The phytochemical curcumin, derived from the rhizome of Curcuma longa, is a constituent of the widely used spice turmeric( Reference Zhang, Browne and Child 1 , Reference Sikora, Scapagnini and Barbagallo 2 ). Curcumin has been extensively reported to demonstrate many beneficial biological effects including anti-cancer, antioxidant and anti-inflammatory activities( Reference Sikora, Scapagnini and Barbagallo 2 – Reference Aggarwal, Sundaram and Malani 5 ). In addition to these properties, both in vitro and in vivo studies have shown that curcumin can bind to the proteins β amyloid (Aβ) and tau as well as inhibit Aβ aggregation and modulate tau processing( Reference Mutsuga, Chambers and Uchida 6 – Reference Huang, Chang and Dai 9 ). Such characteristics are highly desirable, given that cerebral accumulation of Aβ aggregates and intra-neuronal deposits of insoluble hyperphosphorylated tau are pathological hallmarks of Alzheimer’s disease (AD)( Reference Masters, Simms and Weinman 10 , Reference Kosik, Joachim and Selkoe 11 ), whereas both oxidative stress and inflammation are heavily implicated in AD pathogenesis( Reference Zotova, Nicoll and Kalaria 12 – Reference Agostinho, Cunha and Oliveira 14 ).
A recent in vitro study has demonstrated that curcumin can induce structural changes in Aβ aggregates, which likely attenuate Aβ-induced toxicity( Reference Mithu, Sarkar and Bhowmik 15 ). Moreover, several in vivo studies have shown that dietary curcumin crosses the blood–brain barrier and subsequently decreases Aβ deposition and plaque load in the brain of transgenic mouse models of AD( Reference Garcia-Alloza, Borrelli and Rozkalne 8 , Reference Lim, Chu and Yang 16 – Reference Wang, Thomas and Zhong 18 ). Additional transgenic mouse studies have demonstrated marked inhibition of tau phosphorylation( Reference Ma, Yang and Rosario 19 ), reduced soluble tau and elevated levels of molecular chaperones (heat shock proteins) involved in tau degradation( Reference Ma, Zuo and Yang 7 ) following curcumin therapy. These Aβ- and tau-modifying attributes, as well as its anti-inflammatory and antioxidant properties, in combination with an excellent safety profile, have helped make curcumin an appealing target for studies aimed at developing AD prevention and intervention strategies.
Indeed, investigations of the effect of curcumin therapy on cognition and behaviour in animals have revealed positive functional outcomes. Pre-treatment with dietary curcumin, at a dose of 500 parts per million (ppm) for 2 months, prevented Aβ infusion-induced spatial memory deficits in middle-aged female Sprague–Dawley rats( Reference Frautschy, Hu and Kim 20 ). Ma et al. observed prevention of cognitive decline in tests of working memory in curcumin-treated (500 ppm, 4 months) 3xTg-AD mice on a high-fat diet( Reference Ma, Yang and Rosario 19 ), whereas chronic curcumin administration (500 ppm) has also been shown to suppress behavioural deficits in aged human tau transgenic mice( Reference Ma, Zuo and Yang 7 ).
Despite the apparent consensus of promising results among animal studies, these findings have not been translated fully to human studies. An epidemiological study reported better global cognition, as determined by the Mini-Mental State Examination (MMSE) score, among elderly Singaporean individuals consuming higher levels of curcumin in the form of curry compared with those who ‘never or rarely’ consume curcumin( Reference Ng, Chiam and Lee 21 ). Two subsequent 6-month, randomised, placebo-controlled, double-blinded studies conducted in early-to-moderate AD patients found curcumin formulations to be well tolerated and safe; however, no differences in measures were observed between treatment groups( Reference Baum, Lam and Cheung 22 , Reference Ringman, Frautschy and Teng 23 ). The outcome measures included MMSE, the Alzheimer’s Disease Assessment Scale, cognitive sub-portion, the Alzheimer’s Disease Cooperative Study Activities of Daily Living and blood Aβ, as well as cerebrospinal fluid levels of Aβ and tau species. The authors listed limited bioavailability of curcumin (due to dose or formulation), short duration of the studies and the fact that these trials were conducted on individuals already diagnosed with AD in whom cerebral pathology would be considerably advanced as possible explanations for the lack of significant results. In contrast, a recent 4-week, randomised, placebo-controlled, double-blinded study of sixty healthy adults aged 60–85 years showed improved working memory and mood in the curcumin treatment group. Evaluation of the acute effects of curcumin treatment also revealed improved performance on tasks of working memory and attention compared with placebo as early as 1 h after ingestion( Reference Cox, Pipingas and Scholey 24 ). Participants in the curcumin group received a single daily dose of 400 mg Longvida® Optimized Curcumin (Verdure Sciences) – a formulation with previously demonstrated bioavailability( Reference Gota, Maru and Soni 25 , Reference DiSilvestro, Joseph and Zhao 26 ). Whether the observed beneficial effects of curcumin persist longitudinally, however, remains to be determined.
To date, no longitudinal assessment of the effect of curcumin on cognition in a population of aged community-dwelling cognitively healthy individuals has been undertaken. Consequently, the aim of the present study was to conduct a 12-month, randomised, placebo-controlled, double-blinded study in order to investigate the ability of the curcumin formulation BiocurcumaxTM (Arjuna Natural Extracts Ltd) to prevent cognitive decline in a population of community-dwelling older adults. Cognitively healthy older adults were recruited as they represent a population both at risk of developing clinical AD, yet in whom the preclinical disease stage is believed to be early enough to still be responsive to intervention( Reference Villemagne, Burnham and Bourgeat 27 ). The curcumin formulation BiocurcumaxTM was selected based on its enhanced oral bioavailability; after ingestion of 2 g BiocurcumaxTM, a plasma curcumin concentration of 300 ng/g is reached( Reference Antony, Merina and Iyer 28 ); however, participants of the present study were given a dose of 1·5 g.
Methods
Participants
In all, 160 community-dwelling older adults were enrolled in to the study at the McCusker Alzheimer’s Research Foundation, Western Australia. Inclusion criteria were as follows: age 40–90 years, with good health and no significant cerebral vascular disease; no significant cognitive impairments as indicated by the Informant Questionnaire on Cognitive Decline in the Elderly or by objective memory assessment measures; normal general cognitive function as indicated by a Montreal Cognitive Assessment (MoCA) score ≥26; and no or minimal impairment in activities of daily living, as determined by a clinical interview and the 36-Item Short Form Health Survey (SF-36)( Reference Ware and Sherbourne 29 ). Individuals with a MoCA score of 18–25 were discussed by a team of neuropsychologists, and eligibility was determined on a case-by-case basis following stratification of the MoCA score according to age and education( Reference Rossetti, Lacritz and Cullum 30 ). Exclusion criteria included the following: the presence of dementia according to the Diagnostic and Statistical Manual of Mental Disorders, 4th edition( 31 ); previous medical history of stroke; current depressive symptoms based on the Depression Anxiety Stress Scales (DASS)( Reference Lovibond and Lovibond 32 ); presence of acute or untreated chronic psychiatric disorders (including drug and alcohol abuse); anti-coagulant or anti-platelet treatment or bleeding risk factors; obstruction of the biliary tract; and non-fluency in English. This study was conducted according to the guidelines laid down in the Declaration of Helsinki, and all procedures involving human subjects were approved by the Human Research Ethics Committee of Hollywood Private Hospital, Western Australia. Written informed consent was obtained from all subjects. The present study is registered with the Australian New Zealand Clinical Trials Registry (ANZCTR; http://www.anzctr.org.au/) under identification number ACTRN12611000437965.
Intervention
This randomised, double-blind, placebo-controlled study was conducted for a period of 12 months. Eligible participants were identified according to age and sex before being randomly assigned into either the curcumin (n 80) or the placebo (n 80) treatment groups. The curcumin group ingested 1×500 mg BCM-95®CG (BiocurcumaxTM) capsule three times a day (1500 mg/d total), after meals with water. The placebo group ingested 1×placebo capsule three times a day, after meals with water. The placebo (roasted rice powder with no active constituents) and BCM-95®CG capsules were identical in size and appearance (green-coloured hard gelatin shell of ‘0’ size). Each BCM-95®CG capsule contained 88 % total curcuminoids (curcumin, bisdemethoxycurcumin, demethoxycurcumin) and 7 % volatile oils from rhizomes of C. longa Linn. Participants visited the research centre at 3 monthly intervals to return completed capsule diaries and used capsule bottles and to collect a new capsule diary and the following 3 months’ capsule supply. The completed capsule diaries and a count of returned capsules were used to assess intervention compliance. Only the data of participants reaching an intervention compliance threshold of ≥70 % were included in the analysis.
Cognitive and clinical assessment
Comprehensive medical history was captured at baseline by participant self-report, and was subsequently updated at the 6- and 12-month follow-ups. Measurements of blood pressure (mmHg) and weight (kg) were obtained from each participant at baseline and every 3 months thereafter. Pre-morbid verbal intelligence was determined at baseline using the Cambridge Contextual Reading Test( Reference Beardsall 33 ).
Recent mood was assessed by administration of the DASS( Reference Lovibond and Lovibond 34 ) at baseline and at the 6- and 12-month follow-ups. Participant self-reported assessment of physical and mental health at all time points was obtained using the SF-36( Reference Ware and Sherbourne 29 ). The frequency with which participants made errors in prospective as well as retrospective short-term and long-term memory was assessed at baseline and at 6 and 12 months using the sixteen-item self-report Prospective and Retrospective Memory Questionnaire( Reference Smith, Della Sala and Logie 35 ).
An array of cognitive measures was administered at baseline and at the 6- and 12-month follow-up assessments. The MoCA was used to assess general cognitive function( Reference Nasreddine, Phillips and Bedirian 36 ). Verbal learning and memory were examined using the Rey Auditory Verbal Learning Test( Reference Spreen and Strauss 37 ) to provide scores for short-term, long-term and recognition retrieval from memory. Verbal fluency was assessed using the Controlled Oral Word Association Test( Reference Lezak, Howieson and Loring 38 ). The Wechsler Digit Symbol Scale from the Wechsler Adult Intelligence Scale revised (WAIS-R)( Reference Wechsler 39 ) was also administered as a measure of perceptual motor speed. A cognitive composite score (non-computerised) was calculated for each participant by converting the raw scores of the tests listed above to overall sample-based Z scores, and then averaging the Z scores to compute a single composite score.
The computerised CogState battery (CogState) was also administered to each participant at baseline and at the 6- and 12-month follow-ups. CogState tasks included the following: detection, assessing psychomotor speed; one back task to measure working memory; Groton maze learning (GML) for executive functions; GML test recall for identification; and one card learning and the continuous paired associate learning tasks for assessing visual memory. The raw scores of the individual computerised tests were converted to sample-based Z scores, which were then averaged to produce a single computerised composite score.
APOE genotyping
Genotyping was conducted on participants’ DNA samples, which had previously been isolated from whole blood samples using a QIAamp DNA Blood Midi Kit (Qiagen) according to the manufacturer’s protocol. APOE genotype was determined through TaqMan® genotyping assays (Life Technologies) for rs7412 (Assay ID C____904973_10) and rs429358 (Assay ID C___3084793_20). TaqMan® assays were performed on a ViiA™ 7 real-time PCR system (Applied Biosystems).
Statistical analysis
All statistical analyses were performed using IBM SPSS 22 for Windows Vista (SPSS Inc.). A P value of <0·05 determined a significant result for all analyses. Descriptive data analyses (Table 1) were undertaken to provide means, standard deviations and percentages for the treatment and placebo groups. For the evaluation of differences between these groups, independent sample t tests were performed to analyse continuous data and χ 2 tests to analyse categorical data. Repeated-measures ANCOVA was utilised to measure the effect of treatment on change in all measures from baseline to the 6- and 12-month follow-ups. All models included age, sex, years of education and APOE ε4 allele carriage as covariates. Treatment groups were unblinded following completion of data analysis.
Table 1 Demographic data of the treatment group and placebo group (Mean values and standard deviations; percentages and numbers)
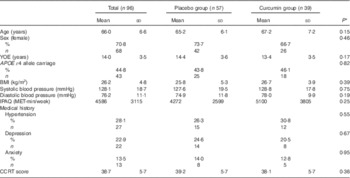
YOE, years of education; IPAQ, International Physical Activity Questionnaire; MET, metabolic equivalent task; CCRT, Cambridge Contextual Reading Test.
* P values are determined by independent samples t test for continuous variables and χ 2 test for categorical variables.
Results
A total of 160 participants were enrolled in to the present study; forty-nine participants were excluded before study completion either because of a baseline assessment result that deemed the participant ineligible to remain in the study (six participants), a suspected adverse event (twenty-three participants; gastrointestinal complaints accounted for the majority of adverse events), or because of other personal or medical reasons unrelated to study participation (twenty participants). Furthermore, an additional eight participants were excluded mid-study because of low intervention compliance rates (<70 %), and the data of a further seven participants who completed the study were excluded from the analysis because of late-onset intervention non-compliance. Compliance rates were based on returned capsule counts and participant capsule logs; ninety-six participants who met all inclusion, exclusion and compliance criteria were included in the present analysis. Fig. 1 provides a summary of the study cohort at all assessment time points and includes a breakdown of reasons for participant exclusion at each stage.
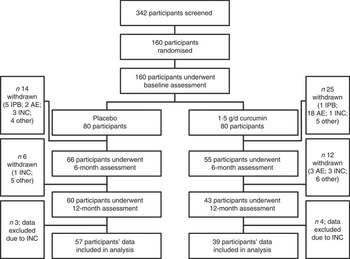
Fig. 1 Study participant flow chart. IPB, ineligible post-baseline assessment; AE, suspected adverse event; INC, intervention non-compliance (<70 %); other, personal or medical reason(s) unrelated to study participation.
No differences between the treatment and placebo groups were observed in terms of the demographic and medical history variables evaluated (Table 1). Nevertheless, the placebo group performed significantly better at baseline in the digit symbol task (t=2·98, P<0·01), the MoCA (t=2·10, P<0·05) and cognitive composite (non-computerised) scores (t=2·40, P<0·05), the latter of which was mainly driven by the MoCA and digit symbol scores. As shown in Table 2, there were no significant interactions of time×treatment group for measures of physical health, mental health, mood and self-reported memory function.
Table 2 Adjusted marginal means of physical health, mental health, mood and self-reported memory function measures at all time points for the treatment group and placebo group (Mean values and standard deviations)
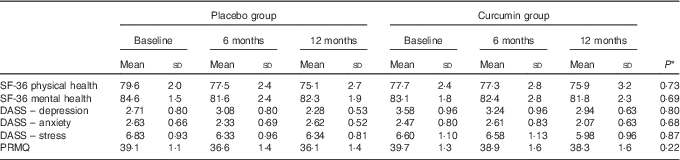
SF-36, 36-Item Short Form Health Survey; DASS, Depression Anxiety Stress Scales; PRMQ, Prospective and Retrospective Memory Questionnaire.
* P values for time×treatment interaction terms determined by repeated-measures ANCOVA, adjusting for age, sex, years of education and APOE ε4 allele carriage.
Table 3 contains the results of the repeated-measures analysis, evaluating the effect of BCM-95®CG on cognitive performance across the three time-points; baseline, 6 months and 12 months. The time×treatment variable was significant for the MoCA score (time×treatment; F=3·85, P<0·05; Table 3 and Fig. 2); however, it is important to note that the time variable itself was not significant (F=1·64, P=0·20). Mean MoCA scores improved by 0·64 points in the curcumin group and by 0·09 points in the placebo group from baseline to 12 months (Table 3 and Fig. 2). To assess whether the significant interaction observed in the repeated-measures analysis was driven by the decreased performance of the placebo group at the 6-month follow-up (a result not consistent with the 12-month follow-up assessment; Table 3 and Fig. 2), the analysis was re-run excluding the 6-month MoCA scores for both treatment groups. Using baseline and 12-month MoCA performance only, no significant interaction between time and treatment groups was observed (F=1·36, P=0·25; Table 3). No other differences in cognitive test performance were observed across the treatment groups.

Fig. 2 Adjusted marginal means of Montreal Cognitive Assessment (MoCA) scores (adjusted for age, sex, years of education and APOE ε4 allele carriage) for both groups at each study time point. Baseline MoCA performance was significantly different between the two groups (P<0·05). A significant interaction of time×treatment group for MoCA scores was also observed (P<0·05). ![]() , Curcumin group;
, Curcumin group; ![]() , placebo group.
, placebo group.
Table 3 Adjusted marginal means of cognitive measures at all time points for the treatment group and placebo group (Mean values and standard deviations)
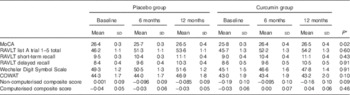
MoCA, Montreal Cognitive Assessment; RAVLT, Rey Auditory Verbal Learning Test; COWAT, Controlled Oral Word Association Task.
* P values for time×treatment interaction terms determined by repeated-measures ANCOVA, adjusting for age, sex, years of education and APOE ε4 allele carriage.
Discussion
This study sought to investigate the effect of a 12-month dietary supplementation of curcumin on cognitive function in a cohort of aged, community-dwelling, cognitively healthy individuals. Furthermore, we examined the influence of curcumin on mood and general quality of life. We observed no differences in placebo and treatment groups in changes in cognitive performance from baseline to the 12-month follow-up. However, a significant time×treatment group interaction was observed for the MoCA. Subsequent analysis revealed that this association was driven by a decline in function within the placebo group at the 6-month follow-up that was not observed in the curcumin treatment group. No differences were observed between the treatment groups in all other clinical and cognitive measures.
A large body of animal studies supports the hypothesis that curcumin is beneficial for cognitive health( Reference Agrawal, Mishra and Tyagi 40 – Reference Yu, Zhang and Luo 42 ). A paucity of evidence is present, however, to support the proposed beneficial effect of curcumin on cognition in humans( Reference Ng, Chiam and Lee 21 , Reference Cox, Pipingas and Scholey 24 ). In the present study, we found no between-treatment group differences in measures of cognition over 12 months. Nevertheless, a decrease in MoCA performance (used to assess general cognitive function) at the 6-month follow-up compared with baseline was observed in the placebo group, which did not manifest in the curcumin treatment group, contributing to a significant time×treatment interaction. Furthermore, at baseline, the placebo group performed significantly better on the MoCA than the curcumin group; however, at 12 months, the MoCA score of both groups was equivalent. Our results differ from a recent study by Cox et al.( Reference Cox, Pipingas and Scholey 24 ), who reported a beneficial effect of acute (1 h after single dose) and chronic (4-week treatment) curcumin treatment on working memory. It should be noted that the effect of curcumin on cognition after 4 weeks of treatment was trend level (unadjusted, reaching significance after adjustment), and that only one task (not administered in the current study), out of a number of cognitive tasks assessed, was shown to be influenced by curcumin treatment. The authors attribute the acute positive effect of curcumin to an up-regulation of monoaminergic neurotransmission. It is possible that short-term effects of curcumin on cognition may indeed be due to increased levels of neurotransmitters; however, whether such transmitter up-regulation can be maintained chronically requires further investigation. Divergent methodologies, most notably, study duration and use of different curcumin formulations, between the present study and that of Cox et al. may account for the differing results.
In the present study, we hypothesised that curcumin would have an effect on attenuating cognitive decline, rather than a direct effect on improving cognitive function. Nevertheless, in our highly educated cohort, we observed little cognitive decline on any tasks in either the placebo or the treatment group. Even though the cognitive battery was carefully selected to assess numerous cognitive functions including global cognition, episodic verbal memory, executive function, working memory and attention, and verbal fluency, it is possible that the magnitude of change exhibited by our high-functioning cohort was too small to be detected. The cognitive performance of our cohort over the follow-up period is, however, consistent with previous reports of a cognitively healthy cohort demonstrating a lack of cognitive decline over 12 months( Reference Maruff, Collie and Darby 43 ). In an attempt to gain a greater understanding of the effect of curcumin on cognition, future studies should consider longer intervention duration than that used in the present study. Furthermore, with longer periods of follow-up or with investigation of an even older cohort at increased risk of cognitive decline, the effect of curcumin treatment on cognition may be more pronounced. Such approaches would also facilitate examination of the effect of curcumin therapy on rate of conversion to mild cognitive impairment or AD, and are feasible considering the excellent safety profile and general tolerability of the curcumin formulation utilised.
A number of animal and human studies indicate that curcumin has a positive effect on symptoms of depression and anxiety( Reference Bergman, Miodownik and Bersudsky 44 – Reference Huang, Zhong and Li 48 ). In the present study, however, curcumin was not shown to influence self-reported measures of depressive and anxiety-related symptoms over 12 months; this result is not entirely unexpected, given that individuals with current depressive symptoms were excluded from the study. Although the majority of human studies yielding positive results in the context of depression and anxiety symptoms were conducted on individuals with major depressive disorder( Reference Bergman, Miodownik and Bersudsky 44 – Reference Sanmukhani, Satodia and Trivedi 46 ), one study showed an improvement in self-reports of mood among non-depressed individuals, after a 4-week curcumin intervention( Reference Cox, Pipingas and Scholey 24 ).
Bioavailability is an important consideration in studies of curcumin supplementation. Poor absorption of orally administered curcumin as well as rapid metabolism in the intestine and liver have long been identified as limitations of curcumin therapy. Thus, a great deal of research activity has focused on the development of formulations, which are more readily absorbed and yield increased levels of curcumin in its unconjugated form. The varying pharmacokinetics of different curcumin formulations likely contributes to the heterogeneity of results among studies of curcumin in humans. Indeed, Ringman et al.( Reference Ringman, Frautschy and Teng 23 ) were unable to determine whether the lack of efficacy observed following Curcumin C3 Complex® (Sabinsa Corporation) administration was due to inefficacy of curcumin as an AD intervention or due to limited bioavailability of the formulation as evidenced by biochemical analysis of blood and cerebrospinal fluid samples. Cox et al.( Reference Cox, Pipingas and Scholey 24 ) utilised Longvida® Optimized Curcumin in their investigation of the effect of curcumin on cognition and mood in healthy older adults; in contrast, the present study utilised BiocurcumaxTM. Both the Longvida® and BiocurcumaxTM formulations are reported to reach plasma concentrations of free curcumin, which are significantly greater than that of unformulated curcumin; there are, however, between-formulation differences in pharmacokinetic parameters such as reported peak plasma concentration, time to peak concentration and elimination rate( Reference Gota, Maru and Soni 25 , Reference Antony, Merina and Iyer 28 ). The curcumin formulation, dosage and frequency of administration are all likely to have impacted the results of the present and previous studies. It is also important to note that the therapeutic concentration of curcumin with regard to enhancing cognition requires further investigation. Although previous reports from Cox et al. describe a positive effect of a daily dose of 400 mg of the Longvida® formulation on cognition, more research in larger cohorts with longer intervention periods is required to identify a therapeutic dose. The three times daily 500 mg BiocurcumaxTM dose utilised in the present study to sustain optimum curcumin blood levels may have also impacted upon intervention compliance and tolerability, contributing to the number of gastrointestinal-related adverse events and subsequent participant withdrawal from the study. Nevertheless, it is unclear whether the high adverse event rate was due to the nature of the BiocurcumaxTM formulation or curcumin itself. At study commencement, participants were blinded to curcumin consumption; steady increase to the eventual BiocurcumaxTM daily dose would have likely reduced the number of participant withdrawals. Indeed, we are currently using this incremental BiocurcumaxTM dosage approach as part of a sister study investigating the role of curcumin in preventing AD (ANZCTR identification number: ACTRN12613000681752), with excellent intervention tolerability observed to date.
Although the results of the present study indicate that, in this cohort of cognitively normal older adults, curcumin had limited influence on cognitive function, mood or general quality of life over 12 months, it is important to note that alterations in cognition manifest up to 20 years after the commencement of AD-related neuropathological changes. Furthermore, other studies have demonstrated positive effects of curcumin on Aβ, tau, inflammation and oxidative stress – factors well known to be associated with AD pathogenesis and pathology( Reference Sikora, Scapagnini and Barbagallo 2 , Reference Ma, Zuo and Yang 7 – Reference Huang, Chang and Dai 9 ). Thus, additional longitudinal studies, which include measurement of biological markers of AD pathology, are warranted, in order to fully elucidate the ability of curcumin to slow neurodegeneration leading to cognitive decline and AD.
Acknowledgements
The authors thank all those who participated in the study for their commitment and dedication to help advance Alzheimer’s disease research. The authors also thank the following individuals for their contribution to the study: Dr Roger Clarnette (medical oversight), Assistant Professor Michael Weinborn (QC of neuropsychological test scores), Assistant Professor Simon Laws and Tenielle Porter (APOE genotyping), Research Assistants, Shaun Markovic, Kerry McCabe, Ellen Putland, Georgia Martins, and Researchers, Dr Sajla Singh, and Dr Cynthia Gregory (neuropsychological test administration and blood sample processing).
This work was funded by the McCusker Alzheimer’s Research Foundation, and a grant awarded to SRS, KGG and RNM by the Hollywood Private Hospital Research Foundation (grant no. RF062). HRS is supported by the Australian CRC for Mental Health Programme. CogState Limited, Melbourne, Victoria, Australia, provided the computerised cognitive assessment battery utilised in this study free of charge. BiocurcumaxTM and placebo capsules were provided free of charge by Arjuna Natural Extracts Limited, Kerala, India. Arjuna Natural Extracts Limited and CogState Limited had no role in the design, analysis or writing of this article.
R. N. M. and H. R. S. designed the study; S. R. R.-S., B. M. B., H. R. S. and T. S. conducted the study; S. R. R.-S., B. M. B. and H. R. S. analysed the data; S. R. R.-S., B. M. B., H. R. S., K. G. G., V. B. G. and R. N. M. wrote the paper; R. N. M. had primary responsibility for final content; and all the authors have read and approved the final version of the manuscript.
The authors declare that there are no conflicts of interest.








