Cocoa, a product derived from the beans of the Theobroma cacao plant, has been consumed since 600 BC, first by ancient civilisations, such as the Mayans and Aztecs(Reference Hurst, Tarka and Powis1). Cocoa consumption in Europe dates from the 16th century when Hernán Cortés introduced it to the Iberian Peninsula; from there its use spread rapidly to Western Europe(Reference Dillinger, Barriga and Escarcega2). Cocoa powder is a rich source of fibre (26–40 %), proteins (15–20 %), carbohydrates (about 15 %) and lipids (10–24 %; most, 10–12 %), and it contains minerals (for example, Ca, Mg, K) and vitamins (A, E, B and folic acid) (Table 1).
Table 1 Cocoa powder: nutritional information per 100 g(93)

Cocoa has become a subject of increasing interest because of its high content of polyphenolic antioxidants, particularly flavonoids. Cocoa powder is reported to contain up to 70 mg polyphenols/g (expressed as catechin)(Reference Vinson, Proch and Zubik3). A serving size portion of certain cocoa-derived products provides more phenolic antioxidants than beverages and fruits such as tea and blueberries, traditionally considered high in antioxidants(Reference Lee, Kim and Lee4, Reference Vinson, Proch and Bose5). Cocoa mainly contains the monomers ( − )-epicatechin and catechin, and various polymers derived from these monomers, known as procyanidins (Figs. 1 and 2). Monomer content ranges from 0·20 to 3·50 mg/g, depending on the type of product, with epicatechin content being higher than (+)-catechin in most cocoa products(Reference Vinson, Proch and Zubik3, Reference Gu, House and Wu6). Procyanidins are the major flavonoids in cocoa and chocolate products, with reported levels ranging from 2·16 to 48·70 mg/g(Reference Gu, House and Wu6). Methylxanthines have also been identified in cocoa powder, and account for 0·5–2 % of the DM(Reference Pura Naik7).
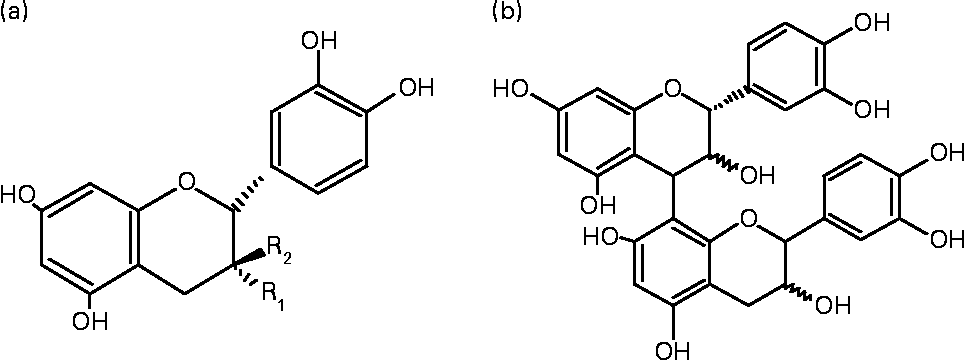
Fig. 1 Chemical structure of the main cocoa flavonoids. (a) R1 = OH corresponds to ( − )-epicatechin and R2 = OH to (+)-catechin. (b) Dimeric procyanidin (4β → 8).

Fig. 2 Flavonoids (a) and non-flavonoid phenols (b) contained in cocoa(Reference Borchers, Keen and Hannum94, Reference Sanchez-Rabaneda, Jauregui and Casals95).
Bioavailability of cocoa flavonoids
The biological effects of flavonoids depend on the bioavailability of the compound. The various manners and rates in which flavonoids are absorbed have been recently reviewed(Reference Hackman, Polagruto and Zhu8). Flavan-3-ols show no changes after 40 min in the human stomach(Reference Rios, Bennett and Lazarus9), indicating that flavonols and procyanidins are stable in the harsh environment of the digestive system. Gut flavonoid absorption basically depends on the chemical structure of the individual type. Monomeric flavonoids and certain dimeric and trimeric procyanidins are absorbed in the small intestine and are rapidly detected in the plasma(Reference Baba, Osakabe and Yasuda10–Reference Tsang, Auger and Mullen13). Certain monomers are better absorbed than others(Reference Baba, Osakabe and Natsume14). For example, epicatechin was the main flavonoid detected in the plasma after intake of a cocoa beverage containing equal amounts of catechin and epicatechin(Reference Holt, Lazarus and Sullards12). Absorption of ( − )-epicatechin in humans is relatively efficient, and the plasma concentration of its primary metabolite, ( − )-epicatechin glucuronide, is about 600 nmol/l at 2 h after the consumption of a cocoa beverage containing 54·4 mg ( − )-epicatechin(Reference Roura, Andres-Lacueva and Jauregui15). Intestinal absorption of epicatechin and catechin has also been demonstrated in rats(Reference Tsang, Auger and Mullen13, Reference Baba, Osakabe and Natsume14, Reference Chang, Zuo and Ho16, Reference Zuo, Zhang and Zhou17).
Short procyanidins (dimers and trimers) are absorbed in the small intestine and rapidly detected in the plasma(Reference Baba, Osakabe and Natsume18) and urine(Reference Tsang, Auger and Mullen13), whereas large procyanidins are less efficiently absorbed, but may have an important local function in the gut, neutralising oxidants and carcinogenic compounds. Moreover, flavonoids can also be metabolised by colon microflora to phenolic acids, which are then absorbed(Reference Manach, Scalbert and Morand19, Reference Gu, House and Rooney20).
The influence of the food matrix on flavonoid absorption has been an issue of discussion in the past few years, particularly after the publication of a study reporting decreased absorption of cocoa flavonoids owing to an interaction with milk proteins, which are present in most cocoa-derived products(Reference Serafini, Bugianesi and Maiani21). Conversely, other studies in human subjects found no significant effects of milk on epicatechin absorption and the total amount of metabolites excreted after ingestion of a cocoa beverage(Reference Keogh, McInerney and Clifton22–Reference Roura, Andrés-Lacueva and Estruch24). Nonetheless, differences in the excreted metabolite profile have recently been described, suggesting that milk components have an effect on cocoa metabolism(Reference Roura, Andrés-Lacueva and Estruch24). Polysaccharides also seem to enhance flavonoid absorption, although the mechanism causing this effect remains uncertain(Reference Schramm, Karim and Schrader25).
Data on the distribution of flavonoid metabolites in tissues after cocoa intake are limited, even in experimental studies. Absorbed flavonoids are widely distributed and can be detected in many organs, including lymphoid tissues, at a concentration of nmol/g tissue (for reviews, see Manach et al. (Reference Manach, Scalbert and Morand19) and de Boer et al. (Reference de Boer, Dihal and van der Woude26)). Levels of flavan-3-ol metabolites have been found in rat liver and kidney over a 24 h period following flavonoid ingestion(Reference Tsang, Auger and Mullen13). In a study in rats and pigs, multiple tissues were found to accumulate quercetin metabolites, with high levels in rat lung and little accumulation in brain, spleen and white fat(Reference de Boer, Dihal and van der Woude26).
Cocoa as an antioxidant
Cocoa has a potent antioxidant capacity as compared with other products, a quality related to its flavonoid content(Reference Lee, Kim and Lee4, Reference Vinson, Proch and Bose5). Procyanidins account for the highest percentage of antioxidants in cocoa products. A serving of dark chocolate (40 g) provides about 517 mg procyanidins with an antioxidant capacity of 9100 Trolox equivalents (TE), and a glass of homemade cocoa milk supplies 108 mg procyanidins and 3200 TE. In fact, one serving of these products imparts greater antioxidant capacity than the average amount of antioxidants consumed daily in the USA(Reference Gu, House and Wu6).
Flavonoids act as antioxidants by directly neutralising free radicals, chelating metals (Fe2+ and Cu+) that enhance highly aggressive reactive oxygen species, inhibiting enzymes responsible for reactive oxygen species production (xanthine oxidase) and up-regulating or protecting antioxidant defences(Reference Cotelle27). Epicatechin and catechin are very effective in chelating Fe(Reference Morel, Lescoat and Cogrel28) and neutralising several types of radicals such as peroxyl, peroxynitrite, superoxide and 1,1 diphenyl-2-picryl-hydrazyl(Reference Hatano, Miyatake and Natsume29–Reference Pollard, Kuhnle and Vauzour31). Epicatechin and catechin are more highly active on alkyl (ROO∙) peroxyl radicals than the well-recognised antioxidants l-ascorbate and β-carotene(Reference Nakao, Takio and Ono32). In addition, epicatechin can regenerate α-tocopherol from its corresponding radical(Reference Pazos, Andersen and Medina33). Cocoa procyanidins also scavenge radicals, such as peroxynitrites, with activity that is proportional to the number of monomeric units they contain(Reference Arteel and Sies34, Reference Counet and Collin35). Even though quercetin is present at a smaller percentage, it may also contribute to cocoa's antioxidant activity by neutralising radicals and chelating metal ions(Reference Formica and Regelson36, Reference Lamuela-Raventos, Andres-Lacueva and Permanyer37). Despite these well-defined antioxidant characteristics, however, flavonoids can become pro-oxidants under certain conditions, such as high flavonoid concentrations and the presence of redox-active metals(Reference Nijveldt, van Nood and van Hoorn38). Lastly, other compounds present in cocoa, particularly methylxanthines (0·5–2 % of cocoa powder), can also contribute to its antioxidant properties(Reference Azam, Hadi and Khan39).
Several in vitro studies have demonstrated the antioxidant capacity of cocoa flavonoids and their metabolites(Reference Yilmaz and Toledo30, Reference Spencer, Schroeter and Rechner40, Reference Natsume, Osakabe and Yasuda41). Epicatechin, catechin and procyanidin B2 reduce oxidant-induced erythrocyte haemolysis in a dose-dependent manner(Reference Zhu, Holt and Lazarus42, Reference Zhu, Schramm and Gross43). Cocoa procyanidins protect intestinal Caco-2 cell monolayers from the loss of integrity induced by a lipophilic oxidant(Reference Erlejman, Fraga and Oteiza44). In addition, cocoa polyphenolic extract inhibits superoxide anion formation and xanthine oxidase activity in stimulated myelocytic leukaemia HL-60 cells(Reference Lee, Kundu and Kim45).
Going beyond these in vitro assays, a small number of studies have investigated the effects of cocoa in vivo. Because it is difficult to isolate large amounts of cocoa polyphenols, almost all in vivo studies are performed using whole cocoa powder. Cocoa intake increases total antioxidant capacity and decreases lipid oxidation products in murine plasma and human plasma from healthy subjects(Reference Baba, Osakabe and Natsume18, Reference Wang, Schramm and Holt46, Reference Lecumberri, Mateos and Ramos47). In these studies, the enhancement of antioxidant capacity was greatest 1–2 h after cocoa administration and gradually decreased thereafter to reach baseline levels at about 6 h post-ingestion. Plasma antioxidant capacity was not enhanced in blood collected more than 6 h after cocoa intake(Reference Orozco, Wang and Keen48–Reference Ramiro-Puig, Urpi-Sarda and Perez-Cano50), probably because of the short plasma half-life of flavonoids and their uptake in cells.
A cocoa-enriched diet increases the antioxidant capacity of cell tissues to varying degrees, with the activity in thymus > spleen > liver(Reference Ramiro-Puig, Urpi-Sarda and Perez-Cano50), an effect that can be attributed to differing levels of flavonoid accumulation(Reference de Boer, Dihal and van der Woude26). Cocoa boosts catalase and superoxide dismutase activity in rat thymus, but not in spleen and liver(Reference Ramiro-Puig, Urpi-Sarda and Perez-Cano50). Although the exact mechanism remains to be established, enhancement of superoxide dismutase and catalase activity by cocoa could be due to direct neutralisation of enzyme substrates (![]() and H2O2, respectively) or to up-regulation of antioxidant enzyme expression. Cocoa phenols could have an important role in these actions, since they are potent antioxidants able to induce enzymes such as superoxide dismutase and glutathione peroxidase(Reference Yeh and Yen51).
and H2O2, respectively) or to up-regulation of antioxidant enzyme expression. Cocoa phenols could have an important role in these actions, since they are potent antioxidants able to induce enzymes such as superoxide dismutase and glutathione peroxidase(Reference Yeh and Yen51).
Cocoa also improves antioxidant defences in experimentally induced oxidative stress. For example, the long-term intake of a cocoa-enriched diet containing 0·3 % polyphenols reduced lipid peroxidation in the plasma and liver of hypercholesterolaemic rats(Reference Mateos, Lecumberri and Ramos52). The consumption of 100 g chocolate containing 0·2 % polyphenols for 2 weeks counteracted oxidative stress in soccer players, as was shown by reductions in plasma malondialdehyde and α-tocopherol increases(Reference Fraga, Actis-Goretta and Ottaviani53).
A recent review of the impact of cocoa on the cardiovascular system has compiled all the interventional studies in human subjects published over the last 10 years. In these trials, cocoa intake was seen to reduce the risk of CVD by a combination of several effects, including improvements in antioxidant status (shown by decreases in oxidative stress biomarkers, such as thiobarbituric acid-reactive species and LDL oxidation), vasodilation, and inhibition of platelet activation and aggregation(Reference Cooper, Donovan and Waterhouse54). Other reviews have examined the beneficial effects of dietary flavonoids on health(Reference Fisher and Hollenberg55–Reference Hodgson and Croft58).
Cocoa as an immunomodulator
Cocoa has exhibited promising regulatory effects on immune cells involved in innate and acquired immunity.
In vitro effects of cocoa on immune cells
In vitro studies have demonstrated the regulatory effects of cocoa on the secretion of inflammatory mediators from macrophages and other leucocytes. Ono et al. (Reference Ono, Takahashi and Kamei59) and Ramiro et al. (Reference Ramiro, Franch and Castellote60) found that flavonoid-rich cocoa extract decreases the secretion of TNF-α, monocyte chemoattractant protein-1 (MCP-1) and NO by lipopolysaccharide-stimulated macrophages (Table 2). The in vitro anti-inflammatory behaviour of cocoa differs from that of individual flavonoids. The influence of cocoa on macrophages is much stronger when cocoa is added before lipopolysaccharide stimulation, whereas when macrophages are treated with epicatechin alone, a stronger effect is achieved with addition after stimulation (Table 2)(Reference Ramiro, Franch and Castellote60). This can be attributed to partial oxidation of epicatechin by reactive oxygen species produced during the pre-stimulation period, and to the effect of cocoa procyanidins, which can be hydrolysed along the pre-stimulation period to monomeric and dimeric compounds that are taken up by macrophages(Reference Mackenzie, Carrasquedo and Delfino61). The inhibitory effect of whole cocoa extract on MCP-1 secretion is higher than that of epicatechin, but lower than that of isoquercitrin (Table 2)(Reference Ramiro, Franch and Castellote60). These findings illustrate the differing potency of the flavonoids for modulating macrophage function, and suggest possible synergism or an influence of the wide spectrum of compounds present in whole cocoa, such as other polyphenols and methylxanthines(Reference Pura Naik7).
Table 2 In vitro studies performed with cocoa investigating inflammatory cytokine secretion

PBMC, peripheral blood mononuclear cells; LPS, lipopolysaccharide; IFN, interferon; SCFF, short-chain flavonol fraction (monomers–pentamers); LCFF: long-chain flavonol fraction (hexamers–decamers); ↓ , decrease; ↑ , increase; = , no effect; GM-CSF, granulocyte-macrophage colony-stimulating factor; MCP-1, monocyte chemoattractant protein-1.
* Cocoa extract has a higher inhibitory effect on TNF-α secretion than epicatechin when it is added before stimulus.
A recent study by Kenny et al. (Reference Kenny, Keen and Schmitz62) investigated the effect of two different fractions of purified cocoa flavonoids on lipopolysaccharide-stimulated human peripheral blood mononuclear cells (PBMC). The short-chain flavonol fraction (including monomers to pentamers), and particularly the long-chain fraction (including hexamers to decamers), enhanced the secretion of TNF-α, IL-1, IL-6 and IL-10 from stimulated human PBMC (Table 2). The differences between these results and those described by Ono et al. (Reference Ono, Takahashi and Kamei59) and Ramiro et al. (Reference Ramiro, Franch and Castellote60) may be attributable to differences in the compounds tested and the cells used, or to the experimental design. Kenny et al. (Reference Kenny, Keen and Schmitz62) tested purified cocoa flavonoid fractions, whereas Ramiro et al. (Reference Ramiro, Franch and Castellote60) used whole cocoa extract, which contained flavonoids and other immunomodulatory compounds. Moreover, the study by Kenny et al. (Reference Kenny, Keen and Schmitz62) was performed on PBMC including several immune cell types that can interact, whereas Ramiro et al. (Reference Ramiro, Franch and Castellote60) used a macrophage cell line, which may not have had the same susceptibility to cocoa compounds. As mentioned above, flavonoids can act as antioxidants or oxidants, depending on certain conditions (for example, a high flavonoid concentration induces an oxidant environment); therefore, it is reasonable to suggest that oligomeric flavonoids could induce an oxidant state in PBMC that would lead to cell activation and production of inflammatory mediators. These results do not reflect what occurs in vivo, however, when tissue cells are under the effect of flavonoid metabolites and/or different concentrations of these compounds.
The effect of cocoa flavonoids on adaptive immunity has been investigated using lymphocyte cultures. In phorbol-myristate acetate-stimulated lymphocytes, cocoa extract reduces lymphocyte proliferation by down-regulating IL-2 secretion and IL-2 receptor expression (Table 3) (Reference Sanbongi, Suzuki and Sakane63–Reference Ramiro, Franch and Castellote65). In keeping with the results obtained in macrophages, the inhibitory effect of whole cocoa extract on lymphocytes is higher than that of epicatechin(Reference Ramiro, Franch and Castellote65). In addition, cocoa procyanidins decrease IL-2 in phytohaemagglutinin-stimulated PBMC (Table 3)(Reference Mao, Powell and Van de Water64), demonstrating that flavonoids are the main compounds responsible for the regulation of lymphocyte activation. Moreover, it has been reported that epicatechin, catechin and dimeric procyanidins, the flavonoid forms usually found in plasma, reduce IL-2 secretion by phorbol-myristate acetate-stimulated Jurkat T cells(Reference Mackenzie, Carrasquedo and Delfino61). Although the exact mechanism is still unclear, cocoa flavonoids have been shown to inhibit IL-2 at the transcriptional level(Reference Mao, Powell and Van de Water64). Moreover, given the regulatory effect of IL-2 on its receptor, IL-2 receptor down-regulation could also be a consequence of the IL-2 decrease.
Table 3 In vitro studies performed with cocoa investigating lymphocyte cytokine secretion

PBMC, peripheral blood mononuclear cells; PHA, phytohaemagglutinin; PMA, phorbol-myristate acetate; ↓ , decrease; ≈ , changes moderately; ( ↓ ), decrease non-statistically significant; ↑ , increase; ( ↑ ), increase non-statistically significant; TGF, transforming growth factor; IL-2R, IL-2 receptor; CD25, cluster of differentiation 25.
Considering the effects of cocoa on cytokines attributed to effector T helper (Th)1 and Th2 cells, cocoa extract has been found to slightly increase in vitro IL-4 secretion and, therefore, Th2-response (Table 3)(Reference Ramiro, Franch and Castellote65). In this setting, cocoa produces a less pronounced effect than epicatechin. The effects of cocoa flavonoids on cytokine secretion seem to be related to the degree of polymerisation: short-chain cocoa procyanidins increase IL-4 and IL-5, whereas long-chain procyanidins reduce both these Th2 cytokines (Table 3)(Reference Mao, Water and Keen66, Reference Mao, Van de Water and Keen67).
The mechanism by which cocoa exerts its opposing effects on Th1/Th2 cytokines remains to be established. It is likely that cocoa differentially modulates transcription factor activation: signal transducer and activator of transcription-4 (STAT4), involved in IL-2 expression, and STAT6, the main IL-4 inducer(Reference Mowen and Glimcher68). Cytokine interactions should also be taken into account; the increase in Th2 cytokines, which are known to be potent down-regulators of Th1, could contribute to IL-2 inhibition.
Cocoa procyanidins promote homeostatic levels of transforming growth factor-β (TGF-β) in PBMC. TGF-β is enhanced in non-stimulated immune cells, whereas it is decreased in stimulated cells (Table 3)(Reference Mao, Water and Keen69). Although TGF-β is generally considered a regulatory cytokine that helps to maintain an appropriate Th1/Th2 balance in certain situations (for example, in advanced CVD), it can act as a pro-inflammatory mediator through the induction of immune cell recruitment and activation(Reference Redondo, Santos-Gallego and Tejerina70, Reference Wahl71).
In summary, cocoa down-regulates both macrophage and lymphocyte activation in vitro. Given their powerful antioxidant activity, flavonoids seem to be the perfect candidates for immune regulation; nonetheless, studies showing opposite effects among different cocoa flavonoid fractions suggest that other compounds may contribute to cocoa's immune effects. In any case, as the profile of flavonoids absorbed in vivo differs from that present in crude cocoa extract, the physiological relevance of these data is limited.
In vivo effects of cocoa on the immune system
A few studies have gone farther than in vitro assays: to investigate the in vivo influence of cocoa on lymphoid organs and immune cell functionality.
The effect of long-term intake (3 weeks) on rat thymus has been recently reported(Reference Ramiro-Puig, Urpi-Sarda and Perez-Cano50). In young rats, an isoenergetic diet containing 10 % cocoa promotes the progression of immature thymocytes (double negative T cell receptor (DN TCR) αβlow and double positive (DP) TCRαβlow cells) towards more mature T cell stages (CD4+CD8− TCRαβhigh cells)(Reference Ramiro-Puig, Urpi-Sarda and Perez-Cano50). Thymus cell differentiation is triggered by complex signalling cascades, most of which are sensitive to changes in the redox environment(Reference Valko, Leibfritz and Moncol72). As was mentioned previously, long-term cocoa intake increases the thymic antioxidant status, as has been shown by enhanced superoxide dismutase and catalase activities(Reference Ramiro-Puig, Urpi-Sarda and Perez-Cano50). These activities might stimulate a slight shift towards a mildly oxidising environment that would favour lymphocyte maturation. In addition, high cocoa intake may promote the differentiation of other immune cell subsets, such as B cells, T cells, myeloid cells, natural killer cells and dendritic cells(Reference Bhandoola and Sambandam73).
The influence of cocoa intake is not only restricted to T cell maturation in the thymus, but also affects lymphocyte composition and function in other immune tissues. High cocoa intake (10 %) for 3 weeks increases the percentage of B cells and decreases the percentage of Th cells in the spleen of young rats (Fig. 3)(Reference Ramiro-Puig, Pérez-Cano and Ramírez-Santana74). In addition, the composition of gut-associated lymphoid tissue, the first line of immune cells to oral challenge and diet(Reference Calder and Kew75), is also influenced by cocoa intake(Reference Ramiro-Puig, Pérez-Cano and Ramos-Romero76). Peyer's patches and mesenteric lymph nodes are gut-associated lymphoid tissue compartments that show changes in lymphocyte composition in young rats fed 10 % cocoa during 3 weeks(Reference Ramiro-Puig, Pérez-Cano and Ramos-Romero76). In Peyer's patches, cocoa intake reduces the TCRαβ+T cell percentage (mainly the Th subset) and increases B and TCRγδ+T cell percentages (Fig. 3). Similarly, in mesenteric lymph nodes, high cocoa intake decreases the Th percentage and raises the percentage of γδ T cells(Reference Ramiro-Puig, Pérez-Cano and Ramos-Romero76).

Fig. 3 Effect of cocoa-enriched diet on lymphocyte percentages in young rats. (a), (c) and (e): Percentages of the main lymphocyte subsets in spleen, mesenteric lymph nodes (MLN) and Peyer's patches (PP), respectively. (b), (d) and (f): Percentages of T cell subsets with respect to total lymphocytes in spleen, MLN and PP, respectively. (□), Reference group (n 10–18); (▧), 10 % cocoa-enriched diet group (n 10–18). NK, natural killer; Th, T helper; Tc, T cytotoxic. Values are means, with standard errors represented by vertical bars. * Mean value was significantly different from that of the reference group (P < 0·05). Adapted from Ramiro-Puig et al. (Reference Ramiro-Puig, Pérez-Cano and Ramírez-Santana74, Reference Ramiro-Puig, Pérez-Cano and Ramos-Romero76).
One of the most important findings of these studies is that a diet containing 10 % cocoa fed to rats increases γδ T cell percentages in gut-associated lymphoid tissue(Reference Ramiro-Puig, Pérez-Cano and Ramos-Romero76). These results are consistent with the effects of apple polyphenol intake in healthy mice(Reference Akiyama, Sato and Watanabe77). Intestinal γδ T lymphocytes are mainly involved in innate immunity, where they have a noteworthy role, with participation in oral tolerance, mucosal tissue repair, and immunity against viral antigens and tumour cells(Reference Boismenu78–Reference Born, Reardon and O'Brien80). In murine models of food allergy, apple polyphenols prevented the development of oral sensitisation, and this inhibition correlated with a rise in the intestinal γδ T cell population(Reference Akiyama, Sato and Watanabe77). Taken together, these results suggest that certain diets rich in flavonoids from cocoa or other sources may increase γδ T cell functionality. This finding could be especially important during childhood when the immune system is maturing(Reference Perez-Cano, Castellote and Marin-Gallen81).
On the other hand, a diet containing 10 % cocoa during 3 weeks seems to produce a relative reduction of Th cells in secondary lymphoid tissues(Reference Ramiro-Puig, Pérez-Cano and Ramírez-Santana74, Reference Ramiro-Puig, Pérez-Cano and Ramos-Romero76). This finding appears to contrast with the impact on thymic tissue, in which cocoa intake promotes Th maturation(Reference Ramiro-Puig, Urpi-Sarda and Perez-Cano50). These contradictory findings may result from a reduction in thymic Th mobility or an increase in maturation speed, leading to a short life of these lymphocytes. The effects of cocoa on Th cell percentage also suggest an influence on the proliferation rate of these cells. In vitro studies have shown that cocoa inhibits Th activation(Reference Ramiro, Franch and Castellote65); this might explain the smaller percentage of Th cells in lymphoid organs. However, research in the proliferative response and secretion of IL-2 (the main cytokine involved in Th proliferation) in the spleen and mesenteric lymph nodes of rats fed high doses of cocoa has not shown any reduction(Reference Ramiro-Puig, Pérez-Cano and Ramírez-Santana74, Reference Ramiro-Puig, Pérez-Cano and Ramos-Romero76). Because the results of these studies were expressed as relative percentages, it is conceivable that the decrease in Th percentage in spleen and gut-associated lymphoid tissue may be due to an increase in the absolute number of other lymphocytes, such as B cells. Nonetheless, the ability of these cells to secret antibodies is down-regulated in rats fed a high-cocoa diet, as was reflected by lower plasma IgG, IgM and IgA levels(Reference Ramiro-Puig, Pérez-Cano and Ramírez-Santana74) and gut secretory IgA and secretory IgM(Reference Ramiro-Puig, Pérez-Cano and Ramos-Romero76) (Fig. 4). This effect cannot be directly related to a decrease of Ig-secreting cells(Reference Ramiro-Puig, Pérez-Cano and Ramírez-Santana74, Reference Ramiro-Puig, Pérez-Cano and Ramos-Romero76); instead, it could be the result of down-regulation of B cell differentiation caused by the decrease in Th2 cytokines, including IL-4(Reference Ramiro-Puig, Pérez-Cano and Ramírez-Santana74, Reference Ramiro-Puig, Pérez-Cano and Ramos-Romero76).

Fig. 4 Effect of cocoa-enriched diet on serum IgG, IgM and IgA levels (a) and on secretory IgA (S-IgA) and secretory IgM (S-IgM) obtained from small intestine lavage (b). (□), Reference group (n 10–18); (▧), 10 % cocoa-enriched diet group (n 10–18). Values are means, with standard errors represented by vertical bars. * Mean value was significantly different from that of the reference group (P < 0·05). Adapted from Ramiro-Puig et al. (Reference Ramiro-Puig, Pérez-Cano and Ramírez-Santana74, Reference Ramiro-Puig, Pérez-Cano and Ramos-Romero76).
A decrease in IL-4 secretion was detected in the spleen and mesenteric lymph nodes of rats fed a 10 % cocoa diet(Reference Ramiro-Puig, Pérez-Cano and Ramírez-Santana74, Reference Ramiro-Puig, Pérez-Cano and Ramos-Romero76). These results suggest that intake of high doses of cocoa in young rats can favour the Th1 response, in contrast to what has been seen in in vitro studies(Reference Ramiro, Franch and Castellote60). The reason for these contradictory results may reside in the differing compounds that reach lymphocytes in in vitro studies, in which cells are directly incubated with cocoa, and in vivo studies, in which cells take up absorbed and metabolised cocoa derivatives. Moreover, in vitro studies use a polyphenol-concentrated cocoa extract, whereas cocoa powder contains other compounds with immunomodulatory properties, such as fibre and lipids(Reference Schley and Field82, Reference Yaqoob83).
The effect of a diet containing 10 % cocoa on the ovalbumin-specific immune response has also been investigated(Reference Pérez-Berezo, Ramiro-Puig and Pérez-Cano84). A cocoa diet started at weaning and maintained throughout the study down-modulated ovalbumin-specific antibody levels of IgG1 (the main subclass associated with the Th2 immune response in rats), IgG2a, IgG2c and IgM isotypes, but led to higher levels of anti-ovalbumin IgG2b antibodies (the subclass linked to the Th1 response). Spleen cells from cocoa-fed animals have shown decreased IL-4 secretion (main Th2 cytokine), and lymph node cells from the same rats displayed increased interferon γ secretion (main Th1 cytokine). Therefore, a cocoa diet attenuates antibody synthesis, and this may be attributable to specific down-regulation of the Th2 immune response. Because IL-4 also induces IgE up-regulation and increases intestinal permeability(Reference Colgan, Resnick and Parkos85), IL-4 down-regulation together with the γδ T cell increase induced by cocoa diet may be beneficial to promote intestinal innate immunity and to reduce certain states of hypersensitivity, such as food allergies, conditions characterised by an immune response against innocuous food antigens and a high IgE response.
In conclusion, a high cocoa intake modulates immune cell function in rats and affects both the intestinal and systemic compartments. Flavonoids seem to be the best candidates as the source of these immune effects; however, other compounds present in cocoa, such as fibre and lipids, should also be taken into account in future studies. To date, evidence of cocoa's immunoregulatory activity has been documented in experimental animal studies. It is difficult to extrapolate these results to human consumption because of the differences in metabolism. However, if flavonoid content were responsible for the immunomodulatory effects, a useful goal for the future could be the design of cocoa formulations with a higher flavonoid content, as has been recently reported(Reference Tomas-Barberan, Cienfuegos-Jovellanos and Marín86).
Are the antioxidant properties of cocoa responsible for its immunoregulatory role?
The exact mechanism by which cocoa modulates innate and acquired immune functions remains unclear. As was indicated above, cocoa is a rich source of flavonoid antioxidants, which might promote changes in redox-sensitive signalling pathways involved in the expression of many genes and, consequently, in several cell functions, such as the immune response.
In macrophages and lymphocytes, cocoa compounds can target transcription factors, such as NF-κB which is redox-sensitive and triggers expression of over 100 genes, many of them involved in the immune response(Reference Pantano, Reynaert and van der Vliet87). NF-κB is found in the cytoplasm of non-stimulated cells bound to κB inhibitor proteins (IκB). Upon cellular stimulation, IκB is phosphorylated by the serine-specific kinase, inhibitor of κB kinase (IKK), allowing NF-κB to translocate to the nucleus where it is reduced to initiate transcription of cellular genes(Reference Nakamura, Nakamura and Yodoi88). Other redox-sensitive kinases, including mitogen extracellular signal-regulated kinase kinase 1 (MEKK-1), protein kinase B (PKB or AKT)/phosphatidylinositol 3-kinase (PI-3-K) and c-Jun N-terminal kinase (JNK), can affect NF-kB activation. Monomeric flavonoids present in cocoa, such as epicatechin, catechin and quercetin, are known to inhibit the NF-κB pathway and decrease TNF-α and NO production in stimulated macrophages(Reference Park, Rimbach and Saliou89, Reference Comalada, Camuesco and Sierra90). Mackenzie et al. (Reference Mackenzie, Carrasquedo and Delfino61) shed light on the regulatory role of cocoa flavonoids on the NF-κB pathway. Epicatechin, catechin and B dimeric procyanidins can act at different stages of NF-κB activation: at early stages, accumulated flavonoids in the cytosol regulate oxidant levels and reduce IKK phosphorylation, and at later stages, flavonoids – mainly dimeric procyanidins, which penetrate the nuclei – selectively prevent NF-κB binding to its consensus sequence(Reference Mackenzie, Carrasquedo and Delfino61). More recently, Kang et al. (Reference Kang, Lee and Lee91) showed that cocoa procyanidins inhibit the kinase activity of mitogen extracellular signal-regulated kinase 1 (MEK1), thus attenuating activation of NF-κB and activator protein-1 (AP-1). These results support an inhibitory effect of cocoa on cytokine production by interacting with NF-κB activation. In addition, apart from the effects on NF-κB, cocoa flavonoids may also have an influence on other transcription factors involved in cytokine production, such as AP-1(Reference Kang, Lee and Lee91) and signal transducer and activator of transcription-4 (STAT4)(Reference Muthian and Bright92).
This evidence is in keeping with the statement that the antioxidant properties of cocoa are responsible for its immunoregulatory role, but it also shows that certain cocoa flavonoids can directly interact with cell signalling and gene expression factors. Therefore, antioxidant-independent mechanisms must also be considered to better understand the effects of cocoa in vivo. In addition, future mechanistic studies should look into the specific metabolites of cocoa that interact with cells in vivo and determine the physiological concentrations of these metabolites after normal cocoa intake.
Conclusions
There is an increasing interest in food compounds that can promote health and reduce the risk of disease. Because of its antioxidant activity, mainly attributed to flavonoids, cocoa is currently attracting considerable attention in this regard. The health benefits of cocoa in reducing cardiovascular risk are emerging. In addition, the influence of whole cocoa and cocoa flavonoids on the immune system is gaining recognition, with the work on this subject being reviewed in the present paper. Cocoa has been shown to have an effect on innate and acquired immune function. Various in vitro studies have attributed down-regulation of the inflammatory response to cocoa compounds. However, more in vivo approaches investigating this anti-inflammatory effect are needed to estimate the true impact of cocoa in this respect.
Cocoa has shown regulatory effects on the acquired immune response in both in vitro and in vivo experiments. In rats, high cocoa intake modulates intestinal and systemic immune cell functionality. Because immune cell function is controlled by redox-sensitive pathways, flavonoids, which are potent antioxidant compounds, seem to be the best candidates as the source of cocoa's beneficial effects. In addition, there is some evidence that certain cocoa flavonoids can directly interact with cell signalling and gene expression factors. Further research is needed to shed light on the interactions between cocoa and cell physiology, contributing thus to the body of knowledge of the effects of food compounds on health.
Acknowledgements
E. R. and M. C. had equal intellectual input and contributed equally to the writing of the manuscript. E. R. is the recipient of a postdoctoral fellowship from the Spanish Ministerio de Educación y Ciencia; M. C. is a professor at the University of Barcelona. The present study was supported by the Ministerio de Ciencia y Tecnología (AGL2005-002823) and the Generalitat de Catalunya (SGR2005-0083).
Both authors declare no conflict of interest.









