The increase in the prevalence of chronic noncommunicable diseases, including diabetes mellitus, results in a concern in the search for strategies for their prevention.
Diabetes is a metabolic disease characterised by chronic high blood glucose, which is associated with the development of long-term complications, if not controlled(Reference Sanz-Paris, Álvarez Hernández and Ballesteros-Pomar1,2) .
According to data from the International Diabetes Federation, there are about 537 million known cases of diabetes worldwide, while approximately 239·7 million remain undiagnosed. In this scenario, Brazil is the sixth country in the world ranking in number of individuals with diabetes (15·7 million), and these data are more worrying when we consider that this contingent could increase to 23·2 million in 2045(3).
Recently, adequate glycaemic control has gained more focus due to the COVID-19 pandemic, since patients with DM are at greater risk of developing the severe form of the disease and have higher mortality(Reference Fleming, Sacks and Pham4).
In addition, coronavirus pandemic has resulted in large numbers of critically ill patients with high blood glucose, even in individuals without diabetes(Reference Morse, Gay and Korwek5). High blood glucose in hospitalised patients is related to worse outcomes, longer length of stay, lower chance to be discharged home and higher in-hospital mortality(Reference Umpierrez, Isaacs and Bazargan6).
In this sense, it is increasingly evident that glycaemic control is essential. The guidelines of the world’s leading nutritional therapy societies for diabetes, as well as for critically ill patients, recommend the use of specialised oral and/or enteral formula for glycaemic control, due to its lower impact on blood glucose(Reference Sanz-Paris, Álvarez Hernández and Ballesteros-Pomar1,2,Reference Castro and Ribeiro7–Reference Campos, Chaer and Hafez9) .
These recommendations are based on studies that demonstrate benefits in reducing postprandial blood glucose, the need for insulin application, low blood glucose episodes, and, consequently, glycaemic variability. In addition, the use of these formulas is related to the reduction of costs and hospitalisation time when compared to the use of standard formulas(2,Reference Campos, Chaer and Hafez9) .
Interest in food glycaemic index (GI) has been growing steadily, as it is a marker of the quality of carbohydrates present in food. Meals with low GI food result in a lower increase in postprandial blood glucose and lower insulin release(Reference Augustin, Kendall and Jenkins10), which avoids hyperinsulinemia peaks.
However, the glycaemic response is also determined by the amount of available carbohydrate consumed. In this sense, we have the concept of glycaemic load (GL), which relates both the quality and quantity of carbohydrates(Reference Foster-Powell, Holt and Brand-Miller11).
Due to the evidence of health benefits of low GI diets, determining the GI and GL of food is important. The International Carbohydrate Quality Consortium states that low GI and GL should be considered in association with other characteristics of carbohydrate foods, such as fibre and wholegrains amount, within the context of healthy diets, being more important for individuals with insulin resistance(Reference Augustin, Kendall and Jenkins10). Researchers have been studying the GI of various foods and compiled into tables, the most recent including over 4000 items(2).
Sanz-Paris et al. (Reference Sanz-Paris, Álvarez Hernández and Ballesteros-Pomar1) recommended the addition of slow-release and low GI carbohydrates to enteral nutrition formulas for glycaemic control. Isomaltulose is a low GI disaccharide(Reference Camps, Kaur and Lim12) composed of a glucose molecule and a fructose molecule, joined by an α-1·6-glycosidic bond, produced by an enzymatic process that generates a bond of more difficult digestion, resulting in slower digestion and absorption, positively impacting the glycaemic response(Reference Atkinson, Brand-Miller and Foster-Powell13,Reference Holub, Gostner and Theis14) .
Therefore, this study aimed to evaluate the glycaemic response (GI and GL) in the ingestion of two formulas for oral and enteral nutrition, a specialised formulation for glycaemic control and its modified version, with the addition of isomaltulose.
Methods
This was a single-blind clinical trial, with a duration of 5 weeks, held at the Food Research Center (FoRC) – School of Pharmaceutical Sciences, University of São Paulo (USP).
Population
Healthy volunteers (n 18) were recruited through advertisements posted on USP’s bulletin boards.
The sample size was determined according to the protocol proposed by Brouns et al. and by ISO 26642 (Reference Brouns, Bjorck and Frayn15,16) , which considers that tests must be performed on at least ten volunteers to increase the accuracy of the results.
Inclusion criteria were age between 18 and 49 years, both sexes, with good health conditions according to a report of absence of diabetes, hyperthyroidism, and renal and gastrointestinal diseases; BMI within the eutrophic range 18·5 ≤ BMI ≥ 24·9 kg/m2; with normal glucose tolerance (between 70–99 mg/100 ml in the morning, after fasting for 10 h), and maximum postprandial blood glucose of 140 mg/100 ml and close to fasting after 2 h(Reference Brouns, Bjorck and Frayn15,17) .
The exclusion criteria were the use of any type of medication that could affect digestion and food absorption (antibiotics, medications for diarrhoea and constipation) during the study period, hormone therapy, pregnancy or breast-feeding, family history of diabetes, and those who showed significant blood glucose variations in the glycaemic response test to the reference food(Reference Brouns, Bjorck and Frayn15). Sixteen volunteers completed the trial.
Experimental protocol for determining glycaemic index
Reference food glycaemic response test (glucose)
Glucose (portion with 25 g of available carbohydrates in 200 ml of water) was ingested by the volunteers in 10–15 min, after fasting for 10 h. Blood samples were collected by fingertip capillary blood sampling and determined by glucometer, before (t = 0) and after ingestion, at the following times: 15, 30, 45, 60, 90 and 120 min, totalling seven collections per d. This procedure was repeated three times, with an interval of 7 d, for each individual(Reference Brouns, Bjorck and Frayn15,17) .
Glycaemic response test with enteral diet
Two formulas for enteral nutrition were consumed in the following 2 weeks, with an interval of 7 d, in an amount sufficient to provide 25 g of available carbohydrates, after 10 h of fasting. Capillary blood samples were obtained following the same protocol described for the reference food(Reference Brouns, Bjorck and Frayn15). The amount of food to be consumed was calculated based on the content of ‘available’ carbohydrates present in the food, calculated only by the sum of the soluble sugars (glucose, fructose and sucrose), since the products do not have available starch.
Analysed formulas
Two formulas for oral or enteral nutrition intended for glycaemic control were analysed: Diamax® Original (DiamaxO) and Diamax® IG (DiamaxIG) (Prodiet Medical Nutrition, Curitiba, Brazil) (Table 1). DiamaxO has 4·2 kJ (1·0 kcal)/ ml, 17 % protein, 44 % carbohydrates, 39 % lipids, and addition to twenty-eight vitamins and minerals in its composition, with vanilla flavour. The 200 ml portion provides 840 kJ (200 kcal), 22 g of carbohydrates (100 % tapioca maltodextrin), 8·6 g of protein, 8·6 g of lipids, comprising 26 % of MUFA and 3 g of dietary fibre. DiamaxIG had its formulation changed with a reduction of carbohydrate content to 40 % and replacement of 20 % of tapioca maltodextrin with isomaltulose, aiming to reduce the glycaemic response to the product, in addition to increasing the protein and lipid content.
Table 1. Nutrition facts of the DiamaxO and DiamaxIG liquid formulas for oral or enteral nutrition (100 ml)

Determination of soluble sugars
Soluble sugars, after tapioca maltodextrin digestion process, were quantified by HPLC(Reference Cordenunsi, Shiga and Lajolo18).
Glycaemic response curve and calculation of the glycaemic index and glycaemic load
The primary outcomes are the determination of glycaemic response, GI and GL.
For the elaboration of the glycaemic response curve, blood glucose at the following times was used: 0, 15, 30, 45, 60, 90 and 120 min. The incremental AUC was calculated geometrically, applying the trapezoidal rule and ignoring the areas below the fasting line(Reference Brouns, Bjorck and Frayn15–17,Reference Jenkins, Wolever and Taylor19,Reference Wolever, Vorster and Björck20) .
The GI of each food was calculated using the following equation(Reference Wolever, Vorster and Björck20): GI = AUC of test food/ AUC of reference food (glucose) × 100.
The average GI of each food was calculated with the individual values of the area under the glycaemic curve of each individual. The value of the area produced by glucose was considered as a reference (100 %).
The GL of each food was calculated using the following equation(Reference Liu, Willett and Stampfer21,Reference Ludwig22) : GL = GI (glucose control) × grams of ‘available’ carbohydrate per serving/100.
The recommended portion for this type of product is 200 ml. The products were classified according to the reference GI and GL(16,17,23) (Table 2).
Ethical aspects
All volunteers were previously informed about the details of the protocol and the risks involved in participating and signed a Free and Informed Consent Form to participate in the study, in accordance with the Declaration of Helsinki. The University’s Research Ethics Committee approved the protocol (CEP/FCF number 2.814.784). The study was registered in ReBEC (Identifier: RBR-6zw2fnb https://ensaiosclinicos.gov.br/rg/RBR-6zw2fnb).
Statistical analysis
GI results were presented as mean ± standard error. Statistical analysis was performed using the Statistica® 12.0 software (StatSoft Inc.). ANOVA with repeated measures was performed, with post hoc Bonferroni test to determine significant differences between the three foods. We used Student’s t test to compare the data from the two supplements. The values of P < 0·05 were considered significant.
Results
Sixteen volunteers were selected (eleven women and five men), with a mean age of 29·9 ± 7·1 years; mean weight of 63·9 ± 10·8 kg and mean height of 1·73 ± 0·1 m.
The average of three batches of the product DiamaxO studied presented an average of 8·7 g/100 ml of available carbohydrates and the product DiamaxIG presented 8·1 g/100 ml (Table 3).
Table 3. Profile of available carbohydrates (g/100 g) present in DiamaxO and DiamaxIG liquid formulas for oral or enteral nutrition

Data presented as mean ± standard error.
Based on these data, the volunteers consumed the 287 ml portion of DiamaxO and 308 ml of DiamaxIG to contain the same 25 g of available carbohydrates as the reference food. The clinical trial showed that the studied samples had a low GI = 37·8 for DiamaxO and GI = 21·5 for DiamaxIG (Table 4), with a significant difference between the GI of the reference food (glucose) and both formulas, but also between the two formulas (Table 5). They also presented low GL = 6·6 for the original formula and GL = 3·5 for the modified one (Table 4).
Table 4. Glycaemic index (GI) and glycaemic load (GL), in healthy volunteers (n 16), of reference food and liquid formula for enteral or oral nutrition

Different letters represent significant differences (ANOVA, post hoc Bonferroni, P < 0·05).
* Data presented as mean ± standard error for reference food(Reference Brouns, Bjorck and Frayn15).
** Reference food.
*** Difference between the two supplements according to Student’s t test (P = 0·0002)
† Portion according to ANVISA.
‡ Classification of GI and GL according to reference values. H = high, M = medium and L = low.
Table 5. Bonferroni test for glucose and formulas for enteral and oral diets, in relation to the glycaemic index (GI)
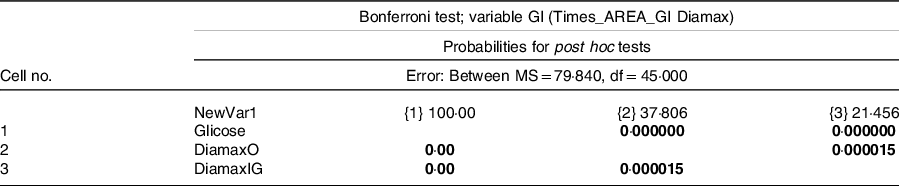
Note: Font in bold represents significant difference (P < 0.05).
Table 6 shows the average glycaemic response values for 120 min of the reference food and the analysed formulas.
Table 6. Glycaemic response (mmol/l) and AUC of healthy volunteers (n 16) during 120 min, after consumption of glucose and Diamax enteral and oral nutrition liquid formulas
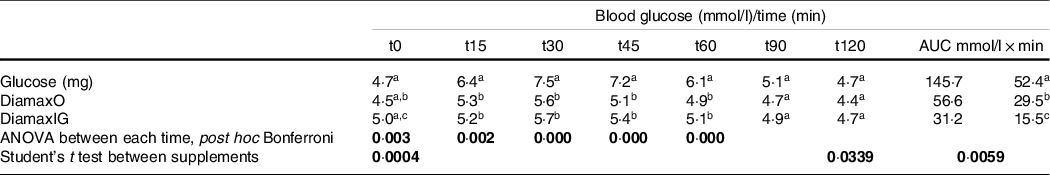
Different letters in the same column represent significant differences (ANOVA, post hoc Bonferroni, P < 0·05), considering glucose and the two supplements. Font in bold represents significant difference (p < 0.05).
Note: There was no difference during ANOVA between the two supplements. However, there are differences between the two Diamax products using Student’s t test, which compares two samples.
Figure 1 shows the glycaemic response curves, which showed a marked difference in relation to all times, except for T0 and T120, which usually approximates the value of fasting blood glucose after 2 h of consumption of food source of carbohydrate. The peak of glycaemic response, both for the reference food and the test foods, occurred 30 min after ingestion, but the difference in blood glucose between the foods at this point is very accentuated. Differences were also significant at times 15, 30, 45 and 60 min in relation to glucose, but not between the two products.

Fig. 1. Mean glycaemic response of volunteers (n 16) to 25 g of available carbohydrates after consumption of reference food and liquid formula for enteral and oral nutrition DiamaxO and DiamaxIG, in 120 min.
We highlight that the two formulations showed a significant difference in blood glucose at time zero (P = 0·000414, t test). The modified product showed less variation between peak glycaemic response and fasting blood glucose (Δ = 0·7 mmol/l for DiamaxO and Δ = 1·1 mmol/l for DiamaxIG). This is reflected in the significant difference in the calculated areas when we compared the original and the modified products.
Discussion
The present study evaluated the glycaemic response to the DiamaxO and DiamaxIG formulas with the determination of GI and GL, resulting in a low glycaemic response, as well as a low GI and low GL, especially for the modified formula.
The postprandial glycaemic response to a food is related both to the quality, assessed by the GI, and to the amount of carbohydrates in the portion, determining the GL. A food can have high GI and low GL, as is the case with fruits such as pineapple(Reference Menezes, Giuntini and Dan24).
Among carbohydrates, the majority mono- and disaccharides are more rapidly absorbed, as they depend only on enzymes and/or brush border transporters. Among oligo- and polysaccharides, researchers believed that the long chain length would result in slower digestion and absorption. After much research over the last few decades, researchers observed that chain size per se does not lead to lower postprandial glycaemia and insulinaemia, which is dependent on carbohydrate quality(Reference Ludwig, Hu and Tappy25).
An example of this are maltodextrins, which are saccharide polymers with linear D-glucose units linked primarily with α-1,4 bonds, but also may have a branched structure through α-1,6 bonds, which will not be hydrolysed. Its digestion begins in the mouth, through the action of salivary α-amylase. In the duodenum, they are hydrolysed to maltose through the action of pancreatic α-amylase, which acts on α-1,4 bonds. Maltose can be absorbed directly by the intestinal epithelium and also degraded by maltase, present in the brush border, resulting in free glucose that will reach the blood. Despite being polysaccharides, available maltodextrins are considered easily digestible carbohydrates, although there may be differences in branching rate(Reference Hofman, van Buul and Brouns26).
Even though the process of digestion and absorption is different from that of glucose, the glycaemic response after ingestion of available maltodextrins can be similar(Reference Hofman, van Buul and Brouns26–Reference Tan, Chia and Ponnalagu28).
Although the specialised formulas Diamax have the highest proportion of carbohydrates in the form of tapioca maltodextrin, both products have low GI and GL. This is possibly due to a combination of factors, since the GI also assesses the influence of the food matrix, which can protect starch from digestion, and the presence of other nutrients or components can affect carbohydrate absorption, depending on the source(Reference Augustin, Kendall and Jenkins10,Reference Ludwig, Hu and Tappy25) . Diamax formulas combine good quality carbohydrates, including dietary fibre and slow-digesting carbohydrate, and good distribution of macronutrients, with only 10 g of carbohydrates available in 100 g of DiamaxIG. Furthermore, it is also important to remember that the gastric emptying rate is regulated by effects related to the volume and composition of macronutrients so that more concentrated beverages have lower emptying rates than more diluted beverages(Reference Hofman, van Buul and Brouns26).
Moreover, we can relate the low glycaemic response of the two specialised formulations to their nutritional characteristics such as lower content and specific type of carbohydrates, high content of MUFA, presence of protein and fibre(Reference Sanz-Paris, Álvarez Hernández and Ballesteros-Pomar1). The partial replacement (20 %) of the carbohydrates in the DiamaxO formula by another carbohydrate with low GI (isomaltulose) resulted in a significant reduction in the glycaemic response(Reference Sanz-Paris, Álvarez Hernández and Ballesteros-Pomar1), which can be observed in both AUC and variation between the peak at 30 min in relation to T0.
Chemically, isomaltulose is an isomer of sucrose, which contains an α-1,6 rather than α-1,2 glycosidic bond between glucose and fructose, which occurs by enzymatic rearrangement. This is a stable and strong bond, so it is hydrolysed slowly in the small intestine – 4 to 5 times slower than sucrose. Hydrolysis occurs through the same sucrose enzyme system, the sucrase–isomaltase complex, and the absorption takes place along the entire small intestine, not only in the upper parts of the small intestine, where the hydrolysis is complete and no significant amounts of isomaltulose reach the large intestine(Reference Sawale, Shendurse and Mohan29).
Due to the slower digestion of isomaltulose, increases in blood glucose and insulin levels after its ingestion are reduced, reaching lower maximum values than those caused by sucrose, being able to acutely reduce glycaemic response and variability. Several studies have demonstrated these and other benefits of the use and consumption of isomaltulose(Reference Camps, Kaur and Lim12,Reference Holub, Gostner and Theis14,Reference Sardá, Giuntini and Nazare30–Reference Henry, Kaur and Quek32) .
In a study that compared a low GI diet with the incorporation of isomaltulose with a high GI diet, the authors observed that the low GI diet resulted in a lower 24-h AUC of glucose (502·5 ± 231·4 v. 872·6 ± 493·1 mmol/l; P = 0·002) and lower glycaemic variability (mean amplitude of glycaemic excursion: 1·67 ± 0·53 v. 2·68 ± 1·13 mmol/l; P < 0·001), showing that the addition of isomaltulose to a low GI meal was able to acutely reduce the glycaemic response and 24-hour glycaemic variability(Reference Henry, Kaur and Quek32).
A review on the beneficial effects of the control of glycaemia by ingestion of isomaltulose on health included an analysis of blood glucose obtained in twelve clinical studies. The authors observed that postprandial glycaemic responses in the first 60 min after ingestion of isomaltulose were 20 % to 52 % lower compared to ingestion of sucrose or maltodextrin. As a result, plasma insulin levels and areas under the glucose curve were also 30 % to 50 % lower(Reference Maresch, Petry and Theis33). In the present study, these values were even more reduced, and the variation of the glycaemic peak in 30 min in relation to T0 was 75 % lower after DiamaxIG consumption in relation to glucose and approximately 93 % in 60 min. The AUC (120 min) of DiamaxIG was reduced by 78 % when compared with glucose, but as it is a formula for a specific public, it has other characteristics in its formulation, such as dietary fibre, monounsaturated fat and protein contents, as recommended by the diabetes nutritional therapy guidelines(Reference Sanz-Paris, Álvarez Hernández and Ballesteros-Pomar1,2) , which may have contributed to increase this difference.
A meta-analysis was performed with eleven clinical trials (n 175 participants), from four countries, to assess the efficacy of isomaltulose and the quality of the evidence. The authors concluded that the replacement of high GI carbohydrates by isomaltulose may be associated with an attenuated and more prolonged glycaemic response and that some people may particularly benefit from its use, such as patients with type 2 diabetes, glucose intolerance, hypertension, as well as elderly, overweight and obese people(Reference Xie, Li and Qin34). The formulation of the products studied here may be indicated for people who have these health problems.
The low GI and low GL results found for the Diamax formulas corroborate data found in the literature. In a study that compared specialised enteral nutrition formulas for glycaemic control with standard formulas, the authors found that the former had a significantly lower GI than the latter (19·4 ± 1·8 v. 42·1 ± 5·9; P = 0·004), with those with lower GI being characterised by a lower carbohydrate content. However, unlike the present study, the formulas with low GI had fructose in their composition(Reference Hofman, de Van Drunen and Kuipers35).
Still, the addition of fructose is controversial. Despite having low GI, sweetening power and insulin-dependent entry into the cell, fructose in high doses can cause hypertriacylglycerolaemia, increased LDL-cholesterol and insulin resistance(Reference Sanz-Paris, Álvarez Hernández and Ballesteros-Pomar1). In addition, the Brazilian Society of Diabetes contraindicates the addition of fructose to foods(2) and the Canadian Diabetes Association recommends limiting its consumption to 10 % of the total energy value(Reference Sievenpiper, Chan and Dworatzek36).
The objective of the present study was to determine the glycaemic response of two specialised formulas, which, as demonstrated, showed low GI, GL and glycaemic response. Several studies have demonstrated the clinical impact of using formulas like these on different clinical outcomes. In a study that compared the administration of a specialised formula for glycaemic control with a standard enteral formula, the authors found that the maximum concentration of serum glucose and mean blood glucose were significantly lower in critically ill patients who received the specialised diet(Reference Egi, Toda and Katayama31).
Another study with a similar objective found that critically ill patients who received low-carbohydrate enteral formulas, in addition to having lower mean glucose (7·8 ± 1·0 v. 8·4 ± 1·1 mmol/l, P = 0·007), also required significantly less insulin (46·8 v. 68·0 μg, P = 0·036) than those who received standard enteral formulas(Reference van Steen, Rijkenberg and Sechterberger37).
In addition, low GI and/or GL diets have been linked to several health benefits. Meta-analyses showed that studies in patients with type 2 diabetes found decreases in HbA1c, fructosamine, fasting blood glucose, BMI, and total and LDL-cholesterol(Reference Livesey, Taylor and Hulshof38–Reference Chiavaroli, Lee and Ahmed42).
In a study that compared a high GL control diet and a low GL diet, it was possible to observe that the latter resulted in a better 24-h glycaemic response, evidenced by reduced glycaemic variability, lower peak glucose levels, longer time to goal and improved postprandial glycaemic response(Reference Camps, Kaur and Lim12). In addition, another study observed greater fat oxidation with the consumption of a low GI diet, even in a sedentary state, favouring weight reduction(Reference Henry, Kaur and Quek32). This corroborates the recommendation to use specialised oral nutritional supplements for glycaemic control for weight loss(2).
The use of these specialised formulas with low GI results in less elevation and variation in blood glucose, leading to reduced insulin release to metabolise glucose from this kind of food, which may be clinically beneficial due to better glycaemic control. Therefore, the use of these formulas should be the preferred option for the nutritional management of diabetic patients or patients with high blood glucose in need of nutritional support(Reference Sanz-Paris, Álvarez Hernández and Ballesteros-Pomar1,Reference Egi, Toda and Katayama31,Reference Hofman, de Van Drunen and Kuipers35) .
Although the increase in blood glucose is related to the release of insulin, the determination of the glycaemic response does not allow an exact assessment of the impact on postprandial insulin after the ingestion of low GI/GL food in relation to the traditional food.
Conclusion
The present study confirmed the low GI and GL of two specialised formulas for glycaemic control, showing that the glycaemic response to the consumption of the products is quite reduced and presenting a curve with a little accentuated shape, without high peak, especially for the modified product, typical of foods with reduced glycaemic response.
Acknowledgements
The authors thank all the volunteers in this clinical trial and all others who directly or indirectly helped us in the preparation of this study.
E. B. G conceived and designed the study, participated in data collection, as well as in the analysis and discussion of the results. B. D. G. M. F. contributed to the conception and design of the study. A. C. Z., H. S. and A. P. M. C. participated in the analysis and discussion of data and drafting of the article. All authors reviewed and agreed to the final version of this manuscript.
The research was on a grant supported by Prodiet Medical Nutrition.
A. C. Z. and H. S. are Prodiet Medical Nutrition Employees; A. P. M. C.: Technical, Director and Co-founder of Prodiet Medical Nutrition; E. B. G. and Franco B. D. G. M.: Food Research Center (FoRC/CEPID/FAPESP) researchers.










