l-Glutamate (l-Glu) is the most abundant free amino acid in protein-containing foods. The oral intake of free l-Glu stimulates gastrointestinal (GI) exocrine secretions, such as saliva, gastric juice, bile and pancreatic juice, as well as endocrine secretions (insulin and gastrin)(Reference Vasilevskaia, Rymshina and Shlygin1–Reference Zolotarev, Khropycheva and Uneyama6). Recently, we reported that a unique glutamate-sensing system might exist within the GI tract, especially in the stomach(Reference Uneyama, Niijima and San Gabriel7), and that the gastric vagal afferents were selectively activated by the intra-gastric administration of l-Glu(Reference Uneyama, Niijima and San Gabriel7). Sensory perception by the abdominal vagus is well known to initiate the coordinated processes of GI motility, exocrine and endocrine secretion, immunity and satiation(Reference Cervero8–Reference Schwartz and Moran10). Therefore, based on the available animal and human evidence, we hypothesised that free l-Glu intake might regulate GI functions for the digestion of dietary protein via visceral glutamate information(Reference Uneyama, San Gabriel and Kawai11).
Diarrhoea is one of the most common GI complications in patients receiving enteral nutrition(Reference Lefron12) and limits the clinical usage of enteral liquid diets in post-operative and elderly patients. Although many animal and clinical trials have been conducted to overcome this critical issue, this side effect still remains unresolved. In clinics, post-vagotomy diarrhoea has been a major nutritional problem associated with abnormal GI functions, such as abnormal regulation of gastric emptying of enteral liquid diets(Reference Loudon13). Normal gastric emptying requires adequate activation of gastric as well as celiac vagus nerves. In the present study, we investigate whether supplementation with free l-Glu can prevent post-operative diarrhoea linked with disrupted vagal regulation caused by repetitive gastric tube feeding, in order to document the potential clinical benefits of l-Glu in enteral nutrition.
Materials and methods
Animals
Male Sprague–Dawley rats weighing 300–350 g were used for all experiments. The rats were housed under a controlled temperature (24°C) and light cycle (lights on between 07.00 and 19.00 hours). The animals had free access to food and water for 1 week before protocol entry. All studies were approved by the Institutional Animal Care and Use Committee of Ajinomoto Company, Inc. (Kanagawa, Japan).
Expt 1: Electrophysiological experiments for vagus nerve recordings
The surgical techniques and electrophysiological experimental methods have been extensively documented elsewhere(Reference Uneyama, Niijima and San Gabriel7, Reference Kitamura, Sato and Uneyama14). Briefly, the rats were anesthetised with urethane (1 g/kg, intraperitoneal), tracheal cannulas were inserted and the pylorus of the stomach was closed with a silk suture. Under a dissecting microscope, a nerve filament was dissected from the peripheral cut end of the gastric branch of the vagus nerve to allow recording of afferent nerve activity via a pair of silver wire electrodes. A rate metre with a reset time of 5 s was used to observe the time course of nerve activity. The discharge rate was expressed as means with their standard errors. The protein-rich diet consisted of 12·5 % dextrin (TK16; Matsutani Chemical, Itami, Japan) and 12·5 % casein–Ca (EN9N; DMV International, Veghel, The Netherlands) supplemented with 0, 0·5 or 1 % (w/v) monosodium l-glutamate (MSG; Ajinomoto Company, Inc.). The food was injected in 2-ml volumes at a flow rate of 0·1 ml/s. The effect of gastric stimulation of MSG on nerve activity was determined by comparing the mean number of spikes in ten successive 5-s bins before and at time points 30 min after the application of the test stimulus. The effects of the treatment were evaluated statistically using a paired t test (P < 0·05).
Expt 2: Functional evaluation of supplementation of the protein-rich liquid diet with l-glutamate
In this experiment, we used 0·5 % (w/v) MSG, which is a normal habitual concentration for rats as well as humans(Reference Giacometti, Filter, Garattini, Kare and Raynolds15, Reference Yamaguchi and Takahashi16). Gastrostomy was performed on the rats utilising sterile techniques. The rats were fasted for 18 h, with free access to tap water, before gastrostomy. Under pentobarbital anesthesia (45 mg/kg, intraperitoneal), the back and abdomen were shaved and a silicon catheter (1·1 mm inner diameter and 2·2 mm outer diameter) was placed in the stomach. The tube was tunnelled subcutaneously to exit at the upper back and was fixed by attachment to the top of the head. A total of sixteen rats were divided into two groups. One group had a complete liquid diet consisting of 10 % protein (mainly casein sodium), 32 % carbohydrate (dextrin), and 6 % fat (soyabean oil) and containing 0·5 % (w/v) MSG (complete diet with MSG, n 8). The other group received an identical diet, except supplemented with 0·2 % glycine and 0·16 % NaCl instead of MSG, to adjust for nitrogen and Na+ sources (complete diet without MSG, n 8). Both diets were isoenergetic and isonitrogenic.
One day after the gastrostomy, the rats in each group received an appropriate complete diet through the gastric tube with a syringe. A 2·7-ml shot of the liquid diet was delivered once per hour for 7 h, and the severity of diarrhoea was scored according to the following standard, as described previously(Reference Trifan, Durham and Salazar17–Reference Zhang, Pan and Gan20): 0 (normal; normal stool or absent); 1 (slight; slightly wet stool without staining of the coat); 2 (moderate, wet and unformed stool with moderate perianal staining of the coat); 3 (severe, watery stool with severe staining of the coat around the anus). The total diarrhoea score area under the curve was calculated during hours 1–7, and the mean score was calculated every hour for 7 h. The faeces were trapped by a nylon mesh, which was changed every hour. Water intake was also measured during the study period. Data are expressed as means with their standard errors. Mann–Whitney's U test was used for statistical significance at P < 0·05.
Results
Expt 1
Afferent activities of the gastric vagus, just before and 30 min after applying the protein-rich liquid diet (2 ml), were 64·2 (se 3·0) and 59·8 (se 4·2) spikes/5 s, respectively (Fig. 1(a) and (d)). Supplementation of the diet with 0·5 and 1 % MSG significantly enhanced the afferent activities of the gastric vagus (0·5 % MSG: 80·5 (se 4·7) compared with 64·6 (se 1·2) spikes/5 s; 1 % MSG: 78·9 (se 5·2) compared with 62·0 (se 3·9) spikes/5 s, P < 0·05, Fig. 1(b–d))

Fig. 1 Vagal gastric activity following intra-gastric application of a protein-rich liquid diet with or without l-glutamate supplementation. (a–c) Representative recording of gastric afferent discharge, displayed as a sequential rate histogram, after intragastric administration of 2 ml of a liquid protein-rich (casein) diet supplemented with 0, 0·5 or 1 % monosodium l-Glu (MSG). Arrowheads indicate the points at which the solution was infused. The vertical bar indicates 100 impulses/5 s. The horizontal bar indicates 30 min. (d) Summary of changes in discharge rate showing the impulse values of diets containing 0, 0·5 and 1 % MSG on gastric afferent activity measured before and after administration. Values are means, with their standard errors represented by vertical bars from five different rats. * Mean values were significantly different by paired t test (P < 0·05).
Expt 2
Feeding the control diet without MSG resulted in a gradual increase in the diarrhoea score after three repetitive injections (3 h), which reached a maximum after six repetitive injections: 3 h: 0·1 (se 0·1) (score 1; 1/8 and 0; 7/8 rats), 4 h: 0·3 (se 0·3) (score 2; 1/8 and 0; 7/8 rats), 5 h: 0·5 (se 0·3) (score 2; 2/8 and 0; 6/8 rats), 6 h: 1·0 (se 0·4) (score 2; 4/8 and 0; 4/8 rats) and 7 h: 0·9 (se 0·4) (score 2; 3/8, 1; 1/8 and 0; 4/8 rats). For the diet with MSG, no change was noted for the diarrhoea score except at 5 h: 0·1 (se 0·1) (score 1; 1/8 and 0; 7/8 rats) and 7 h: 0·3 (se 0·3) (score 2; 1/8 and 0; 7/8 rats) (Fig. 2(a)). At the 6 h point, a significant difference was measured between the diets with and without MSG (P < 0·05; Mann–Whitney's U test). Fig. 2(b) summarises the area under the curve of the diarrhoea score from 1 to 7 h for each diet. The value of the area under the curve was significantly lower for MSG supplementation (0·3 (se 0·3) for the diet with MSG and 2·3 (se 0·8) for the control diet without MSG, P < 0·05; Mann–Whitney's U test). Water intake in both diet groups was not significantly different during the experimental periods (data not shown).
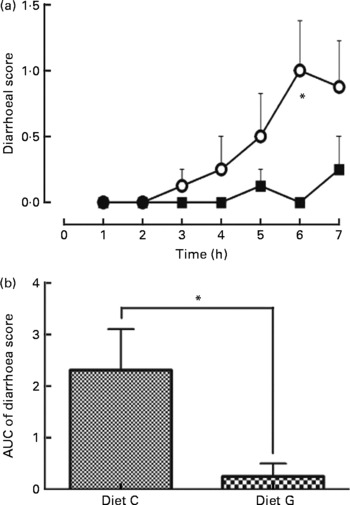
Fig. 2 Effect supplementation of a protein-rich liquid diet with l-glutamate (l-Glu) on postoperative diarrhoea. One day following gastrostomy, rats were delivered a 2·7 ml shot of a liquid diet once per hour for 7 h. Rat injected with a liquid diet supplemented with 0·5 % monosodium l-Glu (MSG; -■-, diet G, n 8); rat injected the diet without MSG (-○-; diet C, n 8). Diarrhoea was scored every hour for 7 h for each animal (0 = normal, 1 = slight, 2 = moderate and 3 = severe). (a) Time course for diarrhoea scores; (b) the area under the curve (AUC) for the diarrhoea scores. Values are means, with their standard errors represented by vertical bars. * Mean values were significantly different between diets C and G by Mann–Whitney's U test (P < 0·05).
Discussion
The results from the present study show that supplementation of an enteral protein-rich liquid diet with l-Glu (as MSG) significantly prevents the incidence of diarrhoea during repetitive intra-gastric tube feeding in rats. Diarrhoea is the most common GI side effect in human patients receiving enteral nutrition(Reference Lefron12). This study provides the first indication that the fortification of an enteral liquid diet with l-Glu might prevent enteral diet-induced diarrhoea in patients with GI troubles. A possible mechanism underlying this effect was revealed by Expt 1 (Fig. 1). The protein-rich liquid diet by itself did not induce the activation of gastric vagal afferents, but supplementation of the diet with l-Glu significantly enhanced afferent activities.
Diarrhoea in patients receiving enteral nutrition generally occurs when the inflow of liquid diets exceeds the capacity of GI digestion and absorption. For this reason, the speed of the administration of a liquid diet is important. The stomach is a storage reservoir as well as a regulator of intestinal nutrient absorption. It is impervious to water and therefore can hold a high osmotic load, which it delivers gradually to the small intestine for steady digestion and absorption. This delivery is strictly regulated by the gastric and celiac vagus nerves. We reported previously that the activation of the gastric vagal afferent, as well as the celiac afferents, by l-Glu might be linked to the gastric digestion of dietary proteins via the gastric vago-vagal reflex. Intra-gastric administration of l-Glu evokes the afferent activity of the gastric vagus(Reference Niijima, Torii and Uneyama21), thereby stimulating gastric secretions and regulating GI motility in animals(Reference Zolotarev, Khropycheva and Uneyama6, Reference Toyomasu, Mochiki and Yanai22). In humans, the supplementation of a protein-rich liquid diet with MSG improved the delayed gastric emptying rate and reduced post-ingestive abdominal discomfort, such as feelings of stomach heaviness and abdominal fullness(Reference Zai, Kusano and Hosaka23). These observations strongly indicate that supplementation with free l-Glu may accelerate the gastric phase of protein digestion and aid in the intestinal digestion and absorption of the diet.
The vagal afferents convey nutrient information from the upper GI to the central nervous system, including information about regulatory functions (e.g. absorption, secretion and emptying) and conscious sensations (e.g. satiety, nausea, discomfort and pain)(Reference Cervero8–Reference Schwartz and Moran10). Well-regulated gastric emptying is important for the prevention of diarrhoea and is partly controlled by the gastric branch that leads to the pyloric antrum and pylorus(Reference Loudon13). The gastric emptying rate is significantly faster in patients who show diarrhoea following selective vagotomy and pyloroplasty than in patients who experience no change in their bowel habits(Reference George and Magowan24). These observations suggest that the regulation of gastric emptying via gastric afferent activation may be responsible for the prevention of diarrhoea induced by l-Glu fortification seen in the present rat diarrhoea model.
Kitamura et al. (Reference Kitamura, Sato and Uneyama14) recently reported that intra-gastric infusion of l-Glu increased the efferent activity of the celiac vagus nerve, which regulates intestinal function, although Boutry et al. (Reference Boutry, Bos and Matsumoto25) reported no effect of MSG on orofaecal transit time. Therefore, the examination of the effects of MSG on water absorption through the intestinal or colonic epithelium would be an interesting future study. Alternatively, a metabolite of l-Glu may exert the observed anti-diarrhoeal effect. Dietary l-Glu is metabolised in the GI tract(Reference Reeds, Burrin and Jahoor26) and it is a specific biosynthetic precursor of glutathione, arginine and proline(Reference Reeds, Burrin and Stoll27). Arginine supplementation has been reported to reduce diarrhoea in young early-weaned pigs(Reference Zhan, Ou and Piao28).
The data from the present study strongly support the possibility that supplementation of enteral liquid diets with a naturally occurring free amino acid, l-Glu, can prevent diarrhoea by sending visceral glutamate information from the stomach to the brain. The observation that purified casein protein alone did not induce any afferent activation of the gastric vagus, is also interesting, as it suggests that protein might be poorly recognised as a nutrient in the stomach. A sharp increase in the gastric concentration of glutamate (before glutamate release would occur from casein through the action of pepsin in the stomach) could explain the effects observed in the present study.
The present study indicates that a combination of l-Glu and casein protein might be a highly suitable nutrient combination for an enteral diet. To date, the physiological importance of l-Glu, which co-exists abundantly both in free form and bound to natural proteins, has largely been ignored, since that the focus of enteral liquid nutrition has typically been on the delivery of simple purified nutrients to patients. Based on our experiments, we strongly recommend the clinical application of l-Glu to medicinal foods, to include hospital meals as well as enteral liquid diets, for the improvement of the nutritional status of all hospitalised patients.
Acknowledgements
The authors are employees of Ajinomoto Company, Inc. and the work described in this article has been funded by the company. There are no other conflicts of interest and funding. S. S., N. H. and A. N. carried out the animal experiments for this study. S. S. drafted the manuscript and N. H., H. U. and K. T. critically evaluated the manuscript. We thank Dr Anil D. Kulkarni (The University of Texas Medical School, Houston, TX, USA) for helpful comments on the manuscript. We are also grateful to Masuyo Yamada, Itaru Kon for technical help and Dr Yukifumi Kokuba and Dr Tatsuro Tanaka for technical advice.




