Maternal alcohol consumption during pregnancy can result in a wide range of birth defects and developmental disabilities in children, collectively known as fetal alcohol spectrum disorders (FASD). In Canada, the prevalence of FASD is estimated as nine per 1000 live births(1). The true prevalence of FASD is unknown, likely due to underreported cases and misdiagnose(Reference Flannigan, Unsworth and Harding2); however, the estimated annual cost of FASD was estimated at approximately $1·8 billion in Canada in 2013(Reference Popova, Lange and Burd3). To this end, many efforts have been made to reduce the incidence of FASD, but none has been successful.
Maternal nutrition during pregnancy is critical for fetal and infant growth and development. While nutrition requirements are increased to meet the physiological demands during pregnancy, poor maternal nutrition can result in adverse birth outcomes, including low birth weight, preterm birth and intra-uterine growth restriction(Reference Abu-Saad and Fraser4). It is well-known that alcohol consumption during pregnancy can decrease food intake and interfere with nutrient digestion and absorption, leading to poor maternal nutritional status and adverse birth outcomes(Reference May, Hamrick and Corbin5–Reference Sebastiani, Borras-Novell and Casanova7). A population-based study in South Africa showed that mothers of children with FASD had a significantly lower nutrient intake than those of children without FASD(Reference May, Hamrick and Corbin5). Another population-based cohort study found that moderate alcohol consumption (one or more drinks per d, with each drink containing about 12 g of alcohol) during pregnancy was associated with low birth weight (<2500 g) and preterm birth (<37 weeks of gestation)(Reference Jaddoe, Bakker, Hofman and Mackenbach6). Conversely, these adverse effects of alcohol consumption during pregnancy may be reversed by maternal nutrition. A prospective cohort study reported that multi-vitamin/mineral supplementation during pregnancy reduced the negative effects of alcohol consumption during pregnancy on cognitive development in 6-month-old children(Reference Coles, Kable and Keen8). Therefore, maternal nutrition plays an important role in the development of FASD.
Because of practical and ethical limitations in studies using humans, various animal models are used to study FASD. Specifically, rodents are commonly utilised due to their short periods of gestation and have shown to mimic most of the features of FASD in humans(Reference Pattern, Fontaine and Christie9). Interestingly, the majority of rodent studies investigating the effect of maternal alcohol (in the form of ethanol, EtOH) consumption during pregnancy have used standard chow diet. These studies often reported detrimental EtOH effects on birth outcomes, such as reduced litter size, low birth weights, reduced fetal and placental weights and fetal malformation(Reference Naassila and Daoust10–Reference Wentzel, Rydberg and Eriksson12). In contrast, studies feeding a formulated semi-purified diet to pregnant rats, commonly used in nutrition studies, found no significant decreases in litter size, placental weight or fetal weight after EtOH exposure for the first 10 d of pregnancy or throughout pregnancy(Reference Feltham, Louis and Kapourchali13,Reference Kapourchali, Louix and Eskin14) . This semi-purified diet was formulated to provide energy representing the current macronutrient intake of pregnant women in North America, providing 17·4–17·9 kJ/g (kJ (%) composed of carbohydrate 50 %, protein (prot) 17·5 % and fat 32·5 %)(Reference Feltham, Louis and Kapourchali13,Reference Kapourchali, Louix and Eskin14) , which is higher than that of standard chow (14·0 kJ/g, LabDiet Rodent 5001). An earlier study which compared a chow (12·5 kJ/g, Panlab, A04) with a semi-purified diet (15·4 kJ/g, increased kJ of carbohydrate and fat of the chow, 4·7 and 2·6 %, respectively; composed of carbohydrate 73 %, prot 12·5 % and fat 14·5 %), without EtOH treatment, showed significant metabolic changes in lactating rats and their offspring(Reference Del Bas, Caimari and Ceresi15), suggesting that there are differential effects by these two diets on the health of dams and offspring. Several studies also reported that EtOH-induced poor maternal outcomes and offspring growth were reversed when a nutritionally adequate diet and energy-dense diet (17·6 kJ/g, kJ (%) composed of carbohydrate 55·2 %, prot 24 % and fat 20·8 %) were provided to dams(Reference Goad, Hill and Slikker16,Reference Wiener, Shoemaker and Koda17) . These findings suggest that a high-energy diet could help to mitigate some of the adverse effects of EtOH during pregnancy. It should be noted that although energy density is not the sole difference between the standard chow and the above formulated semi-purified diet, a direct comparison of these diets in the same EtOH regimen would provide a meaningful diet strategy against FASD.
The objective of this study was to compare the effects of a standard chow with a formulated semi-purified energy-dense (E-dense) diet(Reference Feltham, Louis and Kapourchali13,Reference Kapourchali, Louix and Eskin14) on birth outcomes and metabolic parameters in dams and their offspring after EtOH consumption during pregnancy. The physical and metabolic parameters were measured at postnatal day (PD) 7, which represents the peak of brain growth spurt in rats(Reference Dobbing and Sands18). The study outcomes will indicate whether maternal nutrition is a contributing factor for the severity of FASD.
Methods
Experimental design
Sprague–Dawley rats (10–11 weeks old, twenty-seven females and fourteen males) were purchased from Charles River Laboratories and housed two rats per cage. After 1 week of acclimatisation, female rats were randomised into four groups: (i) chow (n 6); (ii) chow + EtOH (n 7); (iii) E-dense (n 6) and (iv) E-dense + EtOH (n 8). The number of female rats planned was six per group; however, EtOH-treated groups had fewer pups and more neonatal deaths compared with non-EtOH groups. Thus, an additional 1–2 female rats were included in the two EtOH groups. EtOH was provided in drinking water at a final concentration of 20 % (v/v). Female rats were adapted to 20 % (v/v) EtOH over 2 weeks prior to mating: briefly, 5 % EtOH (v/v) for the first 2 d, followed by 10 % EtOH (v/v) for 4 d, 15 % EtOH (v/v) for 4 d and finally reached to 20 % EtOH (v/v) for the last 4 d. The 20 % (v/v) EtOH was continued throughout mating and gestation. Non-EtOH groups were provided with tap water only. Blood alcohol concentration of 80–150 mg/dl is considered a low-to-moderate EtOH intake, whereas heavy binge drinking would provide a blood alcohol concentration >200 mg/dl(Reference Pattern, Fontaine and Christie9). While studies using 20 % (v/v) EtOH reported a blood alcohol concentration in the range of 60–110 mg/dl(Reference Le Duc, Spataru and Ceanga19,Reference Ojeda, Delgado-Willa and Llopis20) , the blood alcohol concentration in this study ranged from 34·6 to 51·2 mg/dl during gestation as measured by an EtOH assay kit (Abcam ab65343).
Following EtOH adaptation, a randomly selected male rat was placed with two female rats for mating. Pregnancy was confirmed by a positive vaginal swab test, and this was considered as gestational day 0. Pregnant rats were housed individually and continued their treatment. The day of parturition was considered as PD0, and EtOH provision was stopped at parturition. At PD3, litters were culled to 12 pups per dam to allow equal access to breast milk to achieve similar growth (one in the chow + EtOH group, one in the E-dense group and four in the E-dense + EtOH group had 9–11 pups, and one in the chow + EtOH had 6 pups due to a smaller litter size at birth or neonatal death). At PD7, lactating dams were fasted overnight and anaesthetised with isoflurane, followed by cardiac puncture and decapitation. Pups were euthanised by decapitation with sharp scissors, and trunk blood was collected.
All animals were kept in a room with maintained temperature (18–23 ºC), relative humidity (40–70 %) and controlled lighting (12 h light–12 h dark cycle). Overall, body conditions and behaviour were monitored every day, and a cage change examination was completed at a minimum once per week. Body weight of dams was recorded once weekly over the study and that of pups was recorded at PD3 and PD7. Food and fluid intakes were noted every 2–3 d over the course of the study. These basic data were collected at about 09.00 hours. The experimental procedures were conducted in accordance with the principles and guidelines of the Canadian Council on Animal Care(21) and were approved by the Office of Research Ethic & Compliance and Animal Care Committee at the University of Manitoba.
Diets
The animals were fed a standard chow (powdered, LabDiet Rodent 5001) or a semi-purified E-dense diet (powdered, formulated) ad libitum over the experimental period (Table 1). Standard chow was made from unrefined ingredients such as ground maize, dehulled soyabean meal and fishmeal, providing 14·0 kJ/g (57·9 % kJ carbohydrate, 28·7 % kJ prot and 13·4 % kJ fat). The E-dense diet was made from refined ingredients and formulated as previously reported(Reference Feltham, Louis and Kapourchali13), providing 17·7 kJ/g (50·0 % kJ carbohydrate, 20·0 % kJ prot and 30·0 % kJ fat). The energy distribution of the E-dense diet is within the recommended Acceptable Macronutrient Distribution Ranges (45–65 % carbohydrate, 10–35 % prot, 20–35 % fat)(22) and is closely representing the current macronutrient intake of pregnant women in North America(Reference Denomme, Stark and Holub23).
Table 1. Composition of the experimental diets
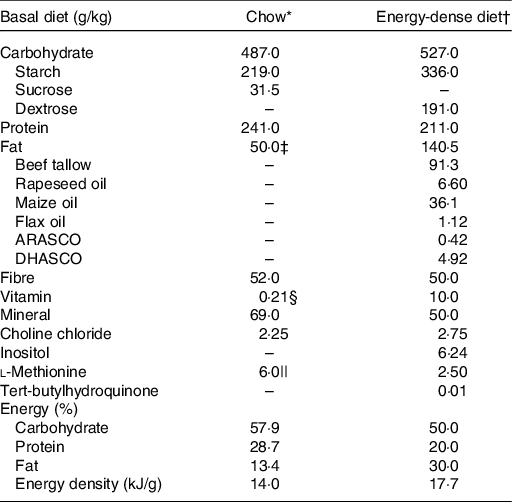
* Chow, powdered, LabDiet Rodent 5001.
† Energy-dense diet, powdered, formulated. Diet ingredients, including vitamin mix (AIN-93M) and mineral mix (AIN-93VX) were purchased from Dyets Inc. Fat mixture was formulated based on the previous study(Reference Feltham, Louis and Kapourchali13), and oils were purchased from Loblaws Inc. and Sobeys Inc. ARASCO and DHASCO, the source of arachidonic acid and DHA, respectively, were donated from DSM.
‡ The source of fat of chow was from unrefined ingredients, including dehulled soyabean meal, fishmeal, porcine animal fat preserved with DHA and citric acid, porcine meat and bone meal.
§ The total amount of vitamin listed in chow was re-calculated to metric units, and ascorbic acid was not included as shown in LabDiet Rodent 5001; the composition of vitamin mixture was available online.
|| Chow contains dl-methionine.
Metabolic parameters
Plasma concentrations of metabolic parameters, including total cholesterol (TC), HDL-cholesterol, LDL-cholesterol, TAG, glucose (Glc) and liver function enzymes alanine aminotransferase and aspartate aminotransferase were measured in dams and pups at PD7 using Cobas c 111 analyzer (Roche Diagnostics).
Statistical analysis
The main effects of diet and EtOH were analysed by two-way ANOVA, followed by Duncan’s multiple range post hoc test. Maternal daily food, energy and fluid intakes and body weights were analysed by repeated-measures ANOVA followed by the Tukey–Kramer multiple comparisons post hoc test. Maternal EtOH intake was tested by one-way ANOVA, as non-EtOH groups were excluded from this test. For pups, the data were presented as a litter mean, representing a litter being an experimental unit. Statistical significance was set at P < 0·05. Results are expressed as mean values and standard deviations. All statistics analyses were performed using SAS 9.4 software. A sample size was calculated based on a similar study(Reference Del Bas, Caimari and Ceresi15) examined metabolic parameters, such as TAG. A minimum sample size of n 6/group will detect significant differences between the two diet groups at a 197·5 % change in TAG at an error rate of 0·05 with a desired power of 80 %.
Results
Maternal intakes and body weights
Maternal intakes and body weights during EtOH adaptation, gestation and the first week of lactation were monitored. Both diet and EtOH significantly affected maternal food intakes during these periods (Table 2, Fig. 1). Without EtOH consumption, the food intake in dams fed the E-dense diet was lower (P < 0·05) during gestation and lactation than those fed chow, while showing no differences during EtOH adaptation. Maternal EtOH consumption significantly reduced food intake in dams (P < 0·0001) compared with those without EtOH consumption during EtOH adaptation and gestation. Dams fed chow while consuming EtOH had the lowest food intake during EtOH adaptation and gestation (Fig. 1), without reaching statistically significance in the latter period compared with dams fed E-dense diet with EtOH (Table 2). Dams fed chow, concurrent with EtOH intake, consumed approximately 34 and 43 % less food than their counterparts without EtOH during adaptation and gestation, respectively. Conversely, the food intake of dams fed the E-dense while consuming EtOH was approximately 22 and 18 % less than their non-EtOH counterparts during EtOH adaptation and gestation, respectively. During lactation, dams fed chow without EtOH had higher food intakes (P < 0·05) than those fed the E-dense diets.
Table 2. Effects of maternal diets and ethanol (EtOH) on average intakes of dams during EtOH adaptation, gestation and lactation*
(Mean values and standard deviations)
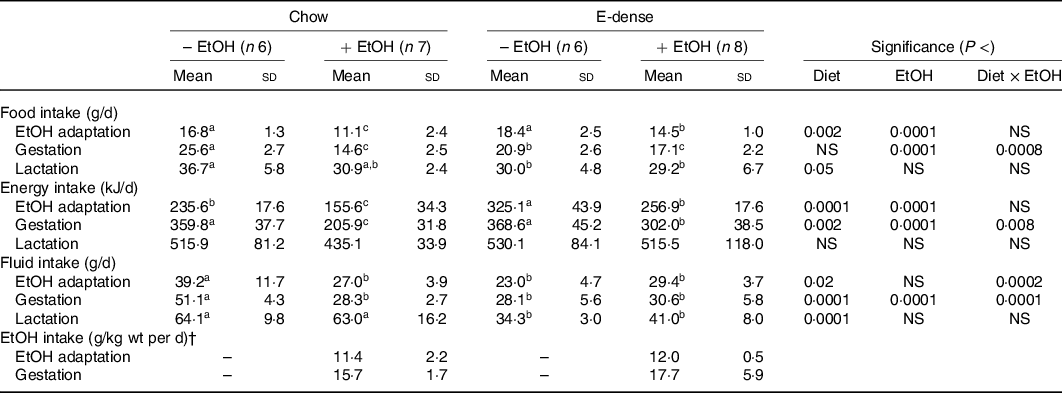
E-dense, energy dense.
a,b,c Mean values within a row with unlike superscript letters are significantly different (P < 0·05).
* The values presented are average food, energy and fluid intakes of dams during each period. EtOH adaptation, gestation and lactation period represented 14, 21 and 7 d, respectively, during the course of experiment. Significant effects of diet and EtOH were analysed using two-way ANOVA followed by Duncan’s multiple range test (P < 0·05).
† EtOH intake was calculated at the days of collecting the dams’ body weights during EtOH adaptation and gestation. Significant differences between the diets were identified by Student’s t test; no significant differences were observed at each period.
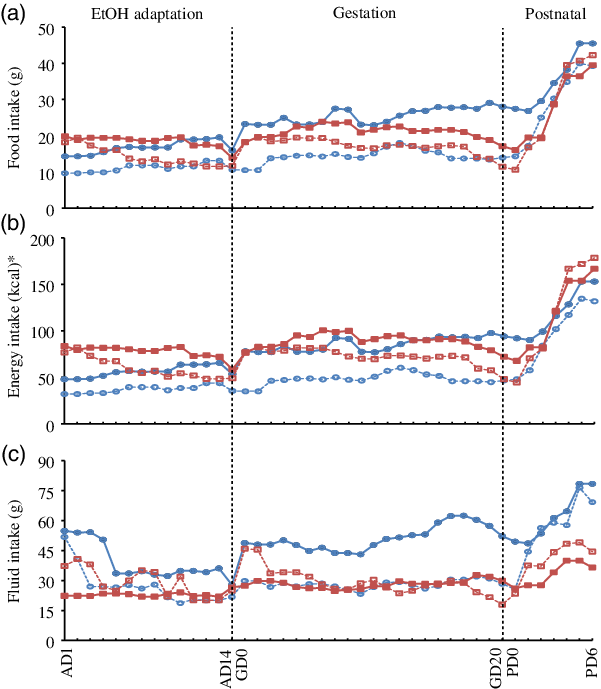
Fig. 1. Effects of maternal diets and ethanol (EtOH) on daily intakes of dams over the study. Data are means (n 6–8 per group at each time point). Repeated-measures ANOVA was performed to evaluate the effects of diet and EtOH on maternal intakes over time. (a) Food intake: significant time effects (P < 0·0001), time × diet effects (P < 0·0001) and time × EtOH effects (P < 0·007). (b) Energy intake: significant time effects (P < 0·0001), time × diet effects (P < 0·0001) and time × EtOH effects (P < 0·03). (c) Fluid intake: significant time effects (P < 0·0001), time × diet effects (P < 0·0001) and time × EtOH effects (P < 0·0001). No effects of time × diet × EtOH were observed in all three intakes. AD, adaptation day; GD, gestational day; PD, postnatal day. ![]() , Chow;
, Chow; ![]() , chow + EtOH;
, chow + EtOH; ![]() , energy-dense (E-dense);
, energy-dense (E-dense); ![]() , E-dense + EtOH. * To convert kcal to kJ, multiply by 4·184.
, E-dense + EtOH. * To convert kcal to kJ, multiply by 4·184.
The average energy intake (kJ/d) during each period was also measured to account for differences in energy density between the two diets. During EtOH adaptation, the energy intake was higher (P < 0·0001) in dams fed the E-dense diet compared with those fed chow, and lower (P < 0·0001) in dams with EtOH consumption than those without EtOH consumption. During gestation, the energy intake was similar among dams without EtOH consumption, whereas with EtOH consumption, dams fed the E-dense diet had a higher energy intake (P < 0·05) than dams fed chow. No significant differences were found in energy intake among dams during lactation.
As for fluid intake in non-EtOH groups, dams fed chow had 1·7–1·9 times higher fluid intake (P < 0·05) compared with those fed E-dense diet during EtOH adaptation, gestation and lactation (Table 2). When administered with EtOH, dams fed chow consumed 30–40 % less fluid than dams fed chow alone, but dams fed the E-dense while consuming EtOH had similar amounts of fluids compared with their non-EtOH counterparts during EtOH adaptation and gestation. When EtOH provision was stopped at parturition, dams fed chow while consuming EtOH during the prenatal period resumed their fluid intake to similar levels as dams fed chow alone. Dams fed the E-dense diet consumed less fluid (P < 0·0001) than dams fed chow during lactation. EtOH intake was similar in dams fed different diets throughout EtOH adaptation and gestation.
Maternal body weights (weekly) changed significantly across EtOH adaptation, gestation and the first week of lactation (P < 0·0001) (Fig. 2). While maternal body weights were maintained in the descending order of E-dense > chow > E-dense + EtOH > chow + EtOH over the experimental period, there were no significant differences identified among dams from the first three groups. Dams fed chow while consuming EtOH had the lowest body weights throughout gestation and lactation (P < 0·0001). Dams fed chow or the E-dense diet while consuming EtOH weighed less compared with their non-EtOH counterparts during gestation and lactation (6·1–12·3 % had 5·9–9·1 % less weight, respectively).

Fig. 2. Effects of maternal diets and ethanol (EtOH) on maternal body weights over the study. Data are means and standard deviations (n 6–8 per group at each time point). Repeated-measures ANOVA was performed to evaluate the effects of diet and EtOH on maternal weights over time. Significant time effects (P < 0·0001), time × diet effects (P < 0·0001) and time × EtOH effects (P < 0·0001) were observed. No effects of time × diet × EtOH were observed. The effects of diet and EtOH at each time point were analysed using Turkey multiple comparisons. a,b Means within a time point with unlike letters are significantly different (P < 0·05). AD, adaptation day; GD, gestational day; PD, postnatal day. ![]() , Chow;
, Chow; ![]() , chow + EtOH;
, chow + EtOH; ![]() , energy-dense (E-dense);
, energy-dense (E-dense); ![]() , E-dense + EtOH.
, E-dense + EtOH.
Litter size and survival data
Birth outcomes including litter size, neonatal deaths and sex ratio of pups were collected. In non-EtOH groups, the litter size of dams was not affected by diet (Table 3). Maternal EtOH consumption significantly decreased litter size compared with those without EtOH consumption in chow groups (Table 3). Neonatal deaths were observed in all dams except those fed the E-dense diet alone. The number of litters affected by neonatal deaths were 2 out of 6 in the chow group (each dam lost 1 pup), 2 out of 7 in the chow + EtOH group (each lost 3 or 10 pups) and 1 out of 8 in the E-dense + EtOH group (lost 7 pups). For litters that lost either 7 or 10 pups, biochemical data from these dams, as well as their pups’ data, were excluded from analysis, due to unequal access to breast milk compared with the other litters. Sex ratio of pups was not affected by maternal diets or EtOH consumption.
Table 3. Effects of maternal diets and ethanol (EtOH) on litter size and survival data
(Mean values and standard deviations)
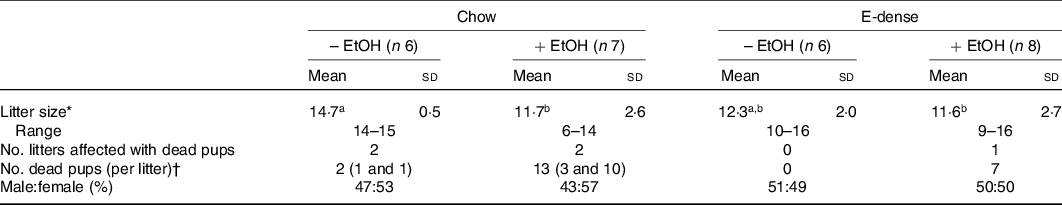
E-dense, energy dense.
a,b Mean values within a row with unlike superscript letters are significantly different (P < 0·05).
* Significant effects of diet and EtOH were analysed using two-way ANOVA followed by Duncan’s multiple range test (significant EtOH effects were observed, P < 0·05).
† Dead pups were observed at postnatal day 1.
Offspring body and organ weights
Birth outcomes including body weights and major organ weights of pups were measured (Table 4). Although maternal EtOH consumption resulted in a 3·7 and 8·4 % reduction in body weight of pups, born to dams fed chow and E-dense diet, respectively, the statistical significances were not reached by both diets or EtOH at PD3. At PD7, pups born to dams fed the E-dense diet had significantly higher body weights than pups born to dams fed chow (16·2 and 15·8 % with and without EtOH, respectively; P < 0·002). Although not significant, maternal EtOH consumption decreased body weights of pups in both diet groups (5·1 and 4·8 % in chow and E-dense diet, respectively) compared with those without maternal EtOH consumption.
Table 4. Effects of maternal diets and ethanol (EtOH) on body and major organ weights (wt) in pups*
(Mean values and standard deviations)

E-dense, energy dense; PD, postnatal day.
a,b,c Mean values within a row with unlike superscript letters are significantly different (P < 0·05).
* Each n represents a litter mean for each group, representing 67–86 pups (31–47 males; 36–46 females) per group at PD3 and 63–76 pups (28–43 males; 33–38 females) per group at PD7. Significant effects of diet and EtOH were analysed using two-way ANOVA followed by Duncan’s multiple range test (P < 0·05).
† Relative organ weight was a percentage of body weight.
Major organ weights including brain and liver weights were weighed at PD7, and organ weights relative to body weights (%) were calculated. Brain weights (g) of pups were not affected by maternal diet or EtOH, but liver weights (g) were significantly higher in pups born to dams fed E-dense diet than those born to dams fed chow (42 and 34 % with and without EtOH, respectively; P < 0·0001). Pups born to dams fed E-dense diet also had a higher relative liver weight (P < 0·0001) and a lower relative brain weight (P < 0·0001) than pups born to dams fed chow, regardless of maternal EtOH consumption. Similar trends in body, brain and liver weights were observed in male and female pups (data not shown).
Metabolic parameters
The effects of maternal diet and EtOH consumption during pregnancy on metabolic parameters were measured in both dams and pups at PD7 (Figs. 3 and 4). Dams fed the E-dense diet had higher concentrations of plasma TC (P < 0·0001), HDL-cholesterol (P < 0·0001) and lower plasma Glc concentrations (P < 0·0001) compared with dams fed chow. Maternal EtOH consumption also increased plasma concentrations of TC (P < 0·03) and Glc (P < 0·05) in dams fed the E-dense diet, whereas no differences were identified in dams fed chow. Maternal plasma LDL-cholesterol concentration was higher in dams fed E-dense diet in comparison with dams fed chow during EtOH consumption. Plasma concentrations of TAG, alanine aminotransferase and aspartate aminotransferase were not affected by either diet or EtOH in dams.

Fig. 3. Effects of maternal diets and ethanol (EtOH) on plasma lipids and glucose (Glc) in dams and pups at PD7. Data are means and standard deviations (n 6–7 per group). For pups, each n represents two pups (one male and one female) per litter. Significant effects of diet and EtOH were analysed using two-way ANOVA followed by Duncan’s multiple range test (P < 0·05). a,b,c Means with unlike letters are significantly different (P < 0·05). Dams: significant diet effects on total cholesterol (TC) (P < 0·0001), HDL-cholesterol (HDL-C) (P < 0·0001), LDL-cholesterol (LDL-C) (P < 0·0001) and Glc (P < 0·0001). Significant diet × EtOH effects on TC (P < 0·03), HDL-C (P < 0·04) and Glc (P < 0·05). No effects of EtOH were observed. Pups: significant diet effects on TC (P < 0·0001), LDL-C (P < 0·004) and TAG (P < 0·003). No effects of EtOH or diet × EtOH were observed. E-dense, energy-dense.
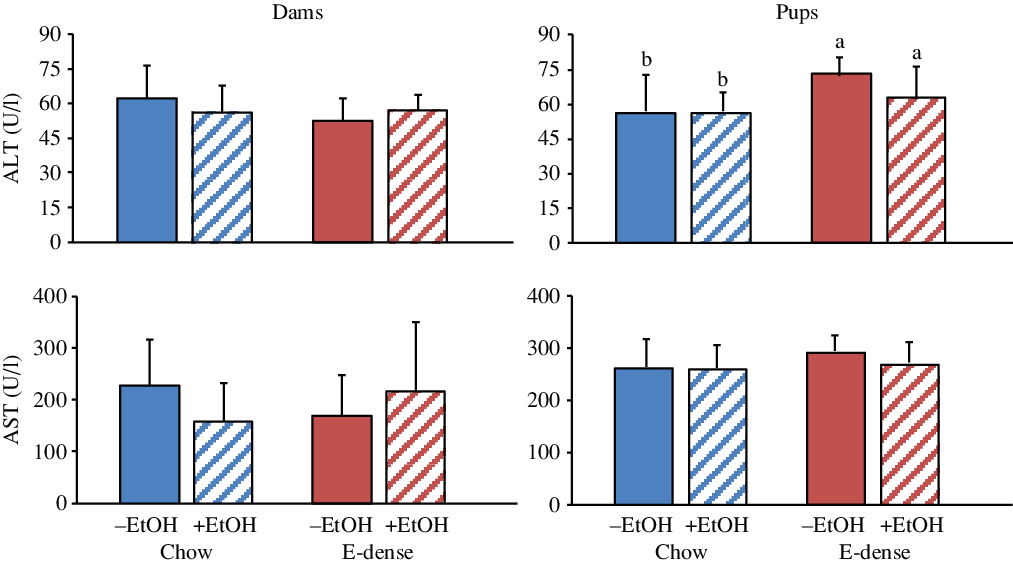
Fig. 4. Effects of maternal diets and ethanol (EtOH) on plasma levels of liver enzymes in dams and pups at PD7. Data are means and standard deviations (n 6–7 per group). For pups, each n represents two pups (one male and one female) per litter. Significant effects of diet and EtOH were analysed using two-way ANOVA followed by Duncan’s multiple range test (P < 0·05). a,b Means with unlike letters are significantly different (P < 0·05). Dams: no effects of diet, EtOH or diet × EtOH were observed. Pups: significant diet effects on alanine aminotransferase (ALT) (P < 0·03). No effects of EtOH or diet × EtOH were observed. AST, aspartate aminotransferase; E-dense, energy-dense.
The metabolic parameters of pups were also significantly affected by maternal diet (Figs. 3 and 4). Pups born to dams fed the E-dense diet had higher plasma concentrations of TC (P < 0·0001) and alanine aminotransferase (P < 0·03) compared with pups born to dams fed chow. Higher plasma LDL-cholesterol (P < 0·004) and TAG (P < 0·003) concentrations were found in pups born to dams fed the E-dense without EtOH and the E-dense diet with EtOH, respectively, in comparison with the pups from chow-fed dams. Plasma concentrations of HDL-cholesterol, Glc and aspartate aminotransferase were not affected by either maternal diet or EtOH.
Discussion
The current study described the effects of different qualities of maternal diets and EtOH consumption during pregnancy on birth and metabolic outcomes in rats and their offspring. Compared with chow, maternal E-dense diet attenuated EtOH-induced weight loss in dams, increased body and liver weights of pups and resulted in negative metabolic outcomes in dams and pups. While it is not known whether these effects can persist into adulthood, this study highlights the importance of maternal diet quality on maternal health and infant growth.
Effects of maternal diet and ethanol on maternal outcomes
A normal weight gain during pregnancy is an effective way to measure a healthy pregnancy. In comparison with non-EtOH groups, dams with EtOH consumption had lower body weights in both diet groups during pregnancy and the first week of lactation, but the significant difference was only identified among dams fed chow. This confirms the previous findings by Feltham et al. (Reference Feltham, Louis and Kapourchali13) and Kapourchali et al. (Reference Kapourchali, Louix and Eskin14) that body weight does not significantly change in dams fed E-dense diet with EtOH consumption during pregnancy. This finding is also in accordance with studies who reported a lower weight gain in dams fed chow while consuming EtOH during pregnancy(Reference Naassila and Daoust10,Reference Wentzel, Rydberg and Eriksson12) . Since dams fed the E-dense diet had a 1·5 times higher energy intake than dams fed chow during EtOH consumption, sufficient energy intake could have attenuated EtOH-induced weight loss in dams. Low maternal weight gain during late pregnancy has been shown to increase the risks of intra-uterine growth retardation(Reference Strauss and Dietz24,Reference Hasan, Khan and Ahmed25) , which is associated with infant mortality as well as other health problems throughout life(Reference Sania, Spiegelman and Rich-Edwards26,Reference Cosmi, Fanelli and Visentin27) . Therefore, this result indicates that increased body weight caused by high energy intake may reduce some of the damages induced by EtOH on offspring. It should be noted that although the current study focused on the energy density between the two experimental diets, other nutrient components and food matrices could also have contributed to the differences observed in this study, which requires further investigation.
Effects of maternal diet and ethanol on birth outcomes
Many studies using a standard chow reported that maternal EtOH consumption during pregnancy is associated with adverse birth outcomes, including a reduced litter size and the occurrence of neonatal deaths(Reference Naassila and Daoust10,Reference Carvalho, Dutra and Andrade28,Reference Furuya, Aikawa and Yoshida29) . In agreement with those studies, these adverse effects were obvious among dams fed chow in the current study. Maternal EtOH consumption also decreased litter sizes in dams fed the E-dense diet; however, the reductions were much smaller than those fed chow (20 v. 6 % in chow and E-dense diet, respectively). Similarly, the previous study by Kapourchali et al. (Reference Kapourchali, Louix and Eskin14) reported that in pregnant dams fed an E-dense diet, EtOH consumption did not affect litter size. Considering that dams fed the E-dense diet also had higher neonatal survival than those fed chow, maternal high energy intake may positively affect birth outcomes in rats with EtOH exposure during pregnancy. Dams fed the E-dense diet had higher food and energy intakes than chow-fed dams during EtOH consumption; thus, it is possible that increased food intake could have reversed some nutritional deficiencies induced by EtOH consumption, thereby improving maternal nutritional status and resulting in a better birth outcome. Furthermore, a small litter size may be a result of fetal resorption, which has been reported to take place in response to EtOH consumption(Reference Furuya, Aikawa and Yoshida29). Since the current study focused on postnatal pups, future studies could also evaluate the effect of the same diet and EtOH regimen on litter size, fetal resorption and intra-uterine growth retardation in the fetus.
Similar to previous studies using standard chow(Reference Furuya, Aikawa and Yoshida29-Reference Testar, Llobera and Herrera32), maternal EtOH consumption during pregnancy decreased body (3·7 % at PD3; 5·1 % at PD7), brain (5·8 %) and liver weights (5·7 %) of pups born to dams fed chow, but these results did not reach statistical significance. In an earlier study, it was reported that maternal EtOH consumption from gestational days 14 to 19 significantly decreased body weight in fetuses but not in postnatal pups(Reference Addolorato, Gasbarrini and Marcoccia33). Although the mechanisms behind this are not completely understood, the authors suggested that the amount of EtOH provided to dams was relatively low; thus, body weights of pups had returned to that of controls after EtOH treatment stopped. Moreover, the previous study using an E-dense diet reported that EtOH increased fetal body and liver weights(Reference Feltham, Louis and Kapourchali13), but the current study showed a non-significant trend towards a reduction in postnatal body (8·4 % at PD3; 4·8 % at PD7) and brain weights (3·0 %) with no changes in liver weight. The variations exist in the administration and dosing of EtOH, and the developmental stages of the offspring that were examined (fetal v. postnatal), which may account for the different results in studies using E-dense diet. It would be of interest to conduct a follow-up study using the same diet and EtOH regimen to determine the effects on fetal parameters.
While the effect of EtOH was minimal, maternal E-dense diet significantly increased body and liver weights of pups than those from dams fed chow at PD7. As expected, this could be due to high energy intake, especially from fat (13·4 v. 30·0 % kJ fat in chow and E-dense diet, respectively). A maternal high-fat diet (28·6–60·3 % kJ) was shown to increase body and liver weights of pups at PD7(Reference Thompson, Cismowski and Trask34) or at weaning(Reference Franco, Fernandes and Rocha35). Although it is controversial whether the energy content of breast milk reflects maternal diet(Reference Butts, Hedderley and Herath36,Reference Bravi, Wiens and Decarli37) , it has been reported that a high-fat diet during pregnancy and lactation increases daily milk volume(Reference Del Prado, Delgado and Villalpando38), as these pups consume more milk than those of control groups(Reference Purcell, Sun and Pass39). Unfortunately, the milk quality of the dams was not measured in this study, but the results suggest that other factors including milk production and pup ingestive behaviour are changed by maternal diets, thereby affecting pups’ weights. Interestingly, the body weights of pups at PD3 were similar regardless of maternal diets. Similar results also showed that a maternal high-fat diet (45–60 % kJ) throughout pregnancy and lactation did not affect birth weight but increased postnatal weight at PD7(Reference Yang, Cai and Xu40) or at weaning(Reference Desai, Jellyman and Han41) compared with a control diet. This raises the question of whether or not a maternal diet during lactation is more critical to infant growth, and thus more research is warranted.
In rats, the period of brain growth spurt, which is characterised by a rapid increase in the brain weight, occurs during early postnatal life and peaks at PD7(Reference Dobbing and Sands18). Although the absolute brain weight of pups was unchanged, the relative brain weight of PD7 pups born to dams fed the E-dense diet was lower compared with those from chow-fed dams. This reduction could be a result of increased body weights in this group, but some studies reported that it is related to the loss of neurons or brain structure(Reference Muralidharan, Sarmah and Zhou42), which then may lead to a number of cognitive and behavioural deficits. Finding signalling mechanisms related to neuronal cell death, such as brain ceramides, are currently under investigation in our laboratory using these animals.
Effects of maternal diet and ethanol on metabolic outcomes
Although several studies have demonstrated that maternal EtOH consumption during pregnancy affects metabolic pathways, leading to adverse metabolic outcomes in offspring(Reference Yao and Nyomba43–Reference Elton, Pennington and Lynch46), these changes were not observed in the current study. However, pups born to dams fed E-dense diet had increased plasma concentrations of TC and TAG compared with those from chow-fed dams, independently of EtOH intake. Considering these pups also had larger livers with higher plasma concentrations of the liver enzyme alanine aminotransferase, it is possible that the E-dense diet induced fatty liver diseases which could contribute to metabolic disorders. Previous studies have made a similar observation of increased metabolic abnormalities in offspring whose mothers consumed a high-fat diet (45–50 % kJ) during pregnancy and lactation(Reference Yang, Cai and Xu40,Reference Desai, Jellyman and Han41,Reference Sheen, Yu and Tain47) . It has also been reported that maternal high fat intake (45 % kJ) decreased fatty acid oxidation and up-regulated hepatic lipogenesis, which contributed to the development of non-alcoholic steatohepatitis and the metabolic syndrome in offspring(Reference Bruce, Cagampang and Argenton48). However, it should be tested whether these metabolic changes can persist into adulthood with the same diets.
Similar to their pups, dams fed the E-dense diet had higher plasma TC concentrations compared with those fed chow. These dams also had elevated plasma HDL-cholesterol but decreased Glc concentrations, possibly due to lowered carbohydrate content in the E-dense diet. Low-carbohydrate diet has been shown to increase plasma HDL-cholesterol and decrease TAG concentrations(Reference Dong, Guo and Zhang49). These effects were not seen in their pups, there were also differences in the concentrations of these metabolic parameters between the dams and pups. For instance, pups had higher plasma TC, LDL-cholesterol and TAG concentrations compared with dams, regardless of treatments. This could result from the experimental procedures, as dams were fasted overnight, whereas pups were allowed to stay with the dams and access milk until the time of kill. It is also possible that the differences were physiological at the specific developmental stage, thus being independent of maternal metabolic outcomes. In terms of EtOH, plasma TC and Glc concentrations were elevated in dams fed E-dense diet but not in those fed chow, indicating that E-dense diet could aggravate metabolic disorders in dams with EtOH consumption during pregnancy. It should be noted that most studies have focused on the offspring less is known about the metabolic outcomes with maternal EtOH consumption; therefore, it is difficult to compare the effect of EtOH in dams among studies. Nevertheless, the current data showed that the two different diets had various effects on metabolic outcomes in dams consuming EtOH during pregnancy.
Parameters were measured only at a single time point at PD7; thus, it is not clear whether those adverse metabolic outcomes of pups could persist into adulthood. It is also difficult to link the differences observed between the two diet groups only to the energy density in the diet, as other dietary components might also have contributed to the differences observed. Further studies could focus on the effect of a single nutrient or combined effects of multiple nutrients on the treatment and prevention of FASD. This study also has some strengths. Chronic drinking model used in the current study mimics the most common route of EtOH consumption in humans compared with other models. Pregnant rats were housed individually and provided with nesting materials, which improved living conditions and reduced stress during pregnancy. Last, the basic data including maternal intakes and weights were collected at about 09.00 hours over the course of the study, ensuring the consistency of the data collection.
In conclusion, the current study demonstrates that the quality of maternal diets (standard chow v. E-dense diet) differently affects the physical and metabolic parameters in dams and pups. Compared with chow, maternal E-dense diet attenuated EtOH-induced maternal weight loss and some adverse birth outcomes, suggesting its potential to reduce certain negative consequences associated with maternal EtOH consumption. While the significant effect of EtOH on maternal body weight was observed, it was not detected in postnatal pups despite there being a trend in reduction of pups’ body and organ weights. It is speculated that the dose of EtOH provided to dams was relatively low, thereby not causing significant changes to postnatal pups, especially when the EtOH treatment was withdrawn at parturition. For this reason, it is of interest to evaluate the fetus with the same diet and EtOH regimen. It is also noteworthy to investigate whether a higher level of EtOH intake during pregnancy has a long-lasting effect in postnatal pups and can persist into adulthood.
Although the effect of EtOH was not observed, maternal E-dense diet increased body and liver weights and exhibited adverse metabolic outcomes in pups. Additional research is needed to examine the long-term impact of maternal E-dense diet on the offspring. As there is limited information available on maternal metabolic outcomes, future studies should include maternal data in order to better understand associations between maternal metabolic outcomes and offspring development. Overall, the current study described that compared with chow, maternal E-dense diet has the potential to attenuate some negative effects of EtOH in dams and pups; however, when EtOH is not present, maternal consumption of E-dense diet causes enlarged liver and metabolic abnormalities in pups, possibly due to excess nutrient intake specifically fat content. Nevertheless, this study highlights the importance of maternal nutrition or a well-balanced diet on maternal health and infant growth. This also suggests that nutritional status of pregnant women who are at risk of EtOH consumption should be determined in order to establish nutritional interventions to prevent and treat FASD.
Acknowledgements
This work was supported by Research Manitoba, Canada-Israel International Fetal Alcohol Consortium and Manitoba Graduate Scholarship.
The authors’ responsibilities were as follows: Y. W. and B. A. F. conducted the research, collected and analysed the data; Y. W. wrote the manuscript; B. A. F., M. N. A. E. and M. S. edited the manuscript. M. S. designed the study, provided financial support, directed the overall study and had primary responsibility for final content. All authors read and approved the final manuscript.
The authors declare no conflicts of interest.










