For thousands of years, humankind has used plants for therapeutics. Recent years have seen the development of highly targeted biological treatments and synthetic therapies, some with serious side effects. At the same time, there is renewed public interest in complementary therapies, naturally occurring treatments with minimal toxicity and diets related to health and disease.
Curcumin is a constituent of the spice turmeric, one of the principal ingredients in curry powder. Turmeric is prepared from the root of the Curcuma longa plant, a member of the ginger family. It is native to India and Southeast Asia, where fresh turmeric root is widely used in a similar way to ginger; in the West, turmeric is much more commonly available as a dried powder. It has been used to treat a broad range of common ailments in Indian Ayurvedic medicine for at least 4000 years, as well as in Chinese, Arabic and other traditional medicines. Curcumin is in modern use worldwide as a cooking spice, flavouring agent and colorant. Dishes traditionally made with turmeric include dahls and most other curries, as well as pickles, relishes and chutneys. It is widely used to colour mustards, mayonnaises and margarines and has been designated as international food additive E100. Because of its resemblance to saffron, curcumin is sometimes referred to as ‘Indian saffron’ and used as a (much less expensive) substitute.
Chemistry
The active ingredient of curcumin is diferuloylmethane, a hydrophobic polyphenol with a characteristic yellow colour. In chemical terms it is bis-α, β-unsaturated β-diketone, a linear diarylheptanoid compound, where two oxy-substituted aryl moieties are linked together through a seven carbon chain (Fig. 1). The aryl rings may be substituted by varying numbers of hydroxy or methoxy groups in a symmetrical or asymmetrical fashion to produce analogues of curcumin or curcuminoids. Curcumin is the most abundantly occurring natural analogue at 77 %(Reference Anand, Thomas and Kunnumakkara1), followed by demethoxycurcumin (17 %) in which one methoxy group is absent, then bis-demethoxycurcumin (3 %) in which the methoxy group is absent from both the aryl rings (Fig. 1).

Fig. 1 Chemical structure of curcumin (diferuloylmethane), its natural analogues and principal metabolites.
There is no explicit evidence that correlates the molecular or stoichiometric properties of curcumin or its analogues with their biological effects. While several groups have studied the differential bioactivities of these different analogues, no single curcuminoid shows overall highest potency. Differential efficacy varies widely according to the cell type, function, disease system and organism in question(Reference Anand, Thomas and Kunnumakkara1). Thus, there is no consensus as to the most effective preparation for human use. Commercially available curcumin preparations are largely derived from natural curcumin sources and therefore contain the three main curcuminoids in approximately the afore mentioned proportions. Indeed, some data suggest that such a mixture of curcuminoids have synergistically greater activity than any of their individual elements(Reference Sandur, Pandey and Sung2).
Dose and safety
The safety, tolerability and non-toxicity of curcumin at high doses are well established. Oral doses up to 12 g/d are well tolerated in human subjects(Reference Lao, Ruffin and Normolle3), although dosing diet regimen above 8 g may be difficult to achieve due to the bulky nature of this quantity of compound(Reference Cheng, Hsu and Lin4). However, drug delivery is a problem and the bioavailability of oral curcumin is low(Reference Anand, Kunnumakkara and Newman5, Reference Sharma, Steward and Gescher6) due to a combination of efficient first pass metabolism, poor gastrointestinal absorption, rapid elimination and poor aqueous solubility. Elimination is largely via hepatic glucuronidation and sulphation. Glucuronidation of curcuminoids preferentially occurs on the phenolic hydroxyl group, when incubated with rat or human liver microsomes(Reference Pfeiffer, Hoehle and Walch7). This produces a strong lipophilic conjugate that is less stable than its unconjugated form and is excreted through stool. Whether such conjugates have pharmacological activity is uncertain(Reference Pfeiffer, Hoehle and Walch7–Reference Aggarwal and Harikumar9). However, other, potentially active, metabolites have been identified (Fig. 1), perhaps the most important and intensively studied of which is tetrahydrocurcumin, a reduction metabolite. It lacks the yellow colour and hydrophobicity of curcumin and does not occur in natural curcumin sources. While it has less anti-inflammatory activity than curcumin in terms of its ability to inhibit NF-κB(Reference Sandur, Pandey and Sung2, Reference Pan, Lin-Shiau and Lin10), it exhibits greater antioxidant potency than curcumin in a number of different models(Reference Osawa, Sugiyama and Inayoshi11–Reference Okada, Wangpoengtrakul and Tanaka13).
After oral curcumin dosing, serum concentrations peak at 1–2 h and are undetectable by 12 h(Reference Cheng, Hsu and Lin4). Some investigators report that serum curcumin is undetectable below oral doses of about 4 g(Reference Lao, Ruffin and Normolle3, Reference Cheng, Hsu and Lin4); however, others have detected curcumin not only in serum, but also in urine, at much lower doses(Reference Sharma, Euden and Platton14). Some studies demonstrate the presence of curcumin in colorectal tissue at oral doses of 3·6 g(Reference Garcea, Berry and Jones15), so the gut may represent a promising local clinical target for curcumin. The pharmacokinetic profile of its major metabolites may also be relevant to the biological effects of curcumin. Most curcumin conjugates produced by in vivo human metabolism are glucuronides (less commonly sulphates), and these are detectable in plasma at greater concentrations than free curcumin with a peak at 4 h after oral dosing(Reference Vareed, Kakarala and Ruffin8).
Thus, the apparent discrepancies in pharmacodynamics observed in different in vivo studies of curcumin may be explained by its high rate of conjugation. Additionally they may relate to the differing formulations used. Curcumin constitutes about 5 % of turmeric root(Reference Ammon and Wahl16, Reference Strimpakos and Sharma17); the remainder is made up of carbohydrates, proteins and essential oils. Preparations used for human consumption are either naturally produced from purified turmeric extract, which contain varying proportions of the different curcuminoids, or are synthetically produced, containing only pure chemically synthesised curcumin. Strategies have also been employed to improve bioavailability based on changes in drug formulation, such as the use of nanoparticles to reduce particle size delivery and micelles to counter hydrophobicity. Recently, it has been reported that heat treatment improves the water solubility of curcumin(Reference Kurien, Singh and Matsumoto18).
In human trials, only minor side effects of curcumin, namely diarrhoea(Reference Sharma, Euden and Platton14), have been reported, and it is considered safe and well tolerated. As a caveat, however, these trials have usually examined short-term outcomes. There is some evidence that long-term, high-dose curcumin administration in rodents can be tumourigenic(19, Reference Somasundaram, Edmund and Moore20). It has also been shown that curcumin's predominant activity switches from antioxidant to pro-oxidant with increasing concentration(Reference Sandur, Ichikawa and Pandey21), which may provide an explanation for its seeminglyopposing biological effects in vivo. These apparent contradictory roles of curcumin, as both anti-cancer and pro-carcinogenic agent, are as yet unexplained, and epitomise the complexity and paradoxical nature of the compound. Nevertheless, there is good evidence from India, at a population level, about the safety of lifelong curcumin ingestion up to about 100 mg/d(Reference Chainani-Wu22), and it is classified ‘Generally Recognised As Safe’ by the United States Food and Drug Administration.
In vitro studies
A wide variety of cellular properties of curcumin have been demonstrated, including antioxidant, anti-inflammatory, anti-proliferative, pro-apoptotic, anti-bacterial and anti-cancer activities (Table 1 and Fig. 2).
Table 1 Molecular targets of curcumin in cell line studies


Fig. 2 Cellular activities of curcumin and molecular mechanisms of action. ROS, reactive oxygen species; HO-1, haem oxygenase; Bax, BCL2-associated X protein; MAPK, mitogen-activated protein kinase; MMP, matrix metalloproteinase; STAT, signal transducer and activator of transcription; NOS, NO synthase; COX2, cyclo-oxygenase-2. ![]() , Activation by curcumin; ↕, effects vary;
, Activation by curcumin; ↕, effects vary; ![]() , suppression by curcumin.
, suppression by curcumin.
Transcription factors
NF-κB
NF-κB is one of the key transcription factors responsive to curcumin. In human myeloid ML-1a cells, curcumin suppresses NF-κB activation induced by TNF-α, phorbol ester and hydrogen peroxide(Reference Singh and Aggarwal23). The mechanism appears to be via reduced IκBα phosphorylation and degradation(Reference Aggarwal, Ichikawa and Takada24), suggesting that curcumin acts at a step above IκB kinase (IKK) in the NF-κB activation pathway. Many of the observed biological effects of curcumin involve processes that are NF-κB-dependent. Therefore, examination of NF-κB signalling was a natural focus and its inhibition by curcumin is a consistent finding in a number of different models. For example, in four different human mantle cell lymphoma lines (an aggressive non-Hodgkin's B cell lymphoma), curcumin down-regulated NF-κB, inhibited IKK and reduced IκBα phosphorylation, leading to cell cycle arrest, apoptosis and suppression of proliferation(Reference Shishodia, Amin and Lai25). The reproducible finding of inhibition of IKK by curcumin suggests that curcumin acts at or above the level of IKK in the NF-κB pathway. Investigators have shown modulation by curcumin of the serine/threonine protein kinase Akt, a ubiquitous cell signalling molecule, which is known to activate NF-κB. Curcumin suppresses both Akt activation and Akt–IKK association(Reference Aggarwal, Ichikawa and Takada24), and thus its effects on NF-κB may be a downstream consequence of true targets that lie higher upstream. The identification not only of NF-κB but also multiple other signalling molecules and transcription factors which are modulated by curcumin further suggests that an upstream direct target (or targets) of curcumin common to these pathways may exist.
Signal transducer and activator of transcription
Signal transducer and activator of transcription (STAT)3 is a transcriptional activator with a ubiquitous role in tumourigenesis. It is involved in dysregulation of cell growth, invasion, angiogenesis, metastasis and resistance to apoptosis(Reference Aggarwal, Sethi and Ahn26). Aberrant STAT3 signalling is an important process in the development and progression of cancer, thus agents that block its activation have therapeutic potential. Curcumin reversibly inhibits STAT3 activation in human multiple myeloma cells and by this mechanism suppresses IL-6-induced cell proliferation(Reference Bharti, Donato and Aggarwal27). It also inhibits STAT3 activation in five different human Hodgkin and Reed-Sternberg lymphoma cell lines(Reference Mackenzie, Queisser and Wolfson28). The down-regulation by curcumin of proteins involved in cell cycling and apoptosis such as cyclin D1 and bcl-XL(Reference Bharti, Donato and Aggarwal27, Reference Han, Chung and Robertson29) may be secondary manifestations of curcumin's inhibitory effects upon STAT3 and NF-κB, which are known to regulate expression of both of these genes(Reference Fujio, Kunisada and Hirota30, Reference Sinibaldi, Wharton and Turkson31).
PPAR-γ
PPAR-γ is a nuclear receptor and transcription factor involved in cell cycle control, proliferation and differentiation, exerting anti-inflammatory, anti-cancer and insulin-sensitising actions. It is highly expressed in adipose tissue and colonic mucosa, where tight control of proliferation, differentiation and apoptosis is vital for homeostasis and prevention of oncogenesis, and here PPAR-γ may have tumour suppressor functions(Reference Sarraf, Mueller and Smith32). It is activated by PG products of the eicosanoid cascade(Reference Nakamura and Omaye33, Reference Ondrey34) and possibly by dietary components such as linolenic and linoleic acids. Curcumin induces and activates PPAR-γ in rat hepatic stellate cells, a liver cell type responsible for fibrosis in liver injury, which contributes to chronic liver damage and cirrhosis. PPAR-γ inhibited the proliferation of stellate cells, and curcumin greatly enhanced this effect(Reference Xu, Fu and Chen35).
PPAR-γ activity in Moser cells (a human colon cancer cell line) is also enhanced by curcumin, interrupting the cell cycle through reduced expression of cyclin D1 and inhibition of epidermal growth factor signalling(Reference Chen and Xu36). Both of these effects were PPAR-γ-dependent. However, the anti-cancer effect of curcumin occurs through multiple mechanisms, and this is supported by the finding that a different human colon cancer cell line, HT-29 cells, despite being more sensitive to curcumin-induced growth suppression, is less responsive to specific PPAR-γ antagonism than Moser cells(Reference Chen and Xu36). These data reflect both that PPAR-γ function is one of the many mechanisms involved in the generation of cancer, and that curcumin exerts its anti-cancer effects through multiple pathways.
Mitogen-activated protein kinase signalling pathways
The mitogen-activated protein kinase (MAPK) cascade is activated by a large number of different types of receptor, including cytokine, growth and toll-like receptors and receptors sensitive to environmental stressors. The precise mechanisms of activation are incompletely understood(Reference Wetzker and Bohmer37). Curcumin modulates MAPK signalling in several different in vitro models, although the data are somewhat contradictory. Under some circumstances, curcumin inhibits MAPK activation, as in a recent study in primary human intestinal microvascular endothelial cells, where curcumin inhibited p38 MAPK activation in response to vascular endothelial growth factor, as well as cyclo-oxygenase (COX)-2 and PGE2 production(Reference Binion, Otterson and Rafiee38). These anti-angiogenic properties of curcumin are of potential clinical benefit in gut inflammation and cancer. Further evidence that curcumin inhibits MAPK pathways includes its inhibition of c-Jun N-terminal kinase activation by a number of different agonists in Jurkat T cells (a human T cell line)(Reference Chen and Tan39). Here the investigators provide evidence that the target of inhibition lies proximally within the pathway, at the level of MAPK kinase kinase or above. Other investigators paradoxically show activation of MAPK by curcumin, for example c-Jun N-terminal kinase in HCT116 cells, a human colon cancer cell line(Reference Collett and Campbell40) and p38 MAPK in primary human neutrophils(Reference Hu, Du and Vancurova41).
Is it feasible that curcumin can both activate and inhibit MAPK signalling? Where MAPK is activated, the biological consequence seen is apoptosis; where MAPK is inhibited, the consequences are anti-inflammatory and anti-angiogenic. The mechanism for the opposing actions of curcumin on MAPK is unexplained, and in both cases its final effects are demonstrably anti-neoplastic and anti-inflammatory. Assuming the primary molecular targets of curcumin lie elsewhere, the MAPK signals observed experimentally may merely represent intermediary pathways by which its ultimate biological effects are mediated. Alternatively, where MAPK activation is seen, it is possible that this has been due to ubiquitous experimental contaminants such as lipopolysaccharide masking the true inhibitory effect of curcumin. In support of this explanation, curcumin-mediated apoptosis (suggested to occur via p38 MAPK activation) was not abrogated by the specific p38 MAPK inhibitor, SB203580(Reference Collett and Campbell40).
Tumour suppressor gene p53
Mutation of the tumour suppressor p53 plays an important role in the evolution of many different human cancers. Once again, the role of curcumin is complex. In an early study of the effects of curcumin on BKS-2 and WEHI-231 cells (both immature B cell lymphoma mouse cell lines), proliferation was inhibited(Reference Han, Chung and Robertson29). Interestingly, and with obvious potential clinical benefit in cancer chemotherapy, this inhibitory effect was much less marked on normal B cells. The investigators demonstrated (unexpected) inhibition of expression of p53 by curcumin, as well as inhibition of various other genes involved in growth, proliferation and transcriptional activation, including early growth response factor (egr)-1, the proto-oncogene c-myc and the transmembrane anti-apoptotic bcl-XL. The finding of reduced p53 activity was confirmed in RKO cells (a colon cancer cell line), where curcumin impairs the post-translational folding of p53 required for its function(Reference Moos, Edes and Mullally42), and in myeloid leukaemic cells, where it induces p53 degradation(Reference Tsvetkov, Asher and Reiss43).
Conversely, other experiments show induction of p53 by curcumin, for example in human epithelial breast cancer, prostate cancer and B cell lymphoma cell lines(Reference Choudhuri, Pal and Das44) and in HT-29 cells (a human colon adenocarcinoma cell line), where it induced apoptosis(Reference Song, Mao and Cai45). In the former work, once again the authors show differential sensitivity of cancer cells compared with healthy cells to curcumin. While some investigators have shown anti-proliferative effects despite inhibition of the tumour suppressor p53(Reference Han, Chung and Robertson29), established precedents exist where an agent that is cancer-preventative in one system can be carcinogenic in another, for example tamoxifen (therapeutic in breast; pro-neoplastic in uterus)(Reference Fisher, Costantino and Wickerham46). Curcumin may be a clinically useful chemopreventive agent, and this might relate specifically to certain types of cancer and not others. Alternatively, it may confer cancer risk that is inseparable from its benefits. These cautions must be borne in mind when considering its human use.
Angiogenesis
There is strong evidence that curcumin is anti-angiogenic. Angiogenesis (the growth of new blood vessels) is required for the development of both inflammation and cancer, where it is crucial for the survival of tumours beyond a certain size. It is also integral to the generation of diabetic eye disease, which is characterised by growth of abnormal vessels across the retina, a major cause of blindness worldwide. In an early study in both primary bovine and immortalised mouse endothelial cells, curcumin inhibited endothelial cell proliferation(Reference Arbiser, Klauber and Rohan47). Curcumin inhibits angiogenesis in response to vascular endothelial growth factor in the human intestinal microvascular endothelium(Reference Binion, Otterson and Rafiee38) and inhibits the angiogenic differentiation of human umbilical vein endothelial cells(Reference Thaloor, Singh and Sidhu48, Reference Bae, Kim and Jeong49). Also in human umbilical vein endothelial cells, curcumin binds to and irreversibly inhibits aminopeptidase N(Reference Shim, Kim and Cho50), a membrane-bound matrix metalloproteinase (MMP), which increases tumour invasiveness and is involved in retinal neovascularisation and tumour angiogenesis(Reference Pasqualini, Koivunen and Kain51). Finally, curcumin decreases hypoxia-inducible factor-1α, an angiogenic transcriptional activator, in human hepatocellular carcinoma cells(Reference Bae, Kim and Jeong49). In this work, curcumin also inhibited the transcriptional action of hypoxia-inducible factor-1α, down-regulating the expression of vascular endothelial growth factor, a potent hypoxia-induced angiogenic factor.
Inflammatory cytokines
Several studies demonstrate the suppression of downstream pro-inflammatory and pro-neoplastic mediators by curcumin. Recent examples include reduced expression of IL-6 and IL-8 in response to acid exposure in a human oesophageal epithelial cell line(Reference Rafiee, Nelson and Manley52) and reduced spontaneous expression of IL-6 and IL-8 in four different head and neck squamous carcinoma cell lines(Reference Cohen, Veena and Srivatsan53). These observations may be secondary to the suppression by curcumin of intermediary signalling pathways such as NF-κB, and some investigators provide evidence to this effect(Reference Cohen, Veena and Srivatsan53). Even if curcumin-mediated cytokine suppression is a later consequence of proximal event(s), this remains a potentially useful clinical application, and the one which is consistently reproduced in pre-clinical models.
Cyclo-oxygenase
COX2 is an inducible form of PGH synthase. It is an early response gene induced by cytokines, growth factors and toxins. COX2 mediates inflammation through production of PG and plays an important role in colon cancer. Over-expression of COX2 in colonic epithelium appears to promote tumour development(Reference Nishisho, Nakamura and Miyoshi54) and non-steroidal anti-inflammatory drugs that inhibit COX2, reduce the risk of colon cancer(Reference Thun, Namboodiri and Heath55). Curcumin inhibits COX2 production in a primary human intestinal microvascular endothelial cell line(Reference Binion, Otterson and Rafiee38) and inhibits COX2 induction in human colonic epithelial cells(Reference Plummer, Holloway and Manson56). In this latter work, the authors note that the COX2 gene promoter contains two NF-κB binding sites and show evidence that the effect of curcumin on COX2 is due to inhibition of NF-κB binding. In agreement with other investigators(Reference Aggarwal, Ichikawa and Takada24), the level of impact of curcumin upon the NF-κB pathway appears to be at or above IKK. Inhibition of COX2 by curcumin, which lacks the adverse effects of chronic aspirin or non-steroidal anti-inflammatory drugs ingestion, holds considerable promise for long-term bowel cancer prevention in human subjects.
Matrix metalloproteinases
In health, fibroblasts produce low levels of MMP that remain largely in latent form and mediate physiological extracellular matrix turnover. In inflammatory disease, MMP are over-expressed and become activated in cascades causing unchecked tissue destruction, fibrosis and further increasing immune cell activation and homing(Reference Pender and MacDonald57). MMP also play a key role in tumour progression, since matrix dissolution is an important step in the conversion of a pre-malignant cell into a frankly malignant one, as well as in tumour growth, invasion, metastasis and angiogenesis(Reference Vihinen, Ala-aho and Kahari58). There are over twenty different types of MMP, which are sub-classified according to the primary stromal substrate upon which they act. Curcumin down-regulates MMP production in various cell types. In human fibrosarcoma cells, it decreases invasion, migration and production of MMP-2 and MMP-9(Reference Yodkeeree, Garbisa and Limtrakul59), and in human and rabbit peripheral blood mononuclear cells, it reduces MMP-9(Reference Saja, Babu and Karunagaran60). Recently, it has been shown to reduce MMP-9 in human intestinal epithelial cells(Reference Claramunt, Bouissane and Cabildo61), and our group has shown dose-dependent inhibition of MMP-3 production by curcumin in primary human colonic myofibroblasts from patients with inflammatory bowel disease (IBD)(Reference Epstein, Docena and MacDonald62). Thus, inhibition of MMP by curcumin is a consistent finding under a range of different cellular conditions. The clinical implications for prevention and treatment of inflammation and cancer are wide ranging.
p300 Acetyl transferase
Lastly, curcumin is a known inhibitor of acetylation, acting on the enzyme p300 acetyl transferase(Reference Lee, Lin and Lin63, Reference Balasubramanyam, Varier and Altaf64). Acetylation modifies proteins when an acetyl group binds to a lysine residue, altering the protein's shape, charge and biological fate in the cell. Traditionally the study of acetylation has examined how the acetylation of histones changes their conformation, loosens their interactions with DNA and thus opens out the nucleosome, exposing DNA for gene transcription(Reference Roth, Denu and Allis65, Reference Sanderson and Naik66). However, recent work shows that other (non-histone) regulatory proteins within the cell are also subjected to acetylation, initiating separate cellular events that regulate for example transforming growth factor-β signalling(Reference Monteleone, Del Vecchio Blanco and Monteleone67) and insulin-like growth factor binding protein-3 expression(Reference Ongeri, Verderame and Hammond68, Reference White, Mulligan and King69). Such events are important in inflammation and cellular proliferation. Another important such non-histone example is the tumour suppressor gene p53 whose capacity to activate transcription and therefore DNA repair is altered by p300 status(Reference Yang70, Reference Brooks and Gu71), and indeed mutations in p300 have been found in several different types of cancer specimen, particularly in gut cancers(Reference Muraoka, Konishi and Kikuchi-Yanoshita72).
p300 Acetyl transferase, as a potent catalyst of acetylation, plays a role in a wide variety of gene transcription and other cellular events. Several effects of curcumin resulting from its p300 inhibitor activity are documented, including inhibition of inflammatory responses in human tracheal smooth muscle cells(Reference Lee, Lin and Lin63), suppression of HIV proliferation(Reference Balasubramanyam, Varier and Altaf64) and inhibition of proliferation of Raji cells (a non-Hodgkin's B cell lymphoma line)(Reference Chen, Shu and Chen73), reflecting once again a broad spectrum of potential clinical applications that might be developed.
Animal models: inflammatory bowel disease
While curcumin has shown benefits in a number of different models of inflammatory disease, particular interest has focused on its use in the gut. IBD (Crohn's disease (CD) and ulcerative colitis (UC)) is a source of considerable morbidity, and its incidence is increasing worldwide. Currently available treatments such as steroids, 5-aminosalicylic acids and immunomodulators do not offer cure, but CD responds well to polymeric or elemental feed that brings about remission in 80 % of paediatric patients(Reference Heuschkel, Menache and Megerian74, Reference Bannerjee, Camacho-Hubner and Babinska75). IBD is less common in developing countries than in the industrialised world(Reference Goh and Xiao76), and individuals emigrating from East to West take on the Western disease risk(Reference Goh and Xiao76, Reference Montgomery, Morris and Pounder77). This holds further relevance to the importance of diet in IBD, and there is keen interest to develop nutritional therapies.
Several studies in various rodent disease models provide strong pre-clinical evidence for the benefit of curcumin(Reference Jian, Mai and Wang78–Reference Venkataranganna, Rafiq and Gopumadhavan81). For example, in multidrug resistance gene-deficient mice, which spontaneously develop colitis, the addition of curcumin to their diet significantly reduced intestinal inflammation(Reference Nones, Knoch and Dommels80). Other investigators used 2,4-dinitrochlorobenzene-induced colitis in rats and showed a dose-dependent improvement in disease activity parameters with dietary curcumin of equal potency to sulfasalazine treatment(Reference Venkataranganna, Rafiq and Gopumadhavan81). Curcumin treatment was associated with a reduction in colonic NF-κB, inducible NO synthase and various measures of oxidative stress, for example myeloperoxidase and lipid peroxidation.
The efficacy of curcumin in IBD may differ according to inflammatory circumstances and dose. For example, trinitrobenzene sulphonic acid colitis in NKT-deficient SJL/J mice exhibits a classic T helper cell (Th)1-type response, while BALB/c mice with trinitrobenzene sulphonic acid colitis exhibit a mixed Th1/Th2 profile(Reference Billerey-Larmonier, Uno and Larmonier82). Curcumin caused improvement in all disease activity parameters only in the BALB/c mice. In simple terms, Th1-type inflammation relates more closely to CD and Th2 to UC, although in real terms the situation is probably more complex with a degree of overlap. The reason for the differential efficacy of curcumin in these two models is unclear. The IL-10 knockout mouse develops spontaneous Th1-type inflammation in large and small bowel, which is dependent on gut bacteria, making it a good model of CD. The protective effect of curcumin in this model (by colon morphology and colonic interferon γ and IL-12/23p40 mRNA) was modest, and paradoxically occurred only at the lowest dietary concentration of 0·1 %(Reference Larmonier, Uno and Lee83). In vivo NF-κB activation in the gut was unaffected by curcumin at any concentration, but curcumin acted synergistically with IL-10 on epithelial cells to decrease NF-κB activity. These data raise once again the suggestion that curcumin can have paradoxically opposing effects at different concentrations, and when clinical studies take place, a wide range of dosages are warranted.
Animal models: cancer
Chemoprevention
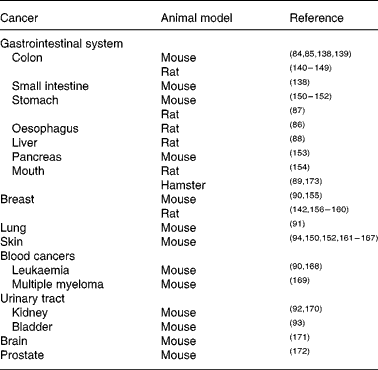
The molecular targets of curcumin include many pathways and processes involved in the generation and propagation of cancer. The observation that many common cancers (including colon, breast, prostate and lung) are commoner in the Western world than in countries such as India, where there is high natural dietary curcumin consumption(Reference Chainani-Wu22), while not indicative of cause and effect, is intriguing. Curcumin has been investigated as both chemotherapeutic and chemopreventive agent in many different animal (largely rodent) models of carcinogenesis. Its chemopreventive efficacy for colon cancer is particularly well established(Reference Rao, Simi and Reddy84, Reference Kim, Araki and Kim85). Other gastrointestinal cancers against which curcumin has shown protective effects include oesophageal(Reference Ushida, Sugie and Kawabata86), stomach(Reference Ikezaki, Nishikawa and Furukawa87), liver(Reference Chuang, Cheng and Lin88) and oral(Reference Azuine and Bhide89); all in rodent models. Curcumin also shows chemopreventive properties in rodent models of various extra-intestinal cancers, including breast(Reference Huang, Lou and Xie90), lung(Reference Hecht, Kenney and Wang91), kidney(Reference Okazaki, Iqbal and Okada92), bladder(Reference Sindhwani, Hampton and Baig93), blood(Reference Huang, Lou and Xie90) and skin(Reference Limtrakul, Lipigorngoson and Namwong94) (Table 2).
Table 2 Animal models in which curcumin has chemopreventive efficacy
Chemotherapy
Curcumin inhibits tumour growth and metastasis, and has chemosensitising and radiosensitising properties. One of the earliest examples of the ability of curcumin to inhibit tumour growth is that of lymphoma cells in a mouse ascites model, when it was administered intraperitoneally at 50 mg/kg(Reference Kuttan, Bhanumathy and Nirmala95). Curcumin also has anti-tumour efficacy against human melanoma cell xenografts if given intraperitoneally(Reference Odot, Albert and Carlier96). Also in xenograft models, sub-cutaneous delivery of curcumin suppresses growth of head and neck squamous carcinoma cells(Reference LoTempio, Veena and Steele97), and when given orally it inhibits proliferation and angiogenesis and induces apoptosis in prostate cancer cells(Reference Dorai, Cao and Dorai98).
Curcumin also suppresses proliferation and angiogenesis and enhances apoptosis in pancreatic cancer; both when given orally in combination with gemcitabine in an orthotopic model(Reference Kunnumakkara, Guha and Krishnan99), and in a xenograft model when given intravenously in a liposomal formulation(Reference Li, Braiteh and Kurzrock100). The same group have also used an intravenous liposomal curcumin preparation in luminal gastrointestinal cancers, where it has chemosensitising properties against colorectal cancer in a mouse xenograft model(Reference Li, Ahmed and Mehta101). In this work, tumour growth and angiogenesis were inhibited and apoptosis enhanced in combination with oxaliplatin. In an orthotopic implantation model of hepatocellular carcinoma, curcumin also prevented intrahepatic metastasis(Reference Ohashi, Tsuchiya and Koizumi102).
Finally, in recent work, oral curcumin has shown efficacy in preventing breast cancer metastasis to lung in orthotopic models, both as chemosensitiser in conjunction with paclitaxel(Reference Aggarwal, Shishodia and Takada103) and in the prevention of its haematogenous spread in immunodeficient mice(Reference Bachmeier, Nerlich and Iancu104). Curcumin given intraperitoneally in combination with docetaxel inhibits tumour growth and angiogenesis in an orthotopic nude mouse model of ovarian cancer(Reference Lin, Kunnumakkara and Nair105).
Human trials
The wealth of in vitro and pre-clinical data has provided a strong basis from which to progress to the trialling of curcumin in human subjects. Many of the molecular efficacies of curcumin demonstrated in cell culture systems and animal models are comparable to those seen in human subjects (Fig. 3). The anti-inflammatory targets of curcumin including reduction of NF-κB, COX2 and pro-inflammatory cytokines such as IL-1, IL-6 and TNF-α, translate into clinical anti-inflammatory efficacy with improvement of rheumatoid arthritis(Reference Kobelt106, Reference Deodhar, Sethi and Srimal107), psoriasis(Reference Heng, Song and Harker108), post-operative inflammation(Reference Satoskar, Shah and Shenoy109), chronic anterior uveitis(Reference Lal, Kapoor and Agrawal110) and orbital inflammatory pseudo-tumours(Reference Lal, Kapoor and Asthana111). Concordant with the finding that high concentrations of curcumin are achievable in gastrointestinal tissue, curcumin shows clinical benefit in irritable bowel syndrome(Reference Bundy, Walker and Middleton112), tropical pancreatitis(Reference Durgaprasad, Pai and Vasanthkumar113), gall bladder and biliary motility(Reference Niederau and Gopfert114–Reference Rasyid, Rahman and Jaalam116), gastric ulceration(Reference Prucksunand, Indrasukhsri and Leethochawalit117) and familial adenomatous polyposis coli(Reference Cruz-Correa, Shoskes and Sanchez118). The in vitro findings of enhanced PPAR-γ expression and modulation of NOS, glutathione and other antioxidant activities are supported by the clinical potency of curcumin to lower serum cholesterol(Reference Soni and Kuttan119) and improve endothelial function in type 2 diabetes mellitus(Reference Usharani, Mateen and Naidu120). Curcumin also enhances early post-transplant renal graft function(Reference Shoskes, Lapierre and Cruz-Correa121), presumably through multiple mechanisms.

Fig. 3 Clinical effects of curcumin: results from human trials (with references).
Consistent with the strong pre-clinical evidence of benefit in animal models of IBD, curcumin is showing early promise as a treatment for CD and UC in human subjects. In a small open-label study of five patients with CD and five with ulcerative proctitis, improvements in clinical and laboratory parameters with reduction in need for concomitant medications were observed in nine out of ten cases(Reference Holt, Katz and Kirshoff122). Further encouraging results came from a larger multicentre, randomised, double-blind, controlled trial of eighty-nine patients with quiescent UC, in which two out of forty-three patients (5 %) taking oral curcumin had relapsed by 6 months compared with eight out of thirty-nine (21 %) in the placebo group(Reference Hanai, Iida and Takeuchi123). The investigators also showed significant clinical and endoscopic improvements in the curcumin-treated group.
There is a strong foundation of evidence from both in vitro and animal models that curcumin has anti-cancer actions, including its pro-apoptotic and anti-angiogenic effects and its modulation of the cell cycle, growth factor expression and signal transduction pathways. Building upon this foundation, curcumin appears to prevent and treat cancer in human subjects. Results from a trial of twenty-five patients with various different pre-malignant or high-risk lesions suggested that oral curcumin may have chemopreventive effects in progression of these lesions(Reference Cheng, Hsu and Lin4). While two of the twenty-five patients progressed to frank cancer, seven regressed; a remarkably high proportion considering the high-grade nature of the lesions (bladder cancer, oral leukoplakia, gastric intestinal metaplasia, cervical intraepithelial metaplasia and Bowen's disease). In another uncontrolled study of fifteen patients with advanced colorectal cancer refractory to standard treatments, the lymphocytic biomarker glutathione S transferase showed a 59 % reduction in activity with low-dose (440 mg daily) oral curcuma extract, and five patients maintained radiologically stable disease over the 2- to 4-month study period(Reference Sharma, McLelland and Hill124). Once again there is a suggestion here that curcumin exhibits paradoxical efficacy at low v. high dose, since this effect was not observed at higher doses. In an interesting, but also uncontrolled, study of sixty-two patients with oral cancerous lesions, topical curcumin application reduced symptoms in the majority (70 %) and caused tumour shrinkage in 10 %(Reference Kuttan, Sudheeran and Josph125). Of twenty-one patients with advanced, normally rapidly fatal, pancreatic cancer treated with high-dose oral curcumin, encouragingly four showed disease stability or regression(Reference Dhillon, Aggarwal and Newman126).
These preliminary data hold promise, and interest in curcumin as a therapeutic agent continues to grow. There are several clinical trials currently ongoing, some involving larger numbers of patients and with a more rigorous, randomised, controlled design. A search on clinicaltrials.gov currently reveals thirty-one human trials using curcumin, of which fourteen are investigating its chemopreventive or chemotherapeutic potential in cancer or pre-malignant conditions. As in the data already reviewed, there is a preponderance of gut cancers; six are in colorectal cancer, two in familial adenomatous polyposis coli, one in UC and three in pancreatic cancer. A novel area of interest is in Alzheimer's disease and cognitive impairment. The first clinical trial failed to show benefit, but this may have been due to an unexpected lack of cognitive decline in the placebo group(Reference Baum, Lam and Cheung127). Three current ongoing trials of curcumin are further assessing its efficacy in age-related cognitive impairment. Interest also continues in systemic inflammatory conditions, and there are two ongoing trials of curcumin in arthritis and one in psoriasis.
Summary and conclusions
Since ancient times, curcumin has been used in a wide range of inflammatory, neoplastic and other conditions. In recent years, the molecular basis for its efficacy has been extensively investigated. Many cellular and molecular targets have been identified and many questions still remain. In complex multifactorial illnesses such as systemic inflammatory diseases and cancer, an agent that acts at a number of different cellular levels offers perhaps a better chance of effective prophylaxis or treatment. Its non-toxicity and good tolerability in human subjects, in combination with strong promising results from cell line, animal and early human clinical studies, support the ongoing research and development of curcumin as a preventive and disease-modifying agent.
Acknowledgements
This work was supported by the Crohn's in Childhood Research Association. There are no conflicts of interest. J. E. prepared and wrote the manuscript and designed the tables and figures. I. R. S. contributed to the preparation of the manuscript and was the Principal Investigator on the grant which funded the work. T. T. M. contributed to the preparation of the manuscript and advised on its structure and organisation. All the authors read and approved the manuscript.







