In addition to the well-established critical role that sufficient Ca intake plays in skeletal development and health( Reference Crenshaw, Lewis and Southern 1 , Reference Soares, Murhadi and Kurpad 2 ), there has been growing evidence that dietary Ca has further benefits, commencing in the intestinal lumen, where Ca was shown to exert anti-inflammatory and cytoprotective effects when it was available at higher luminal concentrations( Reference Schepens, Schonewille and Vink 3 – Reference Ten Bruggencate, Snel and Schoterman 5 ). These effects were linked to the high buffering property of Ca, which abrogated negative aspects of intense fermentation in the intestinal lumen on mucosal integrity in rodent models( Reference Ten Bruggencate, Snel and Schoterman 5 ). Moreover, luminal Ca is essential for intestinal epithelial function, because it affects the composition of intestinal plasma membranes and para- and transcellular Ca transport either directly via the modulation of signalling pathways or indirectly via the modification of the vitamin D endocrine response( Reference Ballard, Hunter and Taylor 6 , Reference Centeno, de Barboza and Marchionatti 7 ). In adult rodents, the colon was proposed as the main intestinal site of action for Ca( Reference van Ampting, Schonewille and Vink 8 ). In a recent study with weaned pigs, however, we found that the main responding intestinal site was the small intestine and not the colon( Reference Metzler-Zebeli, Gänzle and Mosenthin 9 ). Overall, Ca effects on the intestinal mucosa in the juvenile phase have not been explored in detail, and there is little information available about the potential effects of high Ca intake on the intestinal digestive, absorptive, immunal and barrier functions of young animals. Young mammals, like pigs, are very vulnerable to intestinal perturbations in the immediate phase after weaning because of the immaturity of their mucosal immunity, digestive capacity and microbiota( Reference Bauer, Metzler-Zebeli and Verstegen 10 , Reference Kim, Hansen and Mullan 11 ), and they may benefit from the anti-inflammatory and cytoprotective effects of extra dietary Ca. The composition of the dietary complex carbohydrate fractions may mitigate the effect of high dietary Ca supply either by stimulating intestinal Ca absorption or by complexing Ca intestinally( Reference Metzler-Zebeli, Gänzle and Mosenthin 9 ). Whole grains are typical sources of dietary fibre in human and animal diets; however, common dietary cereals, such as wheat, barley and maize, largely differ in their cellulose and hemicellulose fractions( Reference Knudsen 12 ). Therefore, potential Ca effects on intestinal mucosal functions should be investigated when considering differences between the complex carbohydrate fractions of the basal diet to account for the interaction between dietary Ca concentration and dietary cereals. Thus, we hypothesised that a dietary Ca intake that is much higher than the actual requirement may reduce intestinal pro-inflammatory cytokine expression and also up-regulate the expression of nutrient transporters and tight junction proteins in the small and large intestines of young pigs and that dietary cereal composition would interact with dietary Ca concentration in intestinal gene expression.
Aside from direct regulation( Reference Bauer, Metzler-Zebeli and Verstegen 10 , Reference Shimizu 13 ), dietary ingredients modulate the intestinal mucosal response by altering the intestinal microbiota and their metabolites which are generated during the fermentation of diet-derived substrates( Reference Leser and Mølback 14 ). Consequently, dietary composition, including carbohydrate fractions and micronutrient concentrations, can be targeted to selectively stimulate bacteria with known health-promoting effects for the host( Reference Leser and Mølback 14 – Reference Mann, Schmitz-Esser and Zebeli 18 ). Due to their ultimate proximity to the enterocytes, it is accepted that intestinal mucosa-associated microbiota have a significant impact on mucosal gene and protein expression( Reference Leser and Mølback 14 ). Considering these findings, we further hypothesised that the mucosal expression of genes would be connected to specific mucosa-associated bacteria and luminal microbial metabolites independently of the composition of the diet fed to the animals.
To test our hypotheses, the present study was designed to investigate the effect of dietary Ca concentration on the expression of candidate genes that target nutrient absorption, barrier function and mucosal inflammatory response in the jejunum and colon of male weaned pigs. In addition, indirect estimates of digestion and absorption, which were based on apparent total tract digestibility (ATTD) and the retention of nutrients, were applied to study the influence of Ca on intestinal nutrient uptake, and serum acute-phase proteins were determined as markers of systemic inflammation. To evaluate potential Ca × dietary cereal interactions on intestinal mucosa and nutrient digestion, two basal diets with contrasting cereals (wheat–barley v. maize) were formulated. The relationship between mucosal gene expression and mucosa-associated bacteria and luminal microbial metabolites was assessed for the colon using recently published data( Reference Metzler-Zebeli, Mann and Schmitz-Esser 17 , Reference Mann, Schmitz-Esser and Zebeli 18 ).
Materials and methods
The experimental design and methods have been described in detail recently( Reference Metzler-Zebeli, Mann and Schmitz-Esser 17 – Reference Metzler-Zebeli, Ertl and Klein 19 ).
Animals and experimental design
All procedures involving animal handling and treatment were approved by the institutional ethics committee of the University of Veterinary Medicine Vienna and the national authority according to §8ff of the Law for Animal Experiments, Tierversuchsgesetz – TVG (GZ 68.205/0222-II/3b/2011). In the present study, weaned barrows (n 32; (Landrace × Large White) × Piétrain; average body weight 9·5 (sem 0·11) kg; weaning age 28 d) were randomly allocated to four diets (n 8/diet) comprising either wheat and barley or maize as cereals and containing either adequate or high concentrations of Ca (Table 1). The experiment consisted of nine blocks with eight pigs each, with two pigs per run being fed the same diet. In each run, pigs had ad libitum access to the experimental diets for 15 d from day 8 post-weaning (experimental day 1). We used castrated male pigs in order to be able to collect urine and faeces separately.
Table 1 Ingredients (as-fed basis) and analysed chemical composition of the experimental diets( Reference Dostal, Fehlbaum and Chassard 16 – Reference Mann, Schmitz-Esser and Zebeli 18 )

* Provided per kg of complete diet: 10 000 IU (3000 μg) of vitamin A, 2222 IU (56 μg) of vitamin D3, 62·5 mg of vitamin E, 1·67 mg of vitamin B1, 4·45 mg of vitamin B2, 2·22 mg of vitamin B6, 0·022 mg of vitamin B12, 2·2 mg of vitamin K, 22·2 mg of niacin, 11·11 mg of pantothenic acid, 500 mg of choline chloride, 0·05 mg of biotin, 0·56 mg of folic acid, 25 mg of vitamin C, 44 mg of Mn (as MnO), 89 mg of Zn (as ZnSO4), 153 mg of Fe (as FeSO4), 13 mg of Cu (as CuSO4), 0·44 mg of Se (as Na2SeO3), 1·67 mg of I (as Ca(IO3)2).
† Included 500 phytase units (FTU) per kg of complete diet.
‡ Calculated according to Gesellschaft für Ernährungsphysiologie( Reference Metzler-Zebeli, Ertl and Klein 19 ).
Diets met or exceeded the current nutrient requirements for pigs between 10 and 20 kg( 20 ) (Table 1) and were formulated to be isoenergetic and isonitrogenous. Because of the higher fat contencentration in maize, diets differed in their fat source and thus their fatty acid composition. Wheat–barley diets contained 3 % soya oil, whereas maize diets contained only 1·5 % soya oil. Diets adequate in Ca were formulated to meet 100 % of the pigs' Ca and P requirements, and high Ca diets were formulated to contain Ca and P at a concentration of 190 % of the Ca and P requirement for 10–20 kg pigs( 20 ). The calculated Ca and P concentration of adequate Ca diets amounted to 8·0 g Ca and 6·1 g P/kg diet (DM basis), whereas the calculated Ca and P concentration of high Ca diets were 15·1 g Ca and 11·7 g P/kg diet (DM basis). Thus, the calculated Ca:P ratio was 1·3:1 for all diets so that any potential Ca effects on the intestinal mucosa would not be compromised by a diverging Ca:P ratio, as reported in rats( Reference Schepens, Ten Bruggencate and Schonewille 4 ). Microbial phytase was added to the diets to compensate for the different intrinsic phytase activities in wheat, barley and maize( 21 ). Representative diet samples were taken in each experimental block. Feed leftovers (spillage and feed left in the trough) were collected each morning at 08.00 hours before fresh feed was provided. Animals had free access to demineralised water. Pigs were weighed at the commencement, on day 9 and day 15 (end) of the experiment. One pig that had been fed the wheat–barley diet high in Ca was removed from the experiment because it developed meningitis at the commencement of the experiment.
Balance and digestibility study
After 9 d of dietary treatment, pigs' faeces and urine were quantitatively collected from day 10 to day 14 for determination of nutrient digestibility and N, Ca and P balances. The total amounts of faeces and urine produced were recorded during collection. Fresh faecal samples were collected several times daily and frozen at − 20°C. Urine was collected in buckets containing 25 ml of 18·75 m-H2SO4 (VWR International) to minimise microbial fermentation for 24 h, weighed and filtered. Representative subsamples (250 ml) were pooled over the 4 d collection period, kept at 4°C during the collection days and stored at − 20°C until analysis.
Sampling of blood and jejunal and colonic mucosa
On day 15 of the experiment, 3–4 h after receiving fresh feed, animals were anaesthesised (Narketan, 10 ml/kg body weight; Ketamine HCl; Vétoquinol AG and Stresnil, 3 ml/kg body weight; Azaperone; Biokema SA); blood was collected by cardiac puncture into serum collection tubes (Primavette; KABE Labortechnik) and placed on ice until further processing. Blood samples were centrifuged at 1811 g for 10 min at 4°C (Eppendorf Centrifuge 5810 R; Eppendorf) and stored at − 20°C until analysis. Immediately after blood withdrawal, pigs were euthanised by an intracardiac injection of T61 (10 ml/kg, Embutramide; MSD Animal Health) and exsanguinated. The abdominal cavity was opened and the entire gastrointestinal tract was removed. For candidate gene expression, the mid-jejunum (30 cm in the middle of the jejunum) and mid-colon (the middle of the colon after division into three equal segments) were selected as important intestinal sites for digestion and fermentation, respectively. Gut pieces were opened at the mesentery, and digesta were removed. Gut tissues were thoroughly washed in ice-cold PBS, and the mucosa was scraped off using a glass plate. Mucosa samples were immediately snap-frozen in liquid N2 and stored at − 80°C.
Gene expression profiling
Total RNA was extracted and the mRNA expression in jejunal and colonic tissue were determined using quantitative reverse-transcription PCR, which has been described recently(
Reference Metzler-Zebeli, Ertl and Klein
19
). The amplification of target genes for sugar transporters (solute carrier family 2 (facilitated glucose transporter), member 2 (GLUT2) and solute carrier family 5 (sodium/glucose cotransporter), member 1 (SGLT1)), monocarboxylate transporters (monocarboxylate transporter 1 (MCT1) and sodium-coupled monocarboxylate transporter (SMCT)), tight junction proteins (occludin (OCLN) and zonula occludens 1 (ZO1)), cytokines (IL1B, IL6, IL10, interferon-γ (INFG), TNFA and transforming growth factor-β 1 (TGFB1)) and pathogen-associated molecular patterns (toll-like receptor (TLR)2 and TLR4) as well as housekeeping genes (β-actin (ACTB), hypoxanthine phosphoribosyltransferase 1 (HPRT1), glyceraldehyde 3-phosphate dehydrogenase (GAPDH), β-2 microglobulin (B2M) and ornithine decarboxylase antizyme 1 (OAZ1); see online supplementary Table S1) were performed on a ViiA 7 Real-Time PCR system (Life Technologies). Reaction conditions for complementary DNA synthesis and quantitative PCR using the fluorescent DNA stain EvaGreen (Biotium) have been described elsewhere(
Reference Metzler-Zebeli, Ertl and Klein
19
). Reverse transcription controls (RT minus) were included in order to control for residual DNA contamination, and experiments were performed in duplicates. Data were normalised to the expression of the three most suitable housekeeping genes (ACTB, GAPDH and OAZ), which were identified using the geNorm algorithm(
Reference Vandesompele, De Preter and Pattyn
22
). Relative gene expression were calculated using the
![]() $$2^{ - \Delta \Delta C _{ t }} $$
method(
Reference Livak and Schmittgen
23
).
$$2^{ - \Delta \Delta C _{ t }} $$
method(
Reference Livak and Schmittgen
23
).
Chemical analysis
Before proximate analysis, faecal samples were thawed at 4°C and homogenised per pig, and a subsample was taken for DM determination. The remaining faeces were freeze-dried. Diet and freeze-dried faeces were ground into a particle size of less than 1 mm before analysis. Chemical analyses of diets and faeces were performed using classical methods( 24 ) and commercial enzymatic kits, which have been described by Metzler-Zebeli et al. ( Reference Metzler-Zebeli, Mann and Schmitz-Esser 17 , Reference Metzler-Zebeli, Ertl and Klein 19 ). Porcine-specific commercial ELISA kits were used to analyse serum concentrations of the acute-phase proteins haptoglobin and serum amyloid A (GenWay). Serum acute-phase proteins were selected on the basis of previously published data( Reference Chen, Lin and Fung 25 ). Ca and P in serum was determined by enzymatic colorimetric analysis using an autoanalyser for clinical chemistry (Cobas 6000/c501; Roche Diagnostics GmbH).
Calculations
The ATTD of nutrients was calculated as:
 $$\begin{eqnarray} ATTD\,(\%) = ((Daily\,nutrient\,intake - daily\,nutrient\,excretion\,in\,faeces)/daily\,nutrient\,intake)\times 100. \end{eqnarray}$$
$$\begin{eqnarray} ATTD\,(\%) = ((Daily\,nutrient\,intake - daily\,nutrient\,excretion\,in\,faeces)/daily\,nutrient\,intake)\times 100. \end{eqnarray}$$
Daily nutrient excretion in faeces was defined as the amounts of Ca, P and N minerals present in the faeces and urine per d. Daily apparent retention was calculated as the difference between daily nutrient intake and daily nutrient excretion in faeces and urine.
Statistical analyses
To compare the differences between dietary Ca concentrations and cereals, data were subjected to ANOVA using the procedure PROC MIXED of SAS (Statistical Analysis System version 9.3; SAS Institute, Inc.). Fixed effects in the model included the main effects of Ca concentration, dietary cereal, their two-way interaction, the experimental run and the random effect of pig (n 31) nested within sow (n 16). The pig was considered the experimental unit. The Kenward–Rogers method was used to approximate df (ddfm = kr). Data of mucosal gene expression collected from the same pig but at various gut sites were considered as spatial repeated measures using various variance–covariance matrix structures to take into consideration close associations among repeated measures. Gut site repeated measures and corresponding two-way and three-way interactions were analysed. Because there were significant gut site effects on the expression of all genes of interest, except TLR2 expression, gut sites were also individually analysed. Moreover, data on body weight, average daily gain and feed intake from the same pig but on different days of the experimental period were analysed using time-repeated measures and corresponding interactions. Means were reported as least squares means with their standard errors. Differences were considered significant at P≤ 0·05 and as trend at 0·05 < P≤ 0·10.
Linear discriminant analysis by JMP Pro (version 10.0; SAS Institute, Inc.) was applied to demonstrate potential relationships between diets and mucosal gene expression in the jejunum and colon using gene expression as the covariate and diet as the categorical variable. To illustrate the associations between colonic gene expression and mucus-associated bacteria and their metabolites, Spearman's rank correlation analysis was performed in JMP. For the relation between mucosal gene expression and mucus-associated bacteria, Spearman's rank correlation for the fifty most abundant operational taxonomic units (OTU) (published in Mann et al. ( Reference Metzler-Zebeli, Mann and Schmitz-Esser 17 )) and gene expression were estimated. In Mann et al. ( Reference Mann, Schmitz-Esser and Zebeli 18 ), Roche/454-sequencing paired reads were analysed using the software package mothur( Reference Schloss, Westcott and Ryabin 26 ), and OTU were assigned based on a sequence similarity threshold of 97 %. Due to the short reads generated by Roche/454-sequencing, the cut-off of 97 % was taken as a proxy for species-level divergence( Reference Kuczinski, Stombaugh and Walters 27 ) so that the overall species diversity would not be overestimated, which might have been the case with a more stringent cut-off of 98 or 99 %. Obtained OTU were blasted against National Center for Biotechnology Information GenBank numbers to give an approximation of species classification (closest reference strains). Also, Spearman's rank correlations were computed for the relationships between colonic bacterial metabolites (published by Metzler-Zebeli et al. ( Reference Metzler-Zebeli, Mann and Schmitz-Esser 17 )) and gene expression. SCFA concentrations in colonic digesta were analysed using GC, whereas lipopolysaccharides (LPS) were measured using a pyrochrome Limulus amebocyte lysate assay. The Limulus amebocyte lysate assay determines total LPS and does not differentiate between the LPS of different immune reactivities present in the sample.
Results
Dietary composition, feed intake and growth performance
Chemical analyses showed that Ca concentrations were 0·2–0·3 g/kg diet (DM basis) higher for adequate Ca diets and 0·3–1·0 g/kg diet lower for high Ca diets as compared to the calculated dietary Ca concentration (Table 1). Consequently, actual dietary Ca concentrations corresponded to 103, 186, 104 and 176 % of the Ca requirement of 10 kg pigs for the wheat–barley diet adequate in Ca, the wheat–barley diet high in Ca, the maize diet adequate in Ca and the maize diet high in Ca, respectively. The P concentration of the maize diet adequate in Ca was 0·6 g P/kg diet (DM basis) higher, whereas the other three diets closely met the calculated P values (Table 1). Thus, actual P concentrations corresponded to 98, 195, 108 and 192 % of the P requirement of 10 kg pigs for the wheat–barley diet adequate in Ca, the wheat–barley diet high in Ca, the maize diet adequate in Ca and the maize diet high in Ca, respectively. Dietary Ca:P ratios were 1·37:1, 1·24:1, 1·27:1 and 1·21:1 for the wheat–barley diet adequate in Ca, the wheat–barley diet high in Ca, the maize diet adequate in Ca and the maize diet high in Ca, respectively (Table 1). As indicated by the time × cereal interaction (P= 0·032), pigs that were fed the maize-based diets had a greater body weight and a 60 g higher average daily gain (P< 0·05) than pigs that were fed the wheat–barley-based diets, even though the daily feed intake was similar among the feeding groups (Table 2).
Table 2 Body weight, average daily feed intake (ADFI) and average daily gain (ADG) of weaned pigs fed wheat–barley or maize diets adequate or high in calcium (Mean values with their standard errors; n 8 per diet, except wheat–barley diet high in calcium n 7)
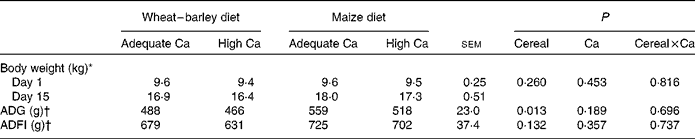
* Data on body weight were analysed using time-repeated measures over time, day P< 0·001, time × Ca P= 0·282, time × cereal P =0·032 and time × Ca × cereal P =0·680.
† Data on ADG and ADFI for experimental days 1–8 and 9–15 can be found in Metzler-Zebeli et al. ( Reference Metzler-Zebeli, Ertl and Klein 19 ). Data on ADG and ADFI were analysed using time-repeated measures.
Apparent total tract digestibility and nitrogen and mineral balances
To establish the actual effect of dietary Ca concentration and cereal composition on digestive and absorptive processes, we determined the ATTD of DM, organic matter, protein, fat, ash, xylose, β-glucan, Ca and P as well as Ca, P and N retention (Tables 3 and 4). When maize was the cereal source, pigs had a 1·5, 5·5 and 7·3 % higher (P< 0·05) ATTD of DM, ash and Ca, and they also tended to have greater (P< 0·1) ATTD of organic matter and fat as compared to the pigs fed wheat–barley-based diets (Table 3). The dietary Ca concentration influenced the pigs' ATTD of DM and ash, resulting in 1·8 and 13 % higher (P< 0·001) ATTD of DM and ash with the high and adequate Ca diets, respectively. The ATTD of protein (Table 3), N intake, and faecal and urinary N excretion (Table 4) did not differ among the diets; however, daily N retention tended (P= 0·074) to be greater by 1·7 g in the pigs that were fed the maize diet as compared to the wheat–barley diet.
Table 3 Apparent total tract digestibility (ATTD) of nutrients and serum haptoglobin, calcium and phosphorus concentration in weaned pigs fed wheat–barley or maize diets adequate or high in calcium (Mean values with their standard errors; n 8 per diet, except wheat–barley diet high in calcium n 7)

a,bMean values within a row with unlike superscript letters were significantly different (P< 0·05).
Table 4 Nitrogen, calcium and phosphorus balances in weaned pigs fed wheat–barley or maize diets adequate or high in calcium (Mean values with their standard errors; n 8 per diet, except wheat–barley diet high in calcium n 7)

Daily Ca (P< 0·001) and P (P< 0·001) intake was greater by 4 and 4·8 g with the high Ca diets as compared to the adequate Ca diets, respectively (Table 4). The pigs fed the high Ca diets excreted more (P <0·001) Ca and P via faeces than those fed the adequate Ca diets, whereas only urinary P excretion increased (P <0·001) with the high Ca diets as compared to the adequate Ca diets. Although total daily absorption of Ca and P were higher with the high Ca diets as compared to the adequate Ca diets, high Ca diets slightly decreased (P= 0·037) the ATTD of Ca by 6·4 % as compared to the adequate Ca diet (Table 3). The pigs fed the high Ca diets had a 2·7 and 2·3 g higher (P< 0·001) total daily Ca and P retention than the pigs fed the adequate Ca diets, respectively (Table 4). The pigs fed maize diets had a 1·1 g higher (P =0·009) Ca retention as compared to those fed the wheat–barley diets. Cereal source did not influence P retention, but P intake was 0·8 g higher (P= 0·042) in the pigs fed maize diets than it was in the pigs fed the wheat–barley diets.
Serum parameters
Acute-phase response was not stimulated by either dietary Ca concentration or cereal composition, as was indicated by the similar serum haptoglobin concentrations among the pigs and serum amyloid A concentrations that were below the detection limit (Table 3). There was a cereal × Ca interaction (P= 0·028), which shows that high Ca concentration reduced serum Ca concentration when it was added to the wheat–barley diets, whereas with maize diets, it increased the serum Ca concentration. Serum P concentration was raised (P< 0·001) in the pigs fed the high Ca diets as compared to those fed adequate Ca diets, but the effect was smaller when pigs were fed the maize diets instead of the wheat–barley diets, as was indicated by the cereal × Ca interaction (P= 0·049).
Mucosal gene expression in the jejunum and colon
Generally, mucosal expression of SGLT1, GLUT2, MCT1 and SMCT was higher (P< 0·05) in the jejunum than in the colon (see online supplementary Table S2). Mucosal expression of ZO1 was higher in the jejunum than in the colon, whereas that of OCLN was lower in the jejunum than in the colon (P< 0·05; see online supplementary Table S2). Except for TLR4 and the cytokines IL10 and INFG, whose mucosal expression showed the opposite effect, the expression of IL1B, IL6 and TGFB1 (P< 0·05) as well as that of TNFA (P< 0·1) was higher in the colon than in the jejunum (see online supplementary Table S2). Mucosal expression of TLR2 was similar in jejunal and colonic tissue. The principal effects of differences in dietary Ca intake occurred on the jejunal expression of tight junction proteins and TLR2. Expression of OCLN (P< 0·031) and ZO1 (P< 0·044) was down-regulated in the jejunal mucosa of the pigs fed the high Ca diets as compared to those fed the adequate Ca diets (Fig. 1(a) and (b)). Mucosal expression of TLR2, in turn, was up-regulated (P< 0·016) in the jejunum of the pigs fed the high Ca diets and was three to four times higher than that of the pigs fed the adequate Ca diets (Table 5). Also, the basal diet affected TLR2 expression; there was greater (P< 0·039) TLR2 expression in the jejunal mucosa of the pigs fed the wheat–barley diets as compared to those fed the maize diets. There was a tendency for a Ca × cereal interaction (P< 0·092) for INFG expression, indicating that with the wheat–barley diet, high Ca concentration down-regulated jejunal INFG expression, whereas high Ca concentration up-regulated its expression with the maize diets. Dietary Ca concentration and basal diet did not affect sugar and monocarboxylate transporter expression in the jejunal mucosa of the pigs, whereas there was a trend (P= 0·076) for a down-regulation of SMCT expression in the colonic mucosa of the pigs fed the high Ca diets as compared to those fed the adequate Ca diets (Table 6). As indicated by the Ca × cereal interaction (P= 0·021), the mucosal IL1B expression responded differently to the dietary Ca concentration depending on the cereal source fed to the pigs. Mucosal IL1B expression was up-regulated by high Ca concentration when combined with a wheat–barley diet, but it was down-regulated when combined with a maize diet as compared to the respective adequate Ca diets. There was a gut site × Ca interaction (P< 0·028) for ZO1 expression, which indicates that ZO1 expression was down-regulated by high Ca diets in the jejunum but not in the colon (Fig. 1), although no further gut site × Ca, gut site × cereal or gut site × Ca × cereal interaction could be found for the expression of the other target genes (data not shown).

Fig. 1 Relative expression of (a) occludin (OCLN) and (b) zonula occludens 1 (ZO1) in the jejunal and colonic mucosa of weaned pigs fed wheat–barley or maize diets adequate or high in Ca. Values are means with their standard errors; n 8 per diet, except wheat–barley diet high in calcium n 7, are represented by vertical bars. (a) Main effects on OCLN expression: jejunum, calcium P =0·031, cereal P =0·269, calcium × cereal P =0·0·942; colon, calcium P =0·816, cereal P =0·443, calcium × cereal P =0·543; gut site effect P =0·028, gut site × calcium P =0·509, gut site × cereal P =0·443, gut site × calcium × cereal P =0·843. (b) Main effects on ZO1 expression: jejunum, calcium P =0·044, cereal P =0·371, calcium × cereal P =0·638; colon, calcium P =0·884, cereal P =0·921, calcium × cereal P =0·950, gut site P <0·001, gut site × calcium P =0·028, gut site × cereal P =0·341, gut site × calcium × cereal P =0·619. ![]() , wheat–barley diet adequate in calcium;
, wheat–barley diet adequate in calcium; ![]() , wheat–barley diet high in calcium;
, wheat–barley diet high in calcium; ![]() , maize diet adequate in calcium;
, maize diet adequate in calcium; ![]() , maize diet high in calcium.
, maize diet high in calcium.
Table 5 Relative expression of cytokines and pathogen-recognition receptors in jejunal and colonic mucosa of weaned pigs fed wheat–barley or maize diets adequate or high in calcium (Mean values with their standard errors; n 8 per diet, except wheat–barley diet high in calcium n 7)

INFG, interferon-γ; TGFB1, transforming growth factor-β 1; TLR, toll-like receptor.
a,bMean values within a row with unlike superscript letters were significantly different (P< 0·05).
Table 6 Relative expression of nutrient transporters related to sugar and fermentation acid absorption in the jejunum and the colon of weaned pigs fed wheat–barley or maize diets adequate or high in calcium (Mean values with their standard errors; n 8 per diet, except wheat–barley diet high in calcium n 7)
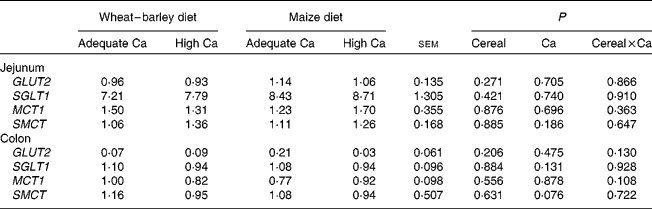
GLUT2, solute carrier family 2 (facilitated glucose transporter), member 2; SGLT1, solute carrier family 5 (sodium/glucose cotransporter), member 1; MCT1, monocarboxylate transporter 1; SMCT, sodium-coupled monocarboxylate transporter.
The linear discriminant analysis of diets and mucosal gene expression in the jejunum (Fig. 2(a)) and the colon (Fig. 2(b)) showed that diet modulated mucosal gene expression and separated the effects of the diets into two and four clusters, respectively. In the jejunum, diets clustered completely separately from one other. The jejunal expression of TLR2, TNFA, INFG, TGFB1 and OCLN discriminated best for the wheat–barley diet with adequate Ca concentration, whereas the expression of TLR4 and SMCT was more associated with the wheat–barley diet high in Ca. The maize diet adequate in Ca, in turn, correlated to jejunal IL1B expression, and the maize diet high in Ca correlated to the jejunal expression of MCT1, GLUT2, SGLT1, IL10, IL6 and ZO1. In the colon, the wheat–barley diet adequate in Ca clustered separately, whereas the other three diets formed one cluster according to their overlapping 95 % CI. In the colon, the wheat–barley diet adequate in Ca was clearly related to the mucosal expression of TGFB1, which distinguishes it from the other three diets.

Fig. 2 Linear discriminant analysis of the wheat–barley and maize diets adequate or high in calcium and data of gene expression in (a) the jejunum and (b) the colon of weaned pigs (n 31). ![]() , Wheat–barley diet adequate in calcium (WB-aCa);
, Wheat–barley diet adequate in calcium (WB-aCa); ![]() , wheat–barley diet high in calcium (WB-hCa);
, wheat–barley diet high in calcium (WB-hCa); ![]() , maize diet adequate in calcium (M-aCa);
, maize diet adequate in calcium (M-aCa); ![]() , maize diet high in calcium (M-hCa); +, treatment mean. Circles indicate 95 % confidence intervals. TLR, toll-like receptor; OCLN, occludin; INFG, interferon-γ; SMCT, sodium-coupled monocarboxylate transporter; TGFB1, transforming growth factor-β 1; MCT1, monocarboxylate transporter 1; GLUT2, solute carrier family 2 (facilitated glucose transporter), member 2; ZO1, zonula occludens 1; SGLT1, solute carrier family 5 (sodium/glucose cotransporter), member 1.
, maize diet high in calcium (M-hCa); +, treatment mean. Circles indicate 95 % confidence intervals. TLR, toll-like receptor; OCLN, occludin; INFG, interferon-γ; SMCT, sodium-coupled monocarboxylate transporter; TGFB1, transforming growth factor-β 1; MCT1, monocarboxylate transporter 1; GLUT2, solute carrier family 2 (facilitated glucose transporter), member 2; ZO1, zonula occludens 1; SGLT1, solute carrier family 5 (sodium/glucose cotransporter), member 1.
Spearman's rank correlations between bacteria and mucosal candicate gene expression in the colon
To identify potential associations between the mucosa-attached bacteria, microbial metabolites and mucosal gene expression in the colon, we performed Spearman's rank correlation. Only Spearman's rank correlation coefficients showing a significant correlation are presented (Tables 7 and 8). Correlations ranged from r± 0·36 to ± 0·62, and correlations were stronger for microbial metabolites. Out of the fifty most abundant OTU, twelve mucosa-associated OTU positively or negatively correlated to the colonic expression of sugar and monocarboxylate transporters, cytokines, pathogen-recognition receptors and tight junction proteins (Table 7). Microbial metabolites (SCFA and LPS) positively or negatively correlated with the colonic expression of cytokines, TLR and GLUT2 (Table 8).
Table 7 Spearman's correlation coefficients for colonic gene expression and mucosa-associated bacterial operational taxonomic units (OTU) in the colon of pigs fed wheat–barley or maize diets adequate or high in calcium

SMCT, sodium-coupled monocarboxylate transporter; TGFB1, transforming growth factor-β 1; ZO1, zonula occludens 1; SGLT1, solute carrier family 5 (sodium/glucose cotransporter), member 1; TLR2, toll-like receptor 2; MCT1, monocarboxylate transporter 1; OCLN, occludin.
* Mucosa-associated OTU are published in Mann et al. ( Reference Mann, Schmitz-Esser and Zebeli 18 ) and were blasted against National Center for Biotechnology Information GenBank numbers. A sequence similarity of 97 % was used as a cut-off for assigning sequences to OTU.
Table 8 Spearman's correlation coefficients for colonic gene expression and bacterial metabolites in the colonic digesta of pigs fed wheat–barley or maize diets adequate or high in calcium
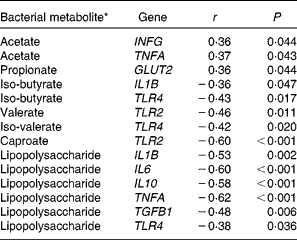
INFG, interferon-γ; GLUT2, solute carrier family 2 (facilitated glucose transporter), member 2; TLR, toll-like receptor; TGFB1, transforming growth factor-β 1.
* Bacterial metabolite concentrations are published in Metzler-Zebeli et al. ( Reference Metzler-Zebeli, Mann and Schmitz-Esser 17 ).
Discussion
The present results demonstrated that dietary Ca concentration modified the jejunal expression of genes related to mucosal permeability and pathogen-associated molecular patterns in young barrows. In addition, dietary cereal composition appeared to interact with the Ca effects on mucosal gene expression response, as was indicated by the distinguishable patterns exhibited by the two Ca concentrations and basal diets in the linear discriminant analysis scores plots, particularly for the jejunum, and as was indicated by the observed Ca × cereals interactions. Correlation coefficients substantiated the link between mucosa-associated bacteria and microbial metabolites and colonic gene expression response. Because we tried to keep the dietary Ca:P ratio similar between the four experimental diets, the effects of Ca and P cannot be seen separately in the present study. Moreover, we would like to point out that we used castrated male pigs, and results obtained in the present study thus might not be totally transferable to young female pigs or intact males, as the P requirements of pigs between 10 and 20 kg differ between the sexes( 21 , Reference Zhai and Adeola 28 ).
To explore the potential Ca effects on digestion and mucosal response in the small and large intestines of young pigs, it was critical to maintain contrasting Ca and P concentrations along the gastrointestinal tract with the two dietary concentrations of Ca and, as a consequence, also of P, and according to the results of the faecal excretion of Ca and P, we were successful in doing so. Both elements are necessary for the formation of the Ca-phosphate complex in the intestinal lumen( Reference Ten Bruggencate, Snel and Schoterman 5 ). In rats, the beneficial cytoprotective effects of high Ca diets in the intestinal lumen have been principally linked to the buffering properties of this Ca-phosphate complex, and the colon has been identified as the main intestinal site that responds to Ca action( Reference Schepens, Ten Bruggencate and Schonewille 4 , Reference Ten Bruggencate, Snel and Schoterman 5 ). In line with our previous study( Reference Metzler-Zebeli, Gänzle and Mosenthin 9 ), the intestinal site where we observed a stronger Ca effect on mucosal gene expression was the small intestine; fewer Ca effects were visible for the colonic mucosa. In contrast to our assumption that high dietary Ca intake would up-regulate the expression of genes related to mucosal barrier function, we found a substantial down-regulation of OCLN and ZO1 expression in the jejunum with the high Ca diets as compared to the adequate Ca diets, whereas the expression of OCLN and ZO1 in the colon was unaffected by the dietary Ca concentration. Bearing in mind the potential translational modification of mRNA, findings of a down-regulation in the expression of genes that encode for tight junction proteins in the jejunum might be counterproductive in the juvenile phase, given that proper barrier function has been suggested as a key mechanism for the development of intestinal disease( Reference Ukena, Singh and Dringenberg 29 ). However, we did not see an enhanced serum acute-phase response or intestinal translocation of LPS( Reference Metzler-Zebeli, Mann and Schmitz-Esser 17 ), which would have been a sign of higher intestinal permeability. Thus, it is possible that alterations in gene expression were not translated into functional protein. We did not scrutinise Ca absorptive pathways in the present study; however, the down-regulation in the expression of ZO1 and OCLN also may have been related to the intestinal availability and absorption of Ca( Reference Anderson, Cerejido, Cereijido and Anderson 30 – Reference Hwang, Yang and Kang 32 ). In pigs, the main site of active Ca absorption is the proximal small intestine( Reference Schröder and Breves 33 ). Transport proteins expression at the apical site of the enterocyte strongly regulates active transcellular Ca uptake, which is adjusted to luminal Ca availability( Reference Schröder and Breves 33 , Reference Breves, Schröder and Muscher 34 ). The latter has also been proposed to influence the rate of paracellular Ca transport by modifying the gene expression of tight junction proteins which form paracellular channels for selective ion transfer between neighbouring epithelial cells in the small intestine( Reference Fujita, Sugimoto and Inatomi 31 , Reference Hwang, Yang and Kang 32 , Reference Kellet 35 ). Evidence is growing for the cooperative functioning of active and passive Ca transport pathways via the regulation of the expression of tight junction proteins by transcellular calbindin-D9K protein expression( Reference Hwang, Yang and Kang 32 ). Diets high in Ca were reported to reduce active transcellular Ca uptake via the down-regulation of membrane Ca channels and calbindin proteins expression in the upper small intestine( Reference Kellet 35 ). Further studies should therefore look more closely at the link between intestinal transcellular and paracellular Ca transfer pathways in juvenile animals, such as weaned pigs, and explore whether there is an association between the regulation of paracellular Ca transport and the barrier function of the intestinal mucosa, thereby focusing not only on gene expression but also on functional protein expression.
Intestinal monocarboxylate transporters expression is substrate induced( Reference Cuff, Lambert and Shirazi-Beechey 36 , Reference Teramae, Yoshikawa and Inoue 37 ). Therefore, lower substrate availability due to the binding of SCFA to Ca-phosphate may explain the down-regulated expression of SMCT in the colonic mucosa of the pigs fed the high Ca diets as compared to those fed the adequate Ca diets. Colonic SCFA concentrations were not altered by dietary Ca concentration( Reference Metzler-Zebeli, Mann and Schmitz-Esser 17 ). The present findings are in line with the trend of a lower ileal MCT1 expression which was recently observed in weaned pigs fed high Ca diets as compared to those fed low Ca diets( Reference Metzler-Zebeli, Gänzle and Mosenthin 9 ). The beneficial cytoprotective effects of high Ca diets, such as the down-regulation of pro-inflammatory cytokine expression, which have been suggested by previous studies in rats( Reference Schepens, Schonewille and Vink 3 , Reference Schepens, Ten Bruggencate and Schonewille 4 ) and weaned pigs( Reference Metzler-Zebeli, Gänzle and Mosenthin 9 ) were not as clearly visible. However, present findings demonstrated that the cereal composition of the diet interacted with the Ca effect on the mucosal expression of pro-inflammatory cytokines, as indicated by the jejunal INFG expression and colonic IL1B expression. In previous research in rodents( Reference Schepens, Schonewille and Vink 3 , Reference Schepens, Ten Bruggencate and Schonewille 4 , Reference van Ampting, Schonewille and Vink 8 ) and weaned pigs( Reference Metzler-Zebeli, Gänzle and Mosenthin 9 ), animals were fed semi-purified diets based on purified starch and casein. Thus, interactions between dietary Ca × composition of the basal diet (corn starch–casein diets v. cereal–soyabean meal-based diets) may explain the diverging mucosal gene expression responses between previous studies and the present experiment. Moreover, differences in the dietary Ca and P concentrations may have also played a role in the varying results between the present and previous studies( Reference Schepens, Ten Bruggencate and Schonewille 4 , Reference van Ampting, Schonewille and Vink 8 , Reference Metzler-Zebeli, Gänzle and Mosenthin 9 ). For instance, in our earlier study in weaned pigs, dietary Ca concentrations were lower compared to those in the present study; they corresponded to 60 and 125 % of the daily Ca requirement, respectively( Reference Metzler-Zebeli, Gänzle and Mosenthin 9 ).
High Ca diets improved the ATTD of DM, which may be mostly a result of improved ash digestibility, as indicated by the similar ATTD of organic matter as compared to adequate Ca diets. Maize, wheat and barley differ in their major nutrient fractions (i.e. carbohydrates, dietary fibre, proteins and fat), which leads to differences in their major nutrient digestibilities( Reference Che, Perez and Song 38 , Reference Wilamil, Badiola and Devillard 39 ) and systemic nutrient metabolism( Reference Metzler-Zebeli, Ertl and Klein 19 ). The higher ATTD of DM and N retention was therefore very likely the reason for the enhanced growth of the pigs fed the maize diets as compared to the pigs fed the wheat–barley diets. Despite the fact that dietary fat concentration was small, it is possible that diverging fatty acid profiles caused by the different dietary fat origins may have influenced the digestibility of Ca, because dietary Ca forms insoluble Ca soaps with fatty acids in the small intestine( Reference Soares, Murhadi and Kurpad 2 , Reference Bendsen, Hother and Jensen 40 ). Likewise, the diverging hemicellulose and cellulose profiles of the maize v. wheat–barley diets might have altered Ca and P absorption because of changes in intestinal digesta viscosity or the complexing of Ca by complex carbohydrates( Reference Grieshop, Reese and Fahey 41 – Reference Metzler-Zebeli, Hooda and Mosenthin 43 ). Furthermore, wheat–barley and maize diets differ in their digestible and resistant starch fractions( Reference Metzler-Zebeli, Mann and Schmitz-Esser 17 ), which could change the jejunal expression of the apical and baso-lateral sugar transporters SGLT1 and GLUT2, respectively, but this was not the case in the present study. Instead, the colonic expression of GLUT2 was down-regulated by the high v. adequate Ca concentration but only in the pigs fed maize diets. Overall, we could not find dietary Ca × cereal interactions for indirect estimates of digestion and absorption, which suggests that Ca effects were similarly directed by, and hence mostly independent of, the cereal composition of the basal diet.
A noteworthy observation of the present study was that the Ca and P balance data suggested that the wheat–barley diet adequate in Ca did not contain sufficient amounts of available P. This was particularly visible when comparing the results for urinary excretion and retention from the pigs fed the wheat–barley diet adequate in Ca with those fed the wheat–barley diet high in Ca. It indicates that the absorbed dietary Ca could not be sufficiently used by the pigs fed the wheat–barley diet adequate in Ca. The pigs fed the wheat–barley diets adequate in Ca excreted more Ca via urine than the pigs fed the corresponding diet high in Ca, although it should have been vice versa. Likewise, P retention increased more than Ca retention in the pigs fed the wheat–barley diet adequate in Ca as compared to the pigs fed the wheat–barley diet high in Ca. Diets were formulated according to current recommendations for the nutrient requirements for pigs( 20 , 21 ), and microbial phytase was added to increase the bioavailability of phytate-P. Dietary Ca and P analysis confirmed that the analysed dietary Ca and P concentrations were very similar as compared to the calculated dietary concentrations. Thus, the dietary Ca and P concentrations in the adequate diet should have met the actual dietary requirements of Ca and available P for 10–20 kg pigs. However, diet analysis also showed that the Ca:P ratio in the wheat–barley diet adequate in Ca was wider, at 1·37:1, as compared to the wheat–barley diet high in Ca, which had Ca and P concentrations in a ratio of 1·24:1. This diverging Ca:P ratio could have contributed to the present results for urinary Ca excretion and Ca and P retention when comparing the wheat–barley diet adequate in Ca to the wheat–barley diet high in Ca. Aside from the ratio between Ca and available P being too wide, high concentrations of dietary protein also could have interfered with bone mineralisation( Reference Varley, Flynn and Callan 44 ) by changing the acid–base balance( Reference Arnett 45 ); this is particularly the case when diets contain rather low concentrations of P, which is the current practice today( 20 , 21 ). Recommendations for the P requirements of pigs have been constantly reduced over the past two decades, mostly because of environmental and economic concerns( Reference Selle and Ravindran 46 , Reference Cordell, Drangert and White 47 ). Because microbial phytase was supplemented to the pigs' diet and wheat contains substantial intrinsic phytase activity, the role of dietary phytate may have been less pronounced, which would explain the lower availability of dietary P with the wheat–barley diets adequate in Ca. Ion-binding capacity and rheological properties, such as viscosity, of dietary complex carbohydrates as well as microbial mineral demands on P and Ca for the fermentation of complex carbohydrates should be mentioned as further factors that may have influenced intestinal Ca and P availability( Reference Metzler-Zebeli, Gänzle and Mosenthin 9 , Reference Grieshop, Reese and Fahey 41 – Reference Metzler-Zebeli, Hooda and Mosenthin 43 , Reference Metzler, Mosenthin and Baumgärtel 48 ). For instance, negative effects of dietary supplementation of 5 % microcrystalline cellulose on the apparent retention of Ca, Mg, P and trace elements were previously reported by our group, which indicated an increased daily requirement for minerals for growing pigs( Reference Metzler-Zebeli, Hooda and Mosenthin 43 ).
The intestinal microbiota interacts with the host mucosa, thereby modulating mucosal gene expression( Reference Bauer, Metzler-Zebeli and Verstegen 10 ). In the present study, the up-regulated TLR2 expression in the jejunum of the pigs fed high Ca diets suggested a higher mucosal abundance of gram-positive bacteria in the jejunum as compared to the pigs fed the adequate Ca diets. Also, the maize diets up-regulated jejunal TLR2 expression as compared to the wheat–barley diets, probably because of the same reason. TLR belong to the family of pathogen-recognition receptors and are involved in the recognition of microbe-associated molecular patterns. TLR2 interacts with the cell wall components of gram-positive bacteria, including lipoteichoic acids, peptidoglycan and lipoproteins( Reference van Bergenhenegouwen, Kraneveld and Rutten 49 ). We did not quantify the bacterial microbiota at the jejunal mucosa. However, results from the stomach mucosa (Pars non-glandularis) and from gastric and ileal digesta indicated changes in the gram-positive bacterial composition as a result of dietary Ca and cereal composition. For instance, the pigs fed the high v. adequate Ca diets had considerably more lactobacilli-related OTU at the Pars non-glandularis of the stomach( Reference Mann, Schmitz-Esser and Zebeli 18 ). Accordingly, we observed an increase in the abundance of bifidobacteria in the gastric and ileal digesta of the pigs fed maize as compared to those fed wheat–barley diets( Reference Metzler-Zebeli, Mann and Schmitz-Esser 17 ). Correlation coefficients for the colon further demonstrated that OTU which were related to the main phyla Bacteroidetes, Firmicutes and Proteobacteria, according to 97 % sequence similarity as the cut-off for species level divergence, affected colonic gene expression. Because monocarboxylate transporters have different affinities for lactic acid and SCFA( Reference Thwaites and Anderson 50 ), differences between the main fermentation metabolites of mucosa-associated OTU 1, 2, 27 and 89, with the closest reference strains being Lactobacillus johnsonii, Lactobacillus amylovorus, Lachnospiraceae bacterium and Eubacterium sp., respectively, likely explained the correlations with colonic monocarboxylate transporters expression. The closest reference strains to OTU 3 and 4, Lactobacillus mucosae and Lactobacillus delbrueckii ( Reference Mann, Schmitz-Esser and Zebeli 18 ), have been shown to have immunomodulatory capacities( Reference Thomas and Versalovics 51 ) which might have been reflected in the negative relation between OTU 3 and 4 and the colonic expression of pro-inflammatory cytokines and TGFB1, respectively. The modification of TGFB1 expression has been proposed as one possible mechanism related to immunological tolerance towards microbial components in the intestine( Reference Schiffrin and Blum 52 ). Known probiotic immunomodulins include lactic acid, surface proteins of lactobacilli, peptidoglycan-derived neuropeptides, capric acid and histamine( Reference Thomas and Versalovics 51 , Reference Thomas, Hong and van Pijkeren 53 ). By contrast, OTU 624, with its closest reference strain being Campylobacter lanienae, might have been recognised by the immune system as a potential threat, and the colonic expression of TGFB1 and TNFA were therefore up-regulated. However, it should always be kept in mind that the taxonomic assignment of OTU to their closest reference strains is only an approximation of species classification. Present negative correlations between colonic cytokines genes and TLR4 gene expression and luminal LPS concentrations, in turn, may suggest that the present LPS was mostly of a non-harmful nature( Reference Gabler and Spurlock 54 ). Sustained exposure to low concentrations of LPS is also known to induce the tolerant states that are typically associated with the decreased expression of pro-inflammatory cytokines( Reference West and Heagy 55 ), and this may also explain the negative correlations observed here. In addition, new data suggest that intestinal alkaline phosphatase play important protective roles in the detoxification of free LPS( Reference Lallès 56 ). Further research focusing on LPS immune reactivity, intestinal alkaline phosphatase and LPS tolerance is needed to establish the role of mucosal and systemic immune responses to luminal LPS in pigs.
In conclusion, high Ca diets increased intestinal Ca and P concentrations and modified the jejunal and colonic mucosal gene expression response. The observed effects of Ca × cereal interaction on intestinal cytokine expression emphasise that the basal diet may intensify or alleviate certain Ca-related physiological effects. Overall, it appears that there is a stronger Ca effect in the small intestine than in the colon, which is in line with our previous study( Reference Metzler-Zebeli, Gänzle and Mosenthin 9 ). Shifts in bacterial composition caused by the diets might have been reflected in up-regulated jejunal TLR2 expression, whereas Spearman's correlations supported the close relationship between mucosal bacterial community and gene expression in the colon.
Supplementary material
To view supplementary material for this article, please visit http://dx.doi.org/10.1017/S0007114515000380
Acknowledgements
The authors thank the lab technicians of the Institute of Animal Nutrition and Functional Plant Compounds and the staff of the University Clinic for Swine for their assistance with sampling and laboratory analysis.
The present study was supported by Profile Lines of the Vetmeduni Vienna (B. U. M.-Z. Start-up project ‘PL effects of calcium in weaned pigs’).
B. U. M.-Z., S. S.-E., M. W., M. R. and Q. Z. designed the research; B. U. M.-Z. and E. M. conducted the research; R. E. and D. K. performed gene expression analysis; B. U. M.-Z. and Q. Z. performed statistical analysis; B. U. M.-Z. wrote the paper; and B. U. M.-Z. had primary responsibility for the final content. All authors read and approved the final manuscript.
The authors declare no conflicts of interest.













