Dairy farming is known to contribute to both atmospheric and hydrospheric pollution( Reference Tamminga 1 ). In spite of improved diet formulation systems and genetic potential of cows, N efficiency for milk protein production has remained relatively low, averaging 25–28 % in North American and North European dairy cow trials( Reference Huhtanen and Hristov 2 ). The reduction in the ruminal degradability of crude protein (CP) is often considered as a strategy to improve N efficiency in milk production. Although a theoretically sound concept, the effects of ruminal CP degradability on milk protein yield and milk N efficiency (milk N/N intake) have been small( Reference Huhtanen and Hristov 2 , Reference Ipharraguerre and Clark 3 ). This can, at least partly, be related to the in situ determinations of protein degradation that can be flawed because of several inherent problems of the in situ technique, including (1) the assumption that the soluble non-NH3-N (SNAN) fractions are completely degraded, (2) the physical restriction of feeds within the bag from microbial interaction and digestion and (3) the imprecise quantification of microbial contamination of the undigested residues( Reference Broderick, Cochran, Theodorou and France 4 ).
The results from studies using rumen digesta kinetics methodology( Reference Chen, Sniffen and Russell 5 – Reference Volden, Mydland and Olaisen 7 ) or omasal sampling( Reference Choi, Ahvenjärvi and Vanhatalo 8 – Reference Reynal, Ipharraguerre and Lineiro 10 ) have indicated a substantial escape of dietary soluble amino acids from ruminal degradation. In addition to the escape of SNAN fractions from ruminal degradation, secondary particle loss from the in situ bags can contribute to the bias of rumen-undegraded protein (RUP) estimates. The use of compartmental flux method, defined as flow divided by pool, is an in vivo alternative to predict the escape of particle-associated N from the rumen. In this method, the flux of an indigestible entity in a particle-size fraction is divided by the ruminal pool of the indigestible entity in that fraction, giving passage rate estimates. The feed N outflow in a fraction is then calculated from the mass of N multiplied by the passage rate of the fraction.
In vivo flow evaluations have shown consistently greater RUP flow for red clover silages than for grass( Reference Dewhurst, Evans and Scollan 11 – Reference Vanhatalo, Gäddnäs and Heikkilä 13 ) or lucerne silages( Reference Brito, Broderick and Olmos Colmenero 14 ), whereas ruminal in situ data do not always support lower ruminal CP degradability of red clover silages compared with grass silages( Reference Dewhurst, Evans and Scollan 11 ). The objectives of the present study were to compare the estimates of feed N outflow from the rumen estimated using the omasal sampling technique, the compartmental flux method and the ruminal in situ incubations, and to evaluate whether the methods ranked similarly different forage types. The diets used in the present study were based on grass and red clover silages harvested at two stages of maturity. As these plant species are usually grown as mixed swards, an additional diet comprising a 1:1 mixture of late-cut grass silage and early-cut red clover silage was used in the study.
Materials and methods
Animals, diets and experimental design
All experimental procedures were approved by the Animal Experiment Committee of MTT Agrifood Research Finland in accordance with the Use of Vertebrates for Scientific Purposes Act 1985. The animals used for the in situ incubations of the feed samples were registered and cared for according to the guidelines approved by the University Animal Care and Use Committee, and the incubations were carried out in accordance with the laws and regulations controlling experiments performed with live animals in Sweden. The details of animals and diets and most of the experimental procedures have been described in more detail elsewhere( Reference Vanhatalo, Kuoppala and Ahvenjärvi 15 ). In brief, five ruminally fistulated multiparous Finnish Ayrshire dairy cows in early lactation with a body weight of 620 (sd 71·3) kg were used in a 5 × 5 Latin square design with 21 d periods. Experimental silages were prepared from the primary growths of timothy (Phleum pratense) and meadow fescue (Festuca pratensis) mixtures (GS) and red clover (Trifolium pratense; RCS) swards grown at Jokioinen, Finland (60°49′N, 23°28′E). During sowing, the seed mixture used for grass sward contained 54 % of timothy and 46 % of meadow fescue. The silages were harvested at two stages of maturity; grasses on 17 (early) or 26 (late) June and red clovers on 2 (early) or 16 (late) July. The four pure silages (early-harvested grass, late-harvested grass, early-harvested red clover and late-harvested red clover) and a mixture of late-harvested grass and early-harvested red clover (1:1 on a DM basis) were fed ad libitum to the cows during days 1 to 15 of each experimental period and restricted to 0·95 of the total intake during sampling days 16 to 21. The cows were fed 9·0 kg/d of a concentrate supplement that was formulated from barley (405 g/kg), oats (400 g/kg), rapeseed expeller meal (160 g/kg) and mineral mixture (35 g/kg). The cows were fed four times daily at 06.00, 09.00, 18.00 and 20.00 hours.
Omasal sampling technique
Digesta flow was assessed by the omasal sampling technique( Reference Ahvenjärvi, Vanhatalo and Huhtanen 16 ) using a triple-marker method( Reference France and Siddons 17 ). Indigestible neutral-detergent fibre (iNDF), Yb-acetate and Cr-EDTA were used as markers for large particles, small particles and fluid phase, respectively. A total of twelve omasal spot samples (500 ml) were collected during 4 d, so that each hour during the daytime, the feeding cycle was sampled. The fractionation of omasal samples into large particles, small particles and fluid phase was done as described by Ahvenjärvi et al. ( Reference Ahvenjärvi, Vanhatalo and Huhtanen 18 ). The determination of microbial N flow using 15N as a marker and the chemical analysis of the feed and faecal samples were carried out as described by Ahvenjärvi et al. ( Reference Ahvenjärvi, Vanhatalo and Huhtanen 18 ). Feed N flow from the rumen was calculated as a difference between non-NH3-N and microbial N flow without any adjustments for endogenous N.
Compartmental flux method
The passage rate of ruminal particle-size fractions was estimated by dividing the flux (faecal output of iNDF of the particle-size fraction) by the ruminal pool of the corresponding iNDF fraction. By determining the corresponding N pool, the flux of N can be calculated as the mass of N in the particle-size fraction multiplied by the passage rate of the corresponding fraction. The details of rumen evacuation and sampling( Reference Kuoppala, Ahvenjärvi and Rinne 19 , Reference Kuoppala, Rinne and Ahvenjärvi 20 ) and sieving( Reference Bayat, Rinne and Kuoppala 21 ) have been described earlier. In brief, ruminal contents were evacuated before the morning feeding at 06.00 hours on day 13 and at 12.00 hours on day 15 of each experimental period. The average weight of the ruminal contents of the two evacuations was used as the estimation of the diurnal mean. Total faeces were collected from day 18 to day 21 of each experimental period, and samples were collected for further analyses. The particle-size distribution of the samples was determined using a Retsch AS200 Digit wet sieving apparatus (Retsch GmbH). The samples were divided into seven particle-size fractions by wet sieving using sieves with pore sizes of 2·5, 1·25, 0·63, 0·315, 0·16, 0·08 and 0·038 mm. The concentrations of iNDF in the ruminal and faecal particle fractions were determined using 12 d ruminal in situ incubation as described by Huhtanen et al. ( Reference Huhtanen, Kaustell and Jaakkola 22 ), except that bags with 17 μm pore size cloth (Swiss Silk Bolting Cloth Manufacturing Company Limited) were used and NDF residues were determined as ash free. Duplicate incubations were conducted in the rumen of two Ayrshire cows fed a grass silage-based diet supplemented with approximately 0·3 kg concentrate DM per kg total diet DM. CP concentration in the particle fractions was determined by the Dumas method using a Leco FP 428 N analyser (Leco Corporation). Compartmental N flow (g/d) was calculated using the compartmental flux method and the following equation:
where DMPool is the rumen DM pool (kg) of the ith particle-size fraction, CP is the CP concentration (g/kg DM) of the ith particle-size fraction, FiNDF is the faecal iNDF output (g/d) of the ith particle-size fraction and RiNDF is the rumen iNDF pool (g) of the ith particle-size fraction, i= 1 to 6 corresponding to the following particle-size ranges: 0·038–0·08; 0·08–0·16; 0·16–0·315; 0·315–0·63; 0·63–1·25; >1·25 mm, respectively. As the proportions of 1·25–2·5 mm ruminal particles and >2·5 mm faecal particles were small (on average, 1·2 and 4·8 % of the particulate matter, respectively), these two fractions were combined into one pool. Faecal iNDF pools were corrected for the complete recovery of dietary iNDF in the faeces and the ruminal pools for the complete recovery of NDF (i.e. sum of NDF pools = total NDF pool determined as rumen DM pool × NDF concentration).
In situ method
A total of three non-lactating Swedish Red cows were fed a diet consisting of grass silage and supplemented with approximately 0·20 kg concentrate DM (Solid 220; Lantmännen Lantbruk AB) per kg total diet DM to meet the maintenance requirements. The cows were fed silage on four occasions daily, and they had free access to barley straw throughout the day.
The ruminal disappearance of CP in the concentrate and forage samples was determined using the in situ method. The feed samples were dried at 60°C for 48 h and ground through a 1·0 mm screen before the in situ incubations. Samples of 2·0 g were weighed into nylon bags with a pore size of 38 μm and a pore area equal to 31 % of the total surface area (Saatifil PES 38/31; Saatitech S.p.A.). The internal dimensions of the nylon bags and sample size were adjusted to give a sample size:surface area ratio of 10 mg/cm2. All the bags were pre-soaked in tap water (approximately 37°C) before being placed in the rumen. For 24–96 h incubations (24, 36, 48, 72 and 96 h), the nylon bags were introduced in a reverse sequence into the rumen of each cow at 07·00 hours and taken out simultaneously. The bags incubated for 3, 6, 12 and 24 h were inserted simultaneously. The concentrate feeds were not incubated in the rumen for more than 48 h. After removal, the bags were immediately rinsed in a household washing machine (the rinsing part of the wool wash programme including washing three times for 2·5 min/washing (including the time for the filling of water) using approximately 8°C water; Electrolux Wascator W75MP; AB Electrolux), dried for 48 h at 60°C and weighed. The in situ residues were analysed for CP as described earlier. Later, 0 h samples of all the feeds were soaked in tap water at approximately 37°C and rinsed in cold water using the household washing machine following the same procedures as those followed for the other bags.
The rate and extent of in situ CP degradation were evaluated using the simple model described by Ørskov & McDonald( Reference Ørskov and McDonald 23 ):
where D (t) is the fraction of CP degraded at time t of incubation (g/kg CP), a is the intercept representing the portion of CP that is soluble and disappeared at the initiation of the incubation (time = 0), b is the potentially degradable CP fraction (g/kg CP), k d is the fractional rate of digestion of fraction b (1/h) and t is the time. The effective degradability of CP (EDP) was calculated using the equation proposed by Ørskov & McDonald( Reference Ørskov and McDonald 23 ):
where a, b and k d are as described previously and k p is the fractional passage rate from the rumen (1/h). The fractional passage rates of forages and concentrates were calculated according to the method of Krizsan et al. ( Reference Krizsan, Ahvenjärvi and Huhtanen 24 ), and the passage rate of concentrate feeds was assumed to be 1·6 times greater than that of forages( Reference Danfær, Huhtanen, Udén, Kebreab, Dijkstra, Bannink, Gerrits and France 25 ). The flow of RUP (N flow) was calculated from the forage and concentrate CP intake by subtracting the amounts of degraded CP.
Calculations and statistical analysis
The true digestibility of N of different particle-size fractions in the lower digestive tract was estimated using the Lucas test. The Lucas test( 26 ) identifies ideal nutritional entities that have uniform digestibility by plotting the digestible nutrient concentration in DM against the nutrient concentration in DM. The slope of regression provides an estimate of true digestibility, and the intercept is an estimate of the metabolic and endogenous faecal output for the nutrient.
Data were subjected to ANOVA for a 5 × 5 Latin square design using the MIXED procedure( Reference Littell, Henry and Ammerman 27 ) using cow and period as random factors. The total feed N flow and ruminal CP degradability data were analysed using the following mixed model:
where C, P, D and M are the effects of cow (random), period (fixed), diet (fixed) and method (fixed). Method was a repeated measurement. Because of a significant diet × method interaction, the data were analysed separately for each method using mixed-model ANOVA for the 5 × 5 Latin square design. The data from one cow fed the mixed diet during the first period were lost due to digestive disorders. Orthogonal contrasts were used to compare the effects of forage species (grass v. red clover), stage of maturity (early v. late harvest) and interactions between forage species and maturity. In addition, the non-additivity of mixed forage was evaluated by a pre-planned non-orthogonal contrast to compare the mixed forage (late grass+early red clover) with the mean of late-cut grass and early-cut red clover diets. In situ data were analysed as a randomised block design with animal as the block.
Results
Composition of experimental feeds and ruminal and faecal particulate matter
The composition of experimental silages and dietary concentrate and the ruminal and faecal particle-size distributions are given in Tables 1 and 2, respectively. The proportions of 0·038–0·08 and < 0·038 mm ruminal particles were greater (P< 0·01) for the RCS diets than for the GS diets at the expense of the 0·08–0·16, 0·16–0·315, 0·315–0·63 and >1·25 mm particles (P< 0·01). The proportion of faecal particles < 0·038 mm was greater (P< 0·01) at the expense of the 0·08–0·16, 0·16–0·315 and 0·315–0·63 mm faecal particles (P< 0·05) when the cows were fed the RCS diets than when fed the GS diets. Generally, there were fewer effects due to maturity compared with those due to forage type on ruminal and faecal particle-size distributions, with the major difference being the smaller (P< 0·01) proportion of < 0·038 mm particles in the late-harvested silages compared with the early-harvested silages.
Table 1 Chemical composition of the experimental silages harvested at early- and late-growth stages and dietary concentrate*
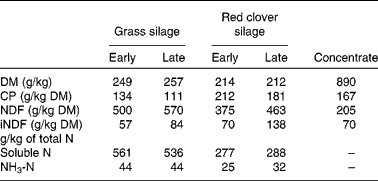
CP, crude protein; NDF, neutral-detergent fibre; iNDF, indigestible neutral-detergent fibre.
* Adopted from Vanhatalo et al. ( Reference Vanhatalo, Kuoppala and Ahvenjärvi 15 ).
Table 2 Ruminal and faecal particle-size distribution (g/kg DM) in cows fed diets containing grass or red clover silages harvested at early- or late-growth stages

* Probability of a significant effect of grass v. red clover (S); early- v. late-harvest time (M); interaction of S and M (S× M); mixture of the late-harvested grass silage and the early-harvested red clover silage v. calculated average response when these silages were fed separately (Mix).
† Growth stage.
‡ Mixture of the late-harvested grass silage and the early-harvested red clover silage (1:1 DM basis).
§ Standard error of means, df = 11. sem of the Mix treatment was 1·15 times greater than the reported value due to a missing observation.
CP concentration in rumen particulate matter was generally reduced with increasing particle size, and it was higher (P< 0·01) for the RCS diets than for the GS diets (Table 3). In particles most eligible for passage (0·038–1·25 mm), the average CP concentrations for the GS and RCS diets were 128 v. 175 g/kg DM (P <0·01), respectively. The effects of maturity on CP concentration in rumen particulate matter were generally small, although sometimes statistically significant. CP concentration in faecal particles was also inversely related to particle size, except for the particles >1·25 mm, which had a higher CP concentration than the particles between 0·63 and 1·25 mm. Except for particles between 0·038 and 0·08 mm, CP concentration was higher (P≤ 0·06) in cows fed the RCS diets than in those fed the GS diets.
Table 3 Crude protein concentrations (g/kg DM) in ruminal and faecal particle-size fractions in cows fed diets containing grass or red clover silages harvested at early- or late-growth stages

S, grass v. red clover; M, early- v. late-harvest time (maturity); S× M, interaction of plant species and forage maturity; Mix, mixture of the late-harvested grass silage and the early-harvested red clover silage v. feeding these silages separately.
* Growth stage.
† Mixture of the late-harvested grass silage and the early-harvested red clover silage (1:1 DM basis).
‡ Standard error of means, df = 11. sem of the Mix treatment was 1·15 times greater than the reported value due to a missing observation.
Estimates of ruminal feed nitrogen outflow by the compartmental flux method
Calculated N flow from the rumen for different particle-size fractions is presented in Table 4. The flow was significantly greater for the RCS diets than for the GS diets for the 0·038–0·08 mm particles, particles most eligible for passage (0·038–1·25 mm) and total particles (P ≤0·03), and it generally increased with advancing maturity at harvest (P≤ 0·13). Advancing maturity had a stronger influence on ruminal N outflow for the RCS diets than for the GS diets as indicated by the frequently significant interaction between diet and maturity (P= 0·02).
Table 4 Nitrogen flow (g/d) determined using the compartmental flux method in different particle-size fractions in cows fed diets containing grass or red clover silages harvested at early- or late-growth stages
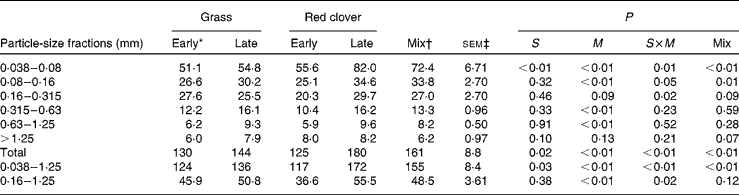
S, grass v. red clover; M, early- v. late-harvest time (maturity); S× M, interaction of plant species and forage maturity; Mix, mixture of the late-harvested grass silage and the early-harvested red clover silage v. feeding these silages separately.
* Growth stage.
† Mixture of the late-harvested grass silage and the early-harvested red clover silage (1:1 DM basis).
‡ Standard error of means, df = 11. sem of the Mix treatment was 1·15 times greater than the reported value due to a missing observation.
When the flow was expressed as a proportion of N intake, the values were generally significantly smaller (P< 0·01) for the RCS diets than for the GS diets, and they increased with advancing maturity of forages at harvest (P≤ 0·01; Table 5). The effects of maturity were marginally greater for the RCS diets than for the GS diets with a significant interaction for the particle-size fractions of 0·038–1·25 and >1·25 mm and a trend (P ≤0·10) for the 0·038–0·08 particles. Generally, the values for the mixture of late-harvested grass and early-harvested red clover did not differ from the mean of early-cut grass and late-cut red clover (P≥ 0·05).
Table 5 Nitrogen (N) flow (g/kg N intake) determined using the compartmental flux method in different particle-size fractions in cows fed diets containing grass or red clover silages harvested at early- or late-growth stages
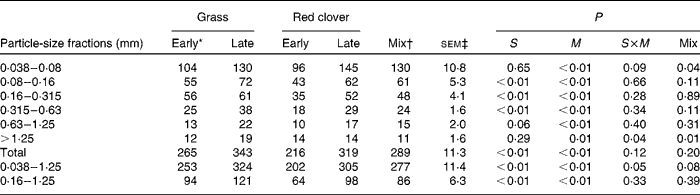
S, grass v. red clover; M, early- v. late-harvest time (maturity); S× M, interaction of plant species and forage maturity; Mix, mixture of the late-harvested grass silage and the early-harvested red clover silage v. feeding these silages separately.
* Growth stage.
† Mixture of the late-harvested grass silage and the early-harvested red clover silage (1:1 DM basis).
‡ Standard error of means, df = 11. SEM of the Mix treatment was 1·15 times greater than the reported value due to a missing observation.
The apparent post-ruminal digestibility of particle-associated CP decreased with increasing particle size, except for the 0·63–1·25 mm particles (Table 6). The post-ruminal digestibility of particle-associated CP in the 0·038–0·08, 0·08–0·16 and 0·63–1·25 mm particles and total particles was higher for the RCS diets than for the GS diets (P≤ 0·02). However, true post-ruminal CP digestibility was not influenced by the forage species or maturity of forages at harvest when estimated using the Lucas test (Fig. 1). True CP digestibility was 0·75 (se 0·042) and 0·90 (se 0·018) for all the particles and for the 0·038–0·08 mm particles, respectively. The data points for the GS and RCS diets were rather evenly distributed across the general regression line. The intercepts of the regression lines were negative (P= 0·03 and 0·09) for the 0·038–0·08 mm and total particles, indicating a post-ruminal metabolic contribution to faecal particle CP.
Table 6 Apparent digestibility of nitrogen of different particle-size fractions in the lower tract in cows fed diets containing grass or red clover silages harvested at early- or late-growth stages
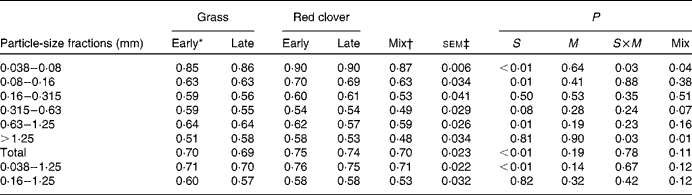
S, grass v. red clover; M, early- v. late-harvest time (maturity); S× M, interaction of plant species and forage maturity; Mix, mixture of the late-harvested grass silage and the early-harvested red clover silage v. feeding these silages separately.
* Growth stage.
† Mixture of the late-harvested grass silage and the early-harvested red clover silage (1:1 DM basis).
‡ Standard error of means, df = 11. sem of the Mix treatment was 1·15 times greater than the reported value due to a missing observation.
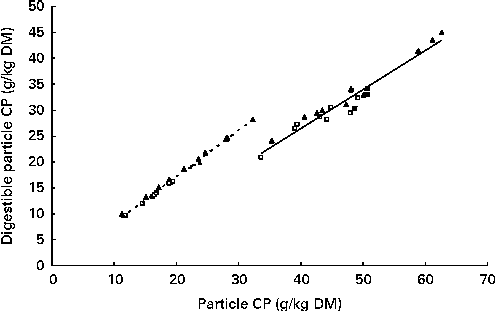
Fig. 1 Lucas test for particle-associated crude protein (CP). —, Total particle-associated CP, y= 0·896x− 0·81; R 2 0·992; root mean square error (RMSE) = 0·43. - - -, 0·038–0·08 mm particle-size fractions, y= 0·751x− 3·58; R 2 0·940; RMSE = 1·44. GS (□), grass silage; RCS (▲), red clover silage; Mix (■), a mixture of late-cut grass and early-cut red clover silage.
Estimates of ruminal feed nitrogen outflow by the in situ method
The immediately disappearing a fraction was significantly (P <0·01) smaller for the red clover silages than for the grass silages (Table 7), and consequently the b fraction was greater (P <0·01) for the red clover silages than for the grass silages. The rate of disappearance was faster (P <0·01) for the red clover silages than for the grass silages, but the differences between forage species in effective protein degradability were small. The a fraction and the degradation rate of the b fraction were higher (P <0·01) for the early-harvested silages than for the late-harvested silages, resulting in a significantly (P <0·01) higher effective protein degradability for the early-harvested silages than for the late-harvested silages (0·804 v. 0·713). The effective protein degradability of the concentrate mixture was 0·810.
Table 7 Effect of silage and maturity stage on in situ degradation characteristics (g/g unless otherwise stated) and effective protein degradability (EPD)* (g/g)
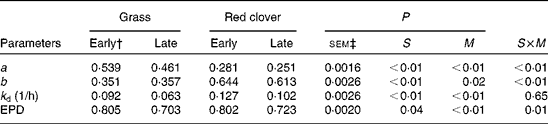
S, grass v. red clover; M, early- v. late-harvest time (maturity); S× M, interaction of plant species and forage maturity; a, intercept representing the portion of crude protein that is soluble and disappeared at the initiation of rumen incubation; b, potentially degradable crude protein fraction; k d, fractional rate of digestion of fraction b (1/h).
* EPD calculated according to the method of Ørskov & McDonald( Reference Ørskov and McDonald 23 ).
† Growth stage.
‡ Standard error of means, df = 11.
The pattern of changes in CP concentration in undegraded in situ residues was different between the red clover silages and grass silages (Fig. 2). In the grass silages, CP concentration was clearly reduced after 3 h of incubation, whereas in the red clover silages, the reduction of CP concentration in undegraded residues took place between 6 and 12 h of incubation.
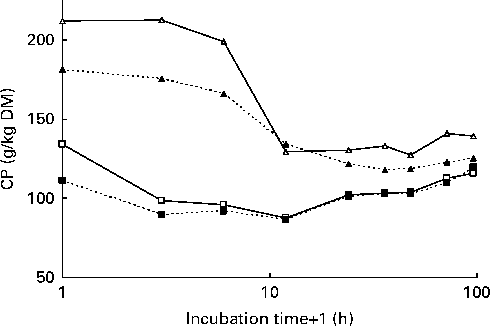
Fig. 2 Crude protein (CP) concentration in the undegraded residues of grass silage harvested at an early stage of maturity (GE, ![]() ) or a late stage of maturity (GL,
) or a late stage of maturity (GL, ![]() ) and red clover silage harvested at an early stage of maturity (RCE,
) and red clover silage harvested at an early stage of maturity (RCE, ![]() ) and a late stage of maturity (RCL,
) and a late stage of maturity (RCL, ![]() ) after different in situ incubation times in the rumen (x-axis is in the logarithmic scale).
) after different in situ incubation times in the rumen (x-axis is in the logarithmic scale).
Comparison of the methods
The mean feed N flow across the diets averaged 177, 108 and 149 g/d (P< 0·01) for the omasal sampling, in situ and compartmental flux methods, respectively. The interaction between diet and method was also significant (P< 0·01). The differences in ruminal CP degradability reflected a similar significant (P< 0·01) pattern (0·664, 0·793 and 0·710, respectively), and the interaction between diet and method was also significant (P< 0·01).
Because of a significant interaction between diet and method, the data were analysed separately for each method. Feed N flow determined using the omasal sampling technique was greater (P< 0·01) in cows fed the RCS diets than in those fed the GS diets, and it tended (P= 0·11) to decrease with advancing maturity at harvest (Table 8). Feed N flow was also greater (P< 0·01) for the RCS diets than for the GS diets, and it increased (P< 0·01) with advancing maturity at harvest when determined using either the in situ or compartmental flux method.
Table 8 Feed nitrogen flow (g/d) estimated using the omasal sampling (in vivo), in situ or compartmental flux technique

S, grass v. red clover; M, early- v. late-harvest time (maturity); S× M, interaction of plant species and forage maturity; Mix, mixture of the late-harvested grass silage and the early-harvested red clover silage v. feeding these silages separately.
* Growth stage.
† Mixture of the late-harvested grass silage and the early-harvested red clover silage (1:1 DM basis).
‡ Standard error of means, df = 11. sem of the Mix treatment was 1·15 times greater than the reported value due to a missing observation.
There were considerable differences between the methods with regard to the estimates of feed N flow. The difference between the RCS and GS diets was markedly greater (P< 0·01) when determined using the omasal sampling method than when determined using the in situ method (87 v. 44 g/d), and the difference was greater (P <0·01) for the early-harvested silages than for the late-harvested silages (86 v. 46 g/d). The difference between the feed N flow estimates based on the omasal sampling and compartmental flux methods was greater (P <0·01) for the RCS diets than for the GS diets (53 v. 1 g/d) and greater (P <0·01) for the diets based on the early-harvested silages than for those based on the late-harvested silages (55 v. − 1 g/d). Feed N flow determined using the omasal sampling technique was 22 g/d greater for the early- v. late-harvested silages, whereas it was 35 and 18 g/d greater for the late- v. early-harvested silages when determined using the compartmental flux and in situ methods, respectively.
Both in situ and compartmental flux methods resulted in greater (P< 0·01) ruminal degradability of feed N for the RCS diets than for the GS diets, whereas the reverse was true for the omasal flow estimates. Ruminal N degradability decreased with advancing maturity of forages at harvest when determined using either the in situ or compartmental flux method, whereas no significant differences were observed when it was determined using the omasal sampling method.
Discussion
Extensive ruminal degradation of CP in ensiled forages has been suggested to impair N utilisation in dairy cows. Red clover has a polyphenol oxidase enzyme that produces protein–quinone complexes that are resistant to degradation in the rumen( Reference Lee, Scott and Tweed 28 ). There is also clear evidence that condensed tannins influence ruminal N metabolism( 29 ). However, the effects of reduced ruminal CP degradability on milk production and N efficiency in lactating dairy cows have been inconsistent. Inconsistent effects of an increased supply of RUP from forages can be due to other nutrients being more limiting, poor quality (amino acid profile and digestibility) of forage RUP and/or the estimates of RUP being biased.
In the present study, we compared feed N flow estimated using the omasal sampling technique, ruminal in situ incubation and a novel method based on compartmental flux. The in situ method with well-known shortcomings( Reference Noziere and Michalet-Doreau 30 ) is a standard method used in most feed protein evaluation systems for estimating protein degradability. The omasal sampling technique( Reference Ahvenjärvi, Vanhatalo and Huhtanen 18 , Reference Huhtanen, Brotz and Satter 31 ) has some advantages compared with the duodenal sampling method as only rumen cannulas are required. In addition, the contribution of endogenous N to N flow is less, and the sampling is carried out before the hydrolysis in the abomasum commences. Digesta flow was determined using a triple-marker method that reduces random variation resulting from unrepresentative sampling. The method is laborious and cannot be used for routine feed evaluation, but it can serve as an in vivo reference method. The compartmental flux method is also laborious, but it can be useful for the evaluation of the strengths and weaknesses of the other methods. It was assumed that the probability of a particle-size fraction to escape out of the rumen is only related to size and not, for example, to specific gravity( Reference Ahvenjärvi, Skiba and Huhtanen 32 ). For dairy cows fed ad libitum, this assumption is likely to be true, since the iNDF concentrations in the particle-size fractions were not related to sampling site in the reticulorumen( Reference Ahvenjärvi, Skiba and Huhtanen 32 ). The limited variation between adjacent sampling sites (dorsal sac, ventral sac and reticulum) with regard to chemical composition within each sieve size compared with the large variation between sieve size fractions within a sampling site tentatively supports this suggestion. Unless young recently ingested particles escaped, the effects of the age of particles on N flow in each particle-size fraction would be rather small based on relatively small changes in CP concentration in undegraded in situ residues after 12 h of incubation.
Comparison of the omasal sampling and in situ techniques
Consistently with other studies that have compared grass and red clover silages, in the present study, omasal N flow was greater and ruminal CP degradability was lower for the red clover silage diets than for the grass silage diets( Reference Dewhurst, Evans and Scollan 11 – Reference Vanhatalo, Gäddnäs and Heikkilä 13 ). In contrast, there was no difference in the in situ CP degradability between the forage types, in agreement with the comparison of red clover and grass silages( Reference Dewhurst, Evans and Scollan 11 ) or dried red clover and grass( Reference Hoffman, Sievert and Shaver 33 ). The greater in situ feed N flow for the RCS diets than for the GS diets resulted from the greater CP concentration in red clover. In situ data predicted increased feed N flow with advancing maturity, whereas an opposite trend was found for the in vivo omasal data. In situ studies have consistently demonstrated reduced CP degradability with advancing maturity of forages( Reference Hoffman, Sievert and Shaver 33 – Reference Yan and Agnew 35 ), whereas the data from in vivo omasal flow studies are inconclusive (present study; Kuoppala et al. ( Reference Kuoppala, Ahvenjärvi and Rinne 19 )).
In addition to interactions between method and diet for feed N flow, in vivo omasal values were much greater (on average, 70 g/d) than in situ values. Omasal values could have been overestimated because no discounts were made for the endogenous contribution while calculating feed N flow. Endogenous non-NH3-N flow to the omasum would be 11 g/d, assuming 0·085 g endogenous N/kg of body weight (BW)0·75 ( Reference Ørskov, MacLeod and Kyle 36 ), or 40 g/d, assuming 2 g endogenous N/kg of DM intake( Reference Hart and Leibholz 37 ). The former is derived using intragastric infusion of protein-free nutrients and the latter by determining omasal non-NH3-N flow on a RUP-free diet using 15N as the microbial marker. Even with the higher estimate of endogenous N, omasal feed N flow was still about 30 g/d greater than the estimates based on the in situ method. On the other hand, microbial contamination of undegraded feed residues( Reference Varvikko and Lindberg 38 ) leads to the overestimation of feed N flow determined using the in situ technique. Assuming an underestimation of ruminal CP degradability by 0·04 units according to Rodríguez & González( Reference Rodríguez and González 39 ) feed N flow was overestimated by 21 g/d (mean N intake 522 g/d). They estimated microbial N contamination of the residues using intraruminal infusions of 15N. The results from in situ studies using 15N-labelled feeds( Reference Varvikko and Lindberg 38 ) suggested even greater microbial contribution. After 24 h of incubation, the proportions of microbial N in undegraded residues were 0·52, 0·58 and 0·11 for ryegrass, barley and rapeseed meal, respectively. Wanderley et al. ( Reference Wanderley, Tuber and Wu 40 ) reported corresponding proportions of 0·19 and 0·47 for maize grain and lucerne, respectively. The extent of microbial contamination is positively related to NDF concentration and negatively to CP concentration( 41 ), suggesting that quantitatively it can be greater for grass silages than for red clover silages. Increasing CP concentration of undegraded feed residues after 12 h of rumen incubation with grass samples rather than with red clover silage samples may support this interpretation in the present study.
Taking into account both endogenous N and microbial contamination, omasal feed N flow would still be at least 50 g/d greater than the estimates based on the in situ technique. The SNAN in the liquid phase is the most likely explanation for this discrepancy. The commonly used Ørskov & McDonald( Reference Ørskov and McDonald 23 ) model assumes that the immediately disappearing proteins, peptides and amino acids in the soluble fraction are degraded at an infinite rate with no escape. There is extensive literature demonstrating that this assumption may not be valid. Chen et al. ( Reference Chen, Sniffen and Russell 5 ) determined the flow of soluble proteins and peptides from rumen fluid kinetics. In their three experiments (in total nine diets), the total flow of these SNAN fractions was, on average, 61 g/kg N intake. Using the omasal sampling technique, Choi et al. ( Reference Choi, Ahvenjärvi and Vanhatalo 8 , Reference Choi, Vanhatalo and Ahvenjärvi 9 ) reported average SNAN flows of 68 and 54 g/kg N intake for four and five diets, respectively. Reynal et al. ( Reference Reynal, Ipharraguerre and Lineiro 10 ) carried out a detailed analysis of different SNAN fractions in omasal digesta and concluded that approximately in total 100 g/kg total amino acid flow escaped as different SNAN fractions (64 g/kg N intake). The average value of SNAN flow from these studies (64 g/kg N intake, se= 2·8, n 22 diets) would correspond to an average of 33 g SNAN flow per d in the present study. Hristov & Broderick( Reference Hristov and Broderick 6 ) observed that 30 % of the feed non-NH3-N that escaped the rumen flowed with the fluid phase.
The passage rate estimates used to calculate in situ CP degradability were based on k p of iNDF derived from rumen evacuation( Reference Krizsan, Ahvenjärvi and Huhtanen 24 ). It could be argued that iNDF does not describe the k p of different insoluble protein fractions. Solubilised protein fractions and small particles escaping from the bags are assumed to be completely degraded as discussed above, but if the k p of some insoluble protein fractions is faster than that of iNDF, ruminal CP degradability values would be underestimated. However, this means that these protein fractions should be physically separated from the cell-wall fraction. On the other hand, we used a one-compartment rumen model that underestimates the degradability for calculating CP degradability compared with a two-compartment rumen model with the same ruminal mean retention time. The excretion curves of 15N-labelled fibre-bound N clearly indicate the selective retention of forage protein in the rumen( Reference Huhtanen and Hristov 42 ).
The greater difference between the omasal and in situ feed N flow for the early-harvested silages than for the late-harvested silages can partly be explained by the greater microbial contamination of the in situ residues with late-harvested silages. However, the greater differences between the omasal sampling technique and the other two methods, especially for the early-harvested red clover silages than for the grass silages, are difficult to explain. The differences could be attributed to greater initial particle losses from the bags for the red clover silages than for the grass silages, but the comparison of the water-soluble N( Reference Vanhatalo, Kuoppala and Ahvenjärvi 15 ) fraction and the in situ a fraction does not support this suggestion. Similarly, the intake of silage SNAN was greater for the GS diets than for the RCS diets (125 v. 93 g/d), precluding that the greater feed N flow for the < 0·038 mm particles with the RCS diets was associated with greater SNAN intake. Secondary particle loss with advanced fermentation is another alternative that could increase the in situ CP degradability of the red clover silages to a greater extent than of the grass silages. It is possible that during advanced fermentation, more red clover particles become eligible for particle loss. The pattern of changes in the CP concentration of undegraded residues indicated clear differences in the degradation of CP in the red clover and grass silages (Fig. 2). It is possible that the protein–quinone complexes produced by the polyphenol oxidase enzyme make the CP in red clover resistant to the initial rate of degradation. The large difference between the omasal and in situ feed N flow estimates for the RC silages suggests that, even though disappearing from the bags between 6 and 12 h of incubation, it could still be eligible for passage as SNAN or small ( < 0·038 mm) particles.
Comparison of the omasal sampling technique and compartmental flux method
Feed N flow estimated using the compartmental flux method was, on average, 29 g/d smaller than that estimated using the omasal sampling technique, and the flow estimates were poorly correlated (R 2 0·05) across the diets. Assuming similar proportions of microbial N in particles as found by Varvikko & Lindberg( Reference Varvikko and Lindberg 38 ) at 24 h of incubation in undegraded residues in situ, proportionally 0·46 of particle N was of microbial origin. This implies that proportionally only 0·16 of N intake escaped ruminal degradation as particle-associated feed N. Rumen digesta samples were frozen, which can lead to detachment of particle-associated microbes. However, the findings of Trabalza-Marinucci et al. ( Reference Trabalza-Marinuccim, Poncet and Delval 43 ) do not support this suggestion. CP concentrations in >0·08 mm ruminal particles (rumen digesta was frozen) and in the undegraded in situ residues at 24 h of incubation do not indicate any greater detachment of microbes from the ruminal particles than from the undegraded in situ residues. CP concentrations were 108 and 101 g/kg DM in the ruminal particles and in situ residues for the GS diets, respectively, and 150 and 126 g/kg DM for the RCS diets, respectively.
As has been found for the in situ method, the compartmental flux method underestimated the omasal feed N flow for the RCS diets compared with the GS diets and overestimated it for the diets based on the late-harvested silages compared with those based on the early-harvested silages. As for the in situ data, the difference between the methods was especially large for the early-harvested RCS diets. Similar differences in the ranking of diets is not surprising, since the same cloth type was used for the in situ incubations and collection of the smallest particle-size fraction in wet sieving. Most probably, the same reasons, the escape of SNAN fractions in the liquid phase and possibly secondary particle loss, explain the difference between the methods. However, the mechanisms for the interactions between method and diet for feed N flow estimates are difficult to explain as discussed previously.
Comparison of the compartmental flux method and in situ method
Feed N flow estimates determined using the compartmental flux and in situ methods were strongly correlated (R 2 for treatment mean data and individual cow data 0·97 and 0·71, respectively). However, the values were, on average, 41 g/d (P< 0·001) greater when determined using compartmental flux data. The difference can be related to greater microbial contribution for the compartmental flux method. The smallest (0·038–0·08 mm) particles had a much higher CP concentration (mean 365 g/kg DM) than the 0·08–0·16 and 0·16–0·315 mm fractions (231 and 144 g/kg DM, respectively). With the RCS diets, the high CP concentration could be associated with the high CP concentration of red clover leaves( Reference Rinne and Nykänen 44 ), but CP concentration in the 0·038–0·08 mm particles was almost as high as that for the GS diets. This suggests that large rumen protozoa could have been harvested in the smallest particle-size fraction. Many rumen protozoa are greater than 38 μm( Reference Church 45 ) and could, therefore, be included in this fraction. Earlier, Huhtanen et al. ( Reference Huhtanen, Dakowski and Vanhatalo 46 ) found 5–7-fold greater particle-associated carboxymethylcellulase and xylanase activities in the 0·04–0·20 mm particles than in the 0·20–0·50 mm particles, which together with the smaller NDF concentration (615 v. 836 g/kg DM, respectively) indicated that this fraction contained more non-feed material. Lower microbial numbers( Reference Meyer and Mackie 47 ) and particle-associated fibrolytic enzyme activity( Reference Noziere and Michalet-Doreau 30 , Reference Huhtanen and Khalili 48 ) within the bags than in the rumen may suggest that microbial colonisation in the in situ bags was less than that in the rumen particles.
Digestibility of particle-associated crude protein in the lower tract
Except in the smallest particle-size fraction, apparent digestibility of particle-associated CP was rather low. The negative intercept of the equation (3·6 g/kg DM) indicates that the post-ruminal digestion of particle-associated CP is associated with faecal metabolic and endogenous CP output, most probably microbial re-colonisation of particles in the hindgut. Consistent with this, Varvikko & Vanhatalo( Reference Varvikko and Vanhatalo 49 ) using 15N-labelled feeds with the mobile bag technique found that a large proportion of N residues were of non-feed origin. Even considering metabolic CP, in the present study, the true digestibility of particle-associated CP was found to remain low (0·75). It could even be lower than this estimate, since protozoa most probably comprised a large fraction of the smallest particle-size fraction resulting in a high (0·90) true CP digestibility of this fraction. When calculated by difference, the true digestibility of CP in particles >0·08 mm was only 0·64. Even this can be an overestimate, since a substantial fraction of particle-associated protein can be of microbial origin( Reference Varvikko and Vanhatalo 49 ) that has a higher true digestibility in the small intestine. Storm et al. ( Reference Storm, Ørskov and Smart 50 ), using the intragastric infusion technique, reported a value of 0·813 for the true digestibility of bacterial protein. According to Varvikko & Vanhatalo( Reference Varvikko and Vanhatalo 49 ), the CP digestibility of intact ryegrass in the intestine was 0·701 as measured using 15N-labelled ryegrass in vivo.
Interestingly, the faecal output of particle-associated N was slightly greater in cows fed the GS diets than in those fed the RCS diets (51·8 v. 46·6 g/d), despite the greater total faecal N output in cows fed the RCS diets (162 v. 148 g/d) as reported in the companion paper( Reference Vanhatalo, Kuoppala and Ahvenjärvi 15 ). In the dataset of Huhtanen et al. ( Reference Huhtanen, Nousiainen and Rinne 51 ), faecal N output was markedly higher for red clover silages than for the primary-growth grass silages (9·1 v. 6·3 g/kg DM intake) in sheep fed at maintenance. In the Lucas test, the difference was in the intercept, whereas the estimates of true digestibility were similar. It seems that the increased faecal N output in animals fed red clover silages is not related to the greater amount of particle-associated CP. Whether the difference is related to the incomplete digestion of the quinone–protein complexes produced by the polyphenol oxidase enzyme needs more detailed analysis of faecal N components.
Conclusions
Feed N flow estimates based on ruminal in situ incubations or those derived from compartmental flux data were poorly correlated with in vivo feed N flow estimates determined using the omasal sampling technique, whereas the two first estimates were well correlated with each other. The results suggested that the in situ and compartmental flux methods resulted in biased estimates of feed N flow, despite the greatest variation being observed with the omasal sampling technique. Markedly greater omasal feed N flow compared with the feed N flow determined using the two other methods supports the conclusions from previous studies that have demonstrated a quantitatively important flow of SNAN in the fluid phase. However, the results of the present study also suggested that this fraction was not related to the intake of silage SNAN or immediately disappearing a fraction in the in situ incubations. The strong correlation between the feed N flow estimates determined using the in situ method and those determined using the compartmental flux method implied that feed N determined using the in situ method only described the flow of feed N in the particle fraction and not the total feed N flow.
Acknowledgements
The authors thank the staff of the experimental unit for the care of experimental animals and the laboratory staff for the chemical analysis of the samples in the original animal experiment.
The present study received partial financial support from the Finnish Ministry of Agriculture and Forestry. The Academy of Finland is greatly acknowledged for the grant provided to A. B. The Finnish Ministry of Agriculture and Forestry and the Academy of Finland had no role in the design and analysis of the study or in the writing of this article.
A. V. and P. H. were responsible for the design of the study. A. V. was responsible for the execution of the dairy cow experiment. A. B. was responsible for wet sieving of the samples. S. J. K. and P. H. designed and conducted the in situ studies. P. H. and A. B. were responsible for the calculation of the results and statistical analysis. All authors were involved in the data interpretation and preparation of the manuscript. All authors approved the final manuscript.
None of the authors had any personal or financial conflict of interest.











