The metabolic syndrome (MetS) is a cluster of cardiometabolic risk factors including abdominal obesity, hyperglycaemia, hypertension and dyslipidaemia(Reference Weiss, Bremer and Lustig1). It has been recognised that children and adolescents with the MetS have an increased risk of adulthood type 2 diabetes and CVD(Reference DeBoer2), which are also prone to the development of pancreatic cancer, colon cancer and other cancers(Reference Uzunlulu, Telci and Oguz3). The prevalence of the MetS in children and adolescents has been growing rapidly globally(Reference Al-Hamad and Raman4). In China, the prevalence of the MetS in children and adolescents was 2·3 %, whereas abdominal obesity and LDL-cholesterol were most prevalent (21·8 and 14·4 %, respectively), and 35·9 % of participants had at least one of the MetS components, making it a serious threat to public health(Reference Zhu, Zheng and Zou5).
Zn, an essential component of numerous enzymes, plays an important role in metabolic processes in human organisms(Reference Prasad6). It is essential for the synthesis, storage and secretion of insulin and as such is important for carbohydrate metabolism(Reference Chabosseau and Rutter7). As an important antioxidant, Zn has membrane stability characteristics, prevents apoptotic cell death and is vital to endothelial cell integrity(Reference Marreiro, Cruz and Morais8). Zn is also engaged in insulin signalling and the redox signalling pathway, which has been proposed as potential mechanisms underlying association between Zn and cardiometabolic risk(Reference Little, Bhattacharya and Moreyra9). Serum Zn concentrations are the best available biomarker to evaluate a population’s Zn status due to their correspondence to dietary Zn intake and Zn supplementation(Reference de Benoist, Darnton-Hill and Davidsson10).
Clinical trials have shown a beneficial effect of Zn supplementation on lipid profile and glycaemic control in adults with metabolic disorders(Reference Khazdouz, Djalalinia and Sarrafi11). However, the associations between serum Zn and metabolic risk factors in population-based observational studies are not consistent. A cross-sectional study from Korea found that serum Zn was negatively associated with fasting glucose (FG) and positively associated with elevated TAG in men, while HDL-cholesterol levels declined in both men and women as serum Zn levels increased(Reference Seo, Song and Han12). A prospective cohort study revealed that serum Zn was positively correlated with blood pressure and negatively correlated with HDL-cholesterol levels in middle-aged and older Finnish men(Reference Yary, Virtanen and Ruusunen13). However, a nested case–control study fails to demonstrate an association between serum Zn and the MetS or its components in middle-aged and older Chinese adults(Reference Fang, Wu and Gu14).
These discrepancies indicate that the association between serum Zn and metabolic risk factors is inconclusive. In addition, most of the previous studies focused on adults, and few studies are available in children and adolescents(Reference Gonoodi, Moslem and Darroudi15,Reference Ho, Baur and Cowell16) . The objective of this study was, therefore, to examine the association between serum Zn and metabolic risk factors in a sample of Chinese children and adolescents. Our findings may provide new insight into the association of Zn homoeostasis with metabolic status.
Methods
Study design and participants
The data were obtained from the 2016–2017 China National Nutrition and Health Surveillance for Children and Nursing in Jiangsu Province, eastern China. A multi-stage stratified random sampling method was used to select a total of twelve sites including two large urban sites, eight small to medium urban sites and two rural sites in the province. These twelve sites represented a geographically, social development and economically diverse population. In each site, at least 270 children and adolescents between 6 and 17 years old were selected using multi-stage stratified probability sampling. At last, a total of 3321 individuals were surveyed, of whom 3246 had complete data of both anthropometric and laboratory measurements. For the purpose of this study, we selected children and adolescents as our participants, which further excluded those who were aged >17 years old (n 5). Thus, the final analytic sample included 3241 participants (1626 males and 1615 females) who were aged between 6 and 17 years old.
The study was reviewed and approved by the Ethical Review Committee of the Chinese Center for Disease Control and Prevention. Written consent was obtained from all participants.
Anthropometric measurements
All participants underwent anthropometric measurements including waist circumference (WC), height, weight and blood pressure by trained nurses and physicians. In order to collect accurate data of body weight and WC, all anthropometric measurements were taken in the morning after an overnight fasting for 12 h. Weight and height were measured in light clothing without shoes and standing straight to the nearest 0·1 kg and 0·1 cm, respectively. BMI was then calculated (weight (kg)/height (m2)). WC was measured midway between the margin of the lowest rib and the iliac crest in a horizontal plane using a non-elastic tape. Blood pressure was measured on the right upper arm using an electronic sphygmomanometer (Omron HBP-1300) with appropriate cuff sizes. After an initial 5-min rest, blood pressure was measured three consecutive times at 1-min intervals and the mean of the three measurements was used in the analyses.
Laboratory measurements
All participants were invited to provide a morning fasting venous blood sample between 07.30 and 09.30 hours. Plasma and serum samples were frozen at −4°C and transported to the laboratory for analysis on the day of sampling. All blood samples were analysed in a single central laboratory. Total cholesterol (TC), HDL-cholesterol, LDL-cholesterol, TAG, FG, serum creatinine and high-sensitive C-reactive protein (hs-CRP) were determined using the Cobas C701 analyzer series (Roche Diagnostics). Serum Zn concentration was tested with an Inductively Coupled Plasma Mass Spectrometer System Agilent 7700×. Estimated glomerular filtration rate (eGFR) was calculated from serum creatinine measurements using the CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) equation(Reference Levey, Stevens and Schmid17).
Definitions
Overweight and obesity were defined by the sex- and age-specific BMI cut-offs for Chinese children and adolescents(18). Weight status was further defined as with/without overweight/obesity. Geographical region was dichotomised into rural and urban area including large urban and small to medium urban area. Participants aged between 7 and 17 years with the MetS were identified according to the modified criteria of the National Cholesterol Education Program (Adult Treatment Panel III) (NCEP-ATP III)(Reference Cook, Weitzman and Auinger19), using at least three of the five components as follows: (1) abdominal obesity: WC ≥ age- and sex-specific 90th percentile(Reference Song, Li and Gasevic20); (2) elevated TAG: TAG ≥ 1·24 mmol/l; (3) low HDL: HDL ≤ 1·03 mmol/l; (4) elevated blood pressure: systolic blood pressure and/or diastolic blood pressure ≥ 90th percentile for sex, age and height(Reference Dong, Ma and Song21) and (5) elevated FG: glucose ≥ 6·1 mmol/l.
Statistical analysis
Participants were classified into quartiles according to serum Zn levels from the lowest (the 1st quartile) to the highest (the 4th quartile). Baseline characteristics of study participants were described as medians and interquartile ranges for continuous variables and numbers and percentages for categorical variables. Differences between groups were analysed by the Kruskal–Wallis test or Mann–Whitney U test for continuous variables and χ 2 test for categorical variables. If significant, the inter-group effect size was calculated according to Cohen’s d for continuous variables. Natural logarithmic transformation was performed on serum Zn, hs-CRP, eGFR and metabolic risk factors (FG, TC, TAG, HDL-cholesterol, LDL-cholesterol, systolic blood pressure and diastolic blood pressure) due to non-normality of the data distribution. Multiple linear regression models were constructed to explore the association between serum Zn concentrations and the metabolic risk factors. Serum Zn concentration is affected by age, sex and systemic inflammation(Reference Hess, Peerson and King22), and metabolic risk factors are related to levels of BMI, eGFR and socio-economic(Reference Zhu, Zheng and Zou5,Reference Song, Yu and Chang23,Reference Shikata, Kodera and Utsunomiya24) . Therefore, the models were adjusted for age and sex and further for hs-CRP, eGFR, BMI and region. Potential effect modification between serum Zn and FG by age group (6–12 years, 13–17 years), sex (male, female), BMI (with/without overweight or obesity), region (urban, rural), hs-CRP (below or above median) and eGFR (below or above median) was assessed in stratified analyses, and significance of interactions was assessed based on first-degree multiplicative models. Additionally, a fully adjusted logistic regression model was performed to evaluate the association of the MetS and its components with serum Zn concentrations. The dependent variable in this model was whether the participants had the MetS or its components. Potential non-linear associations between serum Zn and metabolic risk factors were visualised by smoothed splines from generalised additive models. To determine the robustness of the findings in the primary analysis, sensitivity analyses excluding individuals without overweight or obesity were also performed. Statistically significance was defined as P < 0·05. All statistical analyses were performed using the R software version 3.6.3.
Results
Table 1 shows the baseline characteristics of the study participants across quartiles of serum Zn concentrations. The median concentration of serum Zn for all participants was 14·23 (interquartile range 13·16, 16·83) μmol/l. Compared with participants in the lowest quartile, those with serum Zn levels in the highest quartile had a higher level of weight (P < 0·001, Cohen’s d = 0·21), height (P < 0·001, Cohen’s d = 0·14), BMI (P < 0·001, Cohen’s d = 0·25), WC (P < 0·001, Cohen’s d = 0·27), systolic blood pressure (P = 0·01, Cohen’s d = 0·11), diastolic blood pressure (P = 0·01, Cohen’s d = 0·10), TC (P < 0·001, Cohen’s d = 0·25), LDL-cholesterol (P < 0·001, Cohen’s d = 0·21) and serum creatinine (P < 0·001, Cohen’s d = 0.·15), but a lower level of FG (P < 0·001, Cohen’s d = 0·44) and eGFR (P = 0·02, Cohen’s d = 0·10). Participants with higher serum Zn levels were more likely to be male (P < 0·001, Cohen’s d = 0·15), urban residents (P < 0·001, Cohen’s d = 0·49), with overweight/obesity (P < 0·001, Cohen’s d = 0·23) and had higher hs-CRP levels (P = 0·025, Cohen’s d = 0·08; online Supplementary Table S1).
Table 1. Sample characteristics of Chinese children and adolescents according to serum zinc quartiles
(Numbers and percentages; medians and interquartile ranges (IQR), n 3241)

WC, waist circumference; SBP, systolic blood pressure; DBP, diastolic blood pressure; FG, fasting glucose; TC, total cholesterol; hs-CRP, high-sensitivity C-reactive protein; eGFR, estimated glomerular filtration rate.
* Continuous variables were described as medians and IQR; categorical variables are described as n and %. P values were calculated by the Kruskal–Wallis test (continuous variables) or χ 2 test (categorical variables).
The associations of serum Zn concentrations with metabolic risk factors are presented in Table 2. Serum Zn was positively associated with TC (coefficient = 0·175; 95 % CI 0·127, 0·222), HDL-cholesterol (coefficient = 0·137; 95 % CI 0·082, 0·193) and LDL-cholesterol (coefficient = 0·195; 95 % CI 0·128, 0·263) but negatively associated with FG (coefficient = −0·532; 95 % CI − 0·569, −0·495) in all the models. Also, a slightly weaker but significant negative association between serum Zn and systolic blood pressure was observed only in the fully adjusted model (coefficient = −0·035; 95 % CI −0·059, −0·012). No significant linear association was found between serum Zn with TAG and diastolic blood pressure in multi-variable linear models.
Table 2. Multi-variable associations of serum zinc with metabolic risk factors in children and adolescents in Jiangsu province, 2016–2017
(Coefficients and 95 % confidence intervals, n 3241)*
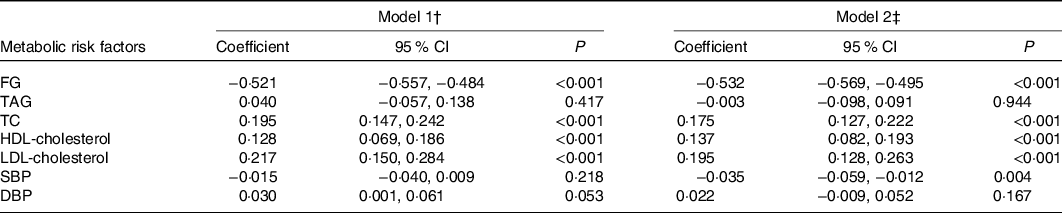
FG, fasting glucose; TC, total cholesterol; SBP, systolic blood pressure; DBP, diastolic blood pressure; hs-CRP, high-sensitivity C-reactive protein; eGFR, estimated glomerular filtration rate.
* Serum Zn, hs-CRP, eGFR and metabolic risk factors were log-transformed.
† Model 1: adjusted for age (continuous), sex (male/female).
‡ Model 2: further adjusted for hs-CRP (continuous, log-transformed), eGFR (continuous, log-transformed), BMI (continuous) and region (urban rural).
Results of the stratified analysis for the association between serum Zn and FG are listed in Table 3. The associations between serum Zn and FG were similar in subgroups stratified by sex (P-interaction = 0·131). However, the association between serum Zn and FG was stronger in participants who were adolescents (13–17 years) (P-interaction < 0·001), without overweight/obesity (P-interaction = 0·013), urban residents (P-interaction < 0·001), had higher hs-CRP levels (>0·34 mg/l) (P-interaction = 0·034) and lower eGFR (≤148·62) (P-interaction < 0·001).
Table 3. Multi-variable associations of serum zinc and fasting glucose (FG): subgroup analysis grouped by potential confounding factors* †
(Coefficients and 95 % confidence intervals; numbers and percentages, n 3241)

hs-CRP, high-sensitivity C-reactive protein; eGFR, estimated glomerular filtration rate.
* Serum Zn, FG, hs-CRP and eGFR were log-transformed.
† Model: adjusted for age (continuous), sex (male/female), hs-CRP (continuous, log-transformed), eGFR (continuous, log-transformed), BMI (continuous) and region (urban/rural).
Fig. 1 shows the association of serum Zn with the MetS and its components in participants. The proportion of participants with abdominal obesity, elevated TAG, low HDL, elevated blood pressure, elevated FG and the MetS was 18·68, 14·98, 3·74, 42·38, 3·31 and 5·12 %, respectively. Logistic regression analyses showed that serum Zn was negatively associated with low HDL-cholesterol (OR 0·74; 95 % CI 0·61, 0·91) and elevated FG (OR 0·61; 95 % CI 0·49, 0·77). Nevertheless, no significant associations were observed between the MetS, abdominal obesity, elevated TAG and elevated blood pressure with serum Zn.

Fig. 1. Forest plot for the associations of serum zinc with the metabolic syndrome and its components in Chinese children and adolescents in Jiangsu Province, 2016–2017 (n 3025). Serum zinc was log-transformed and divided by the standard deviation. The models were adjusted for age (continuous), sex (male/female); high-sensitive C-reactive protein (continuous, log-transformed), estimated glomerular filtration rate (continuous, log-transformed), BMI (continuous) and region (urban/rural). Waist circumference, TAG, HDL, systolic blood pressure, diastolic blood pressure and fasting glucose were used to identify participants with the metabolic syndrome.
A significant non-linear association (Fig. 2) was found between serum Zn and FG as well as TAG. After adjusted for age, sex, hs-CRP, eGFR, BMI and region, the negative correlation between serum Zn and FG was weak at lower serum Zn concentrations and stronger with the increase in serum Zn concentrations. Moreover, a U-shaped curve of serum Zn and TAG was observed in addition to the results of logistic regression (Fig. 1).

Fig. 2. Dose–response curves with 95 % confidence intervals (shaded areas) for the associations of fasting glucose and TAG with serum zinc. Associations were modelled using generalised additive models (n 3241). Analyses were adjusted for age (continuous), sex (male/female), high-sensitive C-reactive protein (continuous, log-transformed), estimated glomerular filtration rate (continuous, log-transformed), BMI (continuous) and region (urban/rural). Both outcome and exposure variables were log-transformed before analysis, and back-transformed values are presented. Density plots indicate the distributions of serum zinc, and dotted lines denote the 5th, 50th and 95th percentiles. * To convert zinc in μg/dl to μmol/l, multiply by 0·153.
Sensitivity analyses were conducted by restricting the analysis to 896 participants with overweight (n 478) or obesity (n 418), yielding slightly attenuated associations for FG (coefficient = −0·474; 95 % CI −0·536, −0·412) and TC (coefficient = 0·164; 95 % CI 0·077, 0·251) with serum Zn, whereas the association between HDL-cholesterol (coefficient = 0·141; 95 % CI 0·032, 0·249) and serum Zn appeared to be slightly enhanced. Additionally, the association of LDL-cholesterol with serum Zn (coefficient = 0·128; 95 % CI −0·009, 0·265) was not maintained after the sensitivity analysis.
The risk associations for incident low HDL-cholesterol with serum Zn (OR 0·91; 95 % CI 0·66, 1·25) were not maintained, while the protective effect of serum Zn (OR 0·50; 95 % CI 0·33, 0·76) with elevated FG was slightly enhanced after sensitivity analysis.
Discussion
In this large-scale cross-sectional study, we observed that serum Zn, a biomarker reflecting Zn status, was negatively associated with FG and hyperglycaemia in Chinese children and adolescents. The association was still present after adjusted for potential confounders including age, sex, hs-CRP, eGFR, BMI and region and was stronger in participants who were aged 7–17 years old, without overweight/obesity, urban residents, had higher hs-CRP levels and lower eGFR.
Our results were consistent with findings from three recent meta-analyses, which reported that Zn supplementation was effective in lowering FG levels in patients with diabetes/prediabetes(Reference Khazdouz, Djalalinia and Sarrafi11,Reference Wang, Wu and Zheng25,Reference Hannon, Fairfield and Adams26) . There are several underlying mechanisms that may explain the effect of Zn on glycaemic metabolism. First, Zn is indispensable in the process of insulin biosynthesis and maturation of insulin secretory granules in pancreatic β-cells and serves to stabilise and alleviate the oxidative damage to insulin sensitivity(Reference Norouzi, Adulcikas and Sohal27). Second, Zn may induce increased glucose transport into cells by the regulation of the insulin-stimulated translocation of GLUT4, thus contributing to the reduction of blood glucose(Reference Wu, Lu and Yang28). Moreover, Zn transporter 8 provides Zn ions to insulin secretory vesicles through its Zn ion transporter and serves a crucial role in stimulating the secretion of insulin(Reference Huang, Merriman and Zhang29). Finally, oxidative stress is a key pathogenetic factor for diabetes; thus, Zn may slow the progression of insulin resistance and diabetes for its antioxidant properties(Reference Marreiro, Cruz and Morais8).
Despite the above studies, some population-level studies reported different results. One cohort study in Finland including middle-aged and older male adults and a cross-sectional study in the USA including adults older than 18 years have shown a positive association between serum Zn levels and risk for developing diabetes(Reference Yary, Virtanen and Ruusunen30,Reference Qu, Yang and Yu31) . Also, two cross-sectional studies, one conducted among Chinese adults over 65 years of age(Reference Fang, Wu and Gu14) and the other among adults aged over 20 years in Iran(Reference Ghasemi, Zahediasl and Hosseini-Esfahani32), did not demonstrate an association between serum Zn and FG or hyperglycaemia. In contrast, a significant negative correlation of serum Zn with FG or hyperglycaemia was found in Korean male adults above 20 years of age(Reference Seo, Song and Han12). For children and adolescents, one of the two published studies found no significant association between serum Zn and FG in Iranian girls(Reference Gonoodi, Moslem and Darroudi15), whereas the other study found plasma Zn was negatively correlated with FG in Australian children(Reference Ho, Baur and Cowell16).
These conflicting findings may result from the differences between ages and regions. Previous studies have shown that insulin resistance is associated with increasing age for reasons of increased abdominal adiposity, reduced physical activity and declines in muscle mass(Reference Barbieri, Rizzo and Manzella33). In addition, the reduced insulin secretion and increased risk for type 2 diabetes caused by the age-dependent decline in the capacity for β-cell mass expansion and proliferation appear to be another potential factor affecting the association of serum Zn with FG at different ages(Reference Li, Liu and Yang34). A positive correlation between red meat and type 2 diabetes has been reported by previous studies(Reference Schwingshackl, H‘‘offmann and Lampousi35). In most developed countries, the main source of Zn is meat, whereas Zn mainly comes from cereals and pulses in developing countries(Reference Nriagu36); thus, the difference of dietary pattern could be one reason for the inconsistent results from different regions. The significant non-linear association between serum Zn and FG we observed in the present study may imply a threshold effect of Zn on FG. Therefore, the lower availability Zn level in the Iranian population could explain the discrepancy between the Iranian study and our findings(Reference Schmidhuber, Sur and Fay37).
We found an intriguing result that the association between serum Zn and FG was much stronger in adolescents than in children. While there are evidences showing that insulin resistance rises before the onset of puberty and reduces significantly with progression to the later stages of puberty and the decrease in insulin sensitivity during adolescence was compensated by a doubling in insulin secretion(Reference Kelsey and Zeitler38), but these do not completely explain the disparate results. Mechanisms for this difference need to be further studied. In the present study, we found that the association was prominent among children and adolescents without overweight/obese, although children and adolescents with overweight/obese had a significantly higher level of serum Zn than those without. The low-grade chronic inflammation in metabolic tissues caused by obesity is related to insulin resistance and has a negative impact on β-cell function, which may contribute to the observed associations(Reference Lee, Wollam and Olefsky39).
In addition to the glucose levels, we also observed a positive linear association of serum Zn levels with TC, HDL-cholesterol and LDL-cholesterol, and a U-shaped association with TAG. To date, there has been no agreed conclusion on the association of serum Zn with lipid profiles. Some studies have reported similar findings to ours. A clinic-based case–control study in the USA that included 778 adults showed that serum Zn levels were positively linked to levels of TC, LDL-cholesterol and TAG(Reference Hiller, Seigel and Sperduto40). A cross-sectional study in Iran involving 2401 adults found a positive correlation between TC, TAG and serum Zn(Reference Ghasemi, Zahediasl and Hosseini-Esfahani32). However, in a South Korean study including1988 adults, a decrease in HDL-cholesterol levels with elevated serum Zn levels was observed. A positive correlation between serum Zn levels and elevated TAG was found only in men(Reference Seo, Song and Han12). Furthermore, some studies demonstrated no statistical correlation between serum Zn level and lipid profiles(Reference Abiaka, Olusi and Al-Awadhi41,Reference He, Tell and Tang42) . Our findings extend the association between serum Zn and lipid profiles to Chinese children and adolescents. Interestingly, clinical and experimental studies have shown that Zn supplementation can reduce levels of TC, LDL-cholesterol and TAG and increase HDL-cholesterol level(Reference Mohy, El-Ashmony and Morsi43,Reference Li, Jiao and Chen44) . Therefore, we speculate that the source of Zn may also be an important factor affecting its association with lipid profiles, which could be verified in future work. Although there is no significant association between serum Zn and the MetS in the present study, the associations of serum Zn with FG and lipid profiles are of great clinical and public health importance for Chinese children and adolescents during the current nutritional transition period(Reference Gordon-Larsen, Wang and Popkin45).
To the best of our knowledge, this is the largest study assessing the associations of serum Zn and metabolic risk factors in children and adolescents worldwide and is the first study on this topic in a Chinese population. Standardised protocols and data collection procedures were adopted in this study, and training and strict quality control were carried out for all the participating staff, which ensures that the results of this study were reliable.
There are also some limitations to this study. First of all, our study used well-known cardiometabolic markers that could predict future CVD instead of clinical outcomes of cardiometabolic events to study its associations with Zn concentrations, since the sample is composed of school-age children and adolescents. Also, despite the fact that we have corrected some known confounding factors, the lack of data on lifestyle factors such as smoking, drinking and physical activity may affect the association between serum Zn and metabolic risk factors(Reference Yu, Xu and Zhu46,Reference Chu, Petocz and Samman47) . Moreover, serum Zn is closely linked to insulin resistance in children and adolescents(Reference Suliburska, Cofta and Gajewska48,Reference Ortega, Rodriguez-Rodriguez and Aparicio49) ; thus, the lack of data on insulin limits our further exploration of the association between serum Zn and insulin in the Chinese population. In addition, our study was conducted among Chinese children and adolescents; the extrapolation of results to other populations should be interpreted cautiously taking into account the possible differences between different populations. Finally, due to the cross-sectional nature of this study, we cannot confirm the causal relationship of serum Zn with FG and lipid profiles.
Conclusions
In summary, we observed an inverse association between serum Zn and FG, especially at higher serum Zn concentrations among Chinese children and adolescents. Additionally, we found a positive linear correlation between serum Zn and TC, HDL-cholesterol and LDL-cholesterol, and a U-shaped correlation of serum with TAG. These associations were still present after adjusted for known risk factors, including age, sex, hs-CRP, eGFR, BMI and region. These findings suggest that lower levels of serum Zn were more likely related to a poor metabolic status in Chinese children and adolescents. Future studies are warranted to confirm this finding and to explore the underlying mechanisms.
Acknowledgements
The authors thank all of the participants and staff members who contributed to this study.
The study was financed by National Key R&D Program (2016YFC1305201) of China.
Y. L. Z., J. Z. and H. Z. contributed to study concept and design as well as critically revised the manuscript for important intellectual content; Y. D., J. X. Z. and W. X. acquired the data; Q. Z. and H. Z. analysed the data; Q. Z. wrote the main manuscript text; all authors reviewed and approved the manuscript.
None of the authors declared conflicts of interest related to this manuscript.
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1017/S0007114521000258








