-challenged control group (piglets fed a control diet and injected with 0·9 % NaCl solution).
* Means are fold changes that were normalised to the control.
† P values were obtained using treatment as the main effect and by analysing the data from the jejunum and ileum as repeated measures.
‡ LPS-challenged (0 % Asn) piglets were compared with CONTR piglets to determine the effects of LPS challenge. Linear and quadratic polynomial contrasts were used to determine the response to Asn supplementation among the LPS-challenged piglets.
No significant treatment × segment interaction was observed for the mRNA abundance of SIRT1 and PGC1α. Compared with CONTR piglets, LPS-challenged (0 % Asn) piglets had increased mRNA abundance of PGC1α (P< 0·05). Among the LPS-challenged piglets, Asn supplementation decreased the mRNA abundance of SIRT1 (linear, P< 0·05) and PGC1α (linear, P< 0·05; quadratic, P< 0·05).
Protein phosphorylation and abundance of AMP-activated protein kinase α
The pAMPKα:tAMPKα ratio in the jejunum were lower than that in the ileum (P< 0·05), and the protein abundance of tAMPKα in the jejunum was higher than that in the ileum (P< 0·001) (Fig. 2). A trend for the treatment × segment interaction was observed for the pAMPKα:tAMPKα ratio (P= 0·073). Compared with CONTR piglets, LPS-challenged (0 % Asn) piglets had increased ileal pAMPKα:tAMPKα ratio (P< 0·05). Among the LPS-challenged piglets, Asn supplementation decreased the ileal pAMPKα:tAMPKα ratio (linear, P< 0·05; quadratic, P< 0·05).

Fig. 2 Effects of asparagine (Asn) supplementation on the (a) phosphorylated AMP-activated protein kinase (pAMPKα):total AMP-activated protein kinase (tAMPKα) ratio and (b) protein abundance of tAMPKα in weaned piglets at 24 h after the administration of Escherichia coli lipopolysaccharide (LPS) challenge. The bands shown are the representative Western blot images of pAMPKα (62 kDa), tAMPKα (62 kDa) and β-actin (42 kDa). β-Actin was from the same blot as the proteins of interest. Data were analysed as repeated measures with treatments (CONTR (![]() ), LPS+0 % Asn (
), LPS+0 % Asn (![]() ), LPS+0·5 % Asn (
), LPS+0·5 % Asn (![]() ) and LPS+1·0 % Asn (
) and LPS+1·0 % Asn (![]() )) as the between-animal effect and segment (jejunum and ileum) as the within-animal effect. LPS-challenged (0 % Asn) piglets were compared with CONTR piglets to determine the effects of LPS challenge. Linear (L) and quadratic (Q) polynomial contrasts were used to determine the response to Asn supplementation among the LPS-challenged piglets. Values are means (n 6; one piglet per pen), with their standard errors represented by vertical bars. a.u., Arbitrary units. The pAMPKα:tAMPKα ratio in the jejunum was lower than that in the ileum (P= 0·006), and protein abundance of tAMPKα in the jejunum was higher than that in the ileum (P< 0·001). A trend for the treatment × segment interaction was observed for the pAMPKα:tAMPKα ratio (P= 0·073). There was no significant treatment × segment interaction observed for protein abundance of tAMPKα (P= 0·947). (a) Jejunum: CONTR v. LPS+0 % Asn, P= 0·879; L, P= 0·957; Q, P= 0·722. Ileum: CONTR v. LPS+0 % Asn, P= 0·001; L, P= 0·007; Q, P= 0·028. (b) CONTR v. LPS+0 % Asn, P= 0·688; L, P= 0·917; Q, P= 0·834. CONTR, non-challenged control group (piglets fed a control diet and injected with 0·9 % NaCl solution).
)) as the between-animal effect and segment (jejunum and ileum) as the within-animal effect. LPS-challenged (0 % Asn) piglets were compared with CONTR piglets to determine the effects of LPS challenge. Linear (L) and quadratic (Q) polynomial contrasts were used to determine the response to Asn supplementation among the LPS-challenged piglets. Values are means (n 6; one piglet per pen), with their standard errors represented by vertical bars. a.u., Arbitrary units. The pAMPKα:tAMPKα ratio in the jejunum was lower than that in the ileum (P= 0·006), and protein abundance of tAMPKα in the jejunum was higher than that in the ileum (P< 0·001). A trend for the treatment × segment interaction was observed for the pAMPKα:tAMPKα ratio (P= 0·073). There was no significant treatment × segment interaction observed for protein abundance of tAMPKα (P= 0·947). (a) Jejunum: CONTR v. LPS+0 % Asn, P= 0·879; L, P= 0·957; Q, P= 0·722. Ileum: CONTR v. LPS+0 % Asn, P= 0·001; L, P= 0·007; Q, P= 0·028. (b) CONTR v. LPS+0 % Asn, P= 0·688; L, P= 0·917; Q, P= 0·834. CONTR, non-challenged control group (piglets fed a control diet and injected with 0·9 % NaCl solution).
Discussion
LPS is the main constituent of the outer membrane of Gram-negative bacteria( Reference Liu, Huang and Hou 3 ). Increasing evidence indicates that LPS is a cofactor in intestinal injury( Reference Liu, Huang and Hou 3 , Reference Liu, Chen and Odle 18 ). Intestinal alterations including morphological injury, increased mucosal permeability and bacterial translocation have often been reported after intraperitoneal injection of LPS( Reference Liu, Huang and Hou 3 ). The pathogenesis of LPS-induced intestinal injury is viewed as a complex event, and it has been correlated with increased inflammation caused by LPS challenge that leads to the expression of pro-inflammatory cytokines( Reference Paszti-Gere, Matis and Farkas 34 , Reference Farkas, Mátis and Pászti-Gere 35 ). In addition, LPS is known to cause significant damage to gastrointestinal oxygen metabolism and mitochondria dysfunction, leading to decreased ATP concentrations and eventually intestinal damage( Reference Duarte, Arango and Parihar 36 ). Our previous studies have suggested that dietary supplementation with 0·5 % Asn alleviated growth suppression, and both 0·5 and 1·0 % dietary Asn supplementation attenuated the changes in total and differential leucocyte counts and serum biochemical parameters in weaned piglets after the administration of LPS challenge( Reference Li, Liu and Shi 22 ). Therefore, we extended the finding to the intestine to explore the effect of Asn supplementation on intestinal injury. To our knowledge, this is the first study to evaluate whether dietary Asn supplementation could attenuate intestinal injury in weanling piglets challenged with LPS.
Villus height, crypt depth and VCR were used to measure intestinal morphology( Reference Liu, Chen and Odle 18 ). Mucosal protein contents, RNA:DNA and protein:DNA ratios are important biochemical indices for intestinal development( Reference Liu, Huang and Hou 3 ). Mucosal disaccharidases, namely lactase, maltase and sucrase, are directly involved in the energy supply of organism, and can mirror intestinal digestive function( Reference Pinheiro, Pacheco and Alvarenga 37 ). In the present study, villus height and disaccharidase activities in the jejunum were higher than those in the ileum, which is similar to the report of Rubio et al. ( Reference Rubio, Ruiz and Peinado 38 ). LPS challenge decreased villus height, VCR, mucosal protein content, RNA:DNA and protein:DNA ratios, and disaccharidase activities, which is consistent with the findings of Liu et al. ( Reference Liu, Huang and Hou 3 , Reference Liu, Han and Huang 29 ). These data indicate that injection of LPS caused intestinal injury in weaned pigs. Asn supplementation to the LPS-challenged pigs increased villus height and VCR, decreased crypt depth, increased RNA:DNA and protein:DNA ratios, and increased disaccharidase activities linearly and quadratically. These data indicate that Asn protected the intestine from damage. Until now, little is known about the nutritional significance of Asn in the intestine. Previous studies have found that Asn stimulates enterocyte proliferation in the small intestine of pigs( Reference Rhoads, Argenzio and Chen 8 , Reference McCormack, Tague and Gragoe 11 ). However, the protective mechanism of Asn remains unknown. Rhoads et al. ( Reference Rhoads, Argenzio and Chen 8 ) reported that Asn can be converted to aspartate, and glutamate can subsequently be generated from α-ketoglutarate and aspartate. In the present study, Asn supplementation to the LPS-challenged pigs increased serum concentrations of aspartate, Asn, glutamine and ornithine linearly and quadratically. In this way, it is possible that Asn may be a precursor for many other amino acids to be produced on demand to meet the requirements of enterocytes.
The intestine takes up a high amount of energy to sustain its integrity, function and health( Reference Blachier, Boutry and Bos 39 , Reference Burrin and Stoll 40 ), and energy deficits in intestinal mucosa may relate to intestinal injury( Reference Hou, Yao and Wang 23 ). Most cellular processes need energy and are driven directly or indirectly by hydrolysing ATP to ADP and phosphate, or less frequently to AMP and pyrophosphate. The AMP:ATP ratio is a sensitive indicator of cellular energy state( Reference Hardie and Hawley 41 ). In comparison with the level of a single nucleotide, the energy charge of the adenyl pool is a better way to measure the energy status of a tissue( Reference Hou, Yao and Wang 23 ). In the present study, LPS challenge decreased ATP concentrations and AEC levels, and increased AMP:ATP ratios. Similarly, Hou et al. ( Reference Hou, Yao and Wang 23 ) reported that LPS challenge altered the cellular energy status in intestinal mucosa. Asn supplementation to the LPS-challenged pigs increased ATP concentrations linearly and AEC levels linearly and quadratically, and decreased AMP:ATP ratios linearly and quadratically. These data support the notion that Asn supplementation attenuated LPS-induced intestinal damage possibly via modulating the adenine nucleotide pool.
The tricarboxylic acid cycle is a central route for energy production in the intestine( Reference Browne, Sanford and Smyth 42 ). Key enzymes involved in the tricarboxylic acid cycle include CS, ICD and α-KGDHC. The enzyme CS catalyses the first step of the tricarboxylic acid cycle by taking molecules of acetate and attaching them to oxaloacetate( Reference Wiegand and Remington 43 ). ICD is responsible for catalysing the oxidative decarboxylation of isocitrate into α-ketoglutarate and CO2 ( Reference Corpas, Barroso and Sandalio 44 ). α-KGDHC is a multi-enzymatic complex that converts α-ketoglutarate to succinyl-CoA( Reference Bunik and Strumilo 45 ). In the present study, Asn supplementation to the LPS-challenged pigs attenuated the decrease in the activities of the key enzymes in the tricarboxylic acid cycle linearly and quadratically. This may due to the conversion of Asn to aspartate( Reference Rhoads, Argenzio and Chen 8 ), which can be converted to tricarboxylic acid cycle intermediates (such as oxaloacetate)( Reference Sivakumar, Anandh Babu and Shyamaladevi 46 ). In the present study, it is possible that dietary supplementation with Asn improved intestinal energy status by enhancing the key enzyme activities of the tricarboxylic acid cycle.
AMPK is an energy regulator whose primary role involves maintaining the intracellular energy balance in eukaryotic evolution( Reference Cantó and Auwerx 12 ). It can be activated by mechanisms including phosphorylation upon allosteric activation by increasing the AMP:ATP ratio( Reference Hardie 47 ). To restore the cellular energy status, the activation of AMPK can switch on ATP-producing processes while synchronously switching off ATP-consuming processes( Reference Hardie 47 ). In addition, AMPK could chronically promote cellular ability to produce ATP and diminish potentially adverse cellular events( Reference Takeuchi, Morizane and Kamami-Levy 48 ). Several reports have revealed that AMPK can enhance the activity of SIRT1 by increasing cellular NAD+ levels( Reference Cantó, Gerhart-Hines and Feige 16 ). SIRT1 was found to be a major regulator of muscle adaptation to nutrient availability( Reference Fulco, Cen and Zhao 49 , Reference Gerhart-Hines, Rodgers and Bare 50 ). When activated, SIRT1 also enhances mitochondrial oxidative function and leads to selective nutrient utilisation to regulate energy balance( Reference Chau, Gao and Yang 13 ). Furthermore, SIRT1 deactetylation has been proposed to be a potential activator for the transcriptional activity of PGC1α( Reference Gurd 51 ). D'Errico et al. ( Reference D'Errico, Lo Sasso and Salvatore 52 ) reported that PGC1α modulates mitochondrial biogenesis and function. In the present study, LPS challenge increased the mRNA abundance of AMPKα1, AMPKα2 and PGC1α, and the phosphorylation of AMPKα. In agreement with the results of the present study, Hou et al. ( Reference Hou, Yao and Wang 23 ) reported that LPS challenge increased intestinal AMPKα phosphorylation. Asn supplementation to the LPS-challenged pigs decreased intestinal AMPKα1, AMPKα2, SIRT1 and PGC1α mRNA abundance, and decreased ileal AMPKα phosphorylation linearly and quadratically. In the present study, consistent with reduced intestinal AMP:ATP ratios and increased ATP concentrations, dietary Asn supplementation inhibited intestinal AMPK signalling pathway in response to LPS treatment. It is possible that the reduced intestinal AMP:ATP ratios and the increased ATP concentrations in enterocytes might be enough to inhibit the AMPK signalling pathway in LPS-challenged piglets fed the Asn diet. Future research is needed to elucidate the mechanisms for the inhibitory effect of Asn on the AMPK signalling pathway.
In the present study, only two doses of Asn (0·5 and 1·0 % Asn) were used. The higher concentration of Asn (1·0 %) had a worse effect on some parameters characteristic for energy status compared with the lower concentration (0·5 %). We speculate that the different effects of the two doses of Asn might be due to the following mechanisms. First, Asn can be converted to glutamine via complex interorgan metabolism( Reference Wu, Bazer and Davis 7 ). In the present study, Asn supplementation to the LPS-challenged pigs increased serum glutamine concentrations. Holecek( Reference Holecek 53 ) reported that enhanced glutamine intake competed with intestinal absorption of a number of amino acids, which might affect amino acid and protein metabolism in the gut, liver and whole body. Second, as with all other nutrients, it is possible that excessive amount of Asn in diets can cause amino acid imbalances and toxicity. In addition, we used an acute model of LPS challenge, which is different from a more chronic LPS challenge situation (e.g. during 8–15 d post-weaning). Thus, future studies including more Asn doses are needed to better understand the effects of Asn supplementation in an acute or chronic LPS challenge situation. Moreover, the effect of dietary Asn supplementation on some variables differed between the jejunum and the ileum. This might be related to different microenvironments among various segments of the gut at molecular and cellular levels( Reference Ren, Chen and Yin 54 ).
In summary, dietary supplementation of Asn mitigates intestinal injury and improves intestinal energy status of weaned piglets challenged by LPS. In addition, Asp supplementation modulates intestinal AMPK signalling pathway. These novel findings not only contribute to the understanding of the mode of action of Asn in the intestine of pigs, but also hold great significance for improving infant nutrition.
Supplementary material
To view supplementary material for this article, please visit http://dx.doi.org/10.1017/S0007114515001877
Acknowledgements
The present study was supported by the National Natural Science Foundation of China (grant no. 31422053, 31372318 and 31172222) and the Project of Natural Science Foundation of Hubei Province (grant no. 2013CFA029).
The authors' contributions are as follows: Y. L. designed the research; X. W., Y. L., S. L., D. P., H. Z., Y. H., H. S. and W. L. conducted the research; X. W., Y. L. and S. L. analysed the data; X. W. and Y. L. wrote the paper; Y. L. had primary responsibility for the final content. All authors read and approved the final manuscript.
The authors declare that they have no conflict of interest.












