The n-3 fatty acid DHA (22 : 6n-3, DHA) increases in the brain grey matter and retina phospholipids (PL) during early development, with a well-known higher proportion of DHA in the brain and retina PL than other organs. Loss of brain DHA results in replacement with the n-6 fatty acids 22 : 4n-6 and 22 : 5n-6 in both animals and humans( Reference Lamptey and Walker 1 , Reference Farquharson, Jamieson and Abbasi 2 ), which has been associated with impaired or reduced visual acuity and aspects of neurological function in animals( Reference Lamptey and Walker 1 – Reference Reisbick, Neuringer and Gohl 4 ). Several randomised intervention trials have reported higher visual acuity and neurological outcomes for infants and children born to mothers given DHA during gestation compared with a placebo( Reference Judge, Harel and Lammi-Keefe 5 – Reference Dunstan, Simmer and Dixon 7 ), whereas others have found no benefit( Reference Helland, Saugstad and Smith 8 – Reference Smithers, Gibson and Makrides 10 ) or a negative effect( Reference Gould, Treyvaud and Yelland 11 , Reference Makrides, Gould and Gawlik 12 ). Similarly, several studies have provided evidence that addition of DHA to infant formula fed in the weeks or months after birth leads to greater visual and neurological development in term and preterm infants compared with formula with no DHA( Reference Agostoni, Trojan and Bellu 13 – Reference Carlson, Werkman and Rhodes 18 ).
It is well known that nutritional deficiency of certain essential nutrients during development can have lasting, irreversible effects on the brain, which do not occur when the same deficiency occurs later on. For example, folate deficiency occurring within 28 d post-conception may interfere with closure of the neural tube( Reference Osterhues, Ali and Michels 19 ), whereas iodine deficiency before birth is associated with irreversible neurological consequences( Reference Cao, Jiang and Dou 20 ). Deficiency of DHA in the brain may be caused by an inadequate source of DHA or its precursor α-linolenic acid (18 : 3n-3), which synthesises DHA via EPA (20 : 5n-3, EPA) through desaturation and elongation( Reference Innis 21 ). A high intake of the n-6 fatty acid linoleic acid (18 : 2n-6) has also been shown to be associated with DHA deficiency, with increased arachidonic acid (20 : 4n-6) in the brain during early development( Reference Martinez 22 ). Loss of neural DHA when an n-3 fatty acid-deficient or high n-6 fatty acid diet is fed to the mother during gestation has also been shown to alter neurotransmitter metabolism, neural function and behaviour in animals, including rodents and non-human primates( Reference Lamptey and Walker 1 , Reference Neuringer, Connor and Van Petten 3 , Reference Coti Bertrand, O’Kusky and Innis 23 , Reference Chalon 24 ).
The infant brain, however, is only about 25 % of the adult brain weight at term gestation, being about 370 g and increasing to about 70 % of adult brain weight by 12 months( Reference Dekaban 25 ). Synapses, which are rich in DHA, increase with remarkable rates of formation of 40 000 synapses/second during the first 2 years, with structural reorganisation and pruning of synapses leading to maximum synaptic density from about 6–7 years( Reference Levitt 26 ). Both observational and randomised intervention studies have provided evidence that DHA intake and status of children up to about 10 years are also related to child neural function, including verbal learning ability( Reference Dalton, Wolmarans and Witthuhn 27 ), language( Reference Ryan and Nelson 28 ), reading( Reference Sinn, Cooper and O’Dea 29 , Reference Richardson, Burton and Sewell 30 ), spelling( Reference Sinn, Cooper and O’Dea 29 ), non-verbal intelligence( Reference Sinn, Cooper and O’Dea 29 , Reference Kirby, Woodward and Jackson 31 ) and memory( Reference Baym, Khan and Monti 32 ). Reaction time and prefrontal cortex activation during a sustained attention test has also been associated with baseline erythrocyte DHA in boys aged 8–10 years, and 400 or 1200 mg/d DHA for 8 weeks led to increased prefrontal cortex activation from baseline compared with controls( Reference McNamara, Able and Jandacek 33 ). This suggests that although DHA deficiency during gestation may have lasting effects, continued brain development after birth with child diet potentially impacting brain PL DHA may in turn impact child brain function.
We previously reported that DHA insufficiency in some pregnant women in our population was low enough to limit infant neurodevelopment to 18 months of age( Reference Mulder, King and Innis 34 ). The primary purpose of the current study was to determine whether fetal DHA insufficiency extends to neurological effects in children assessed at 5·75 years. The analysis of both the previous and the present studies are based on the concept that infants with the highest scores on neurodevelopmental tests were unlikely to be nutrient deficient (Fig. 1). In addition, this study also sought to determine whether the child’s DHA intake and erythrocyte status was related to the mother’s DHA intake and erythrocyte status during gestation.
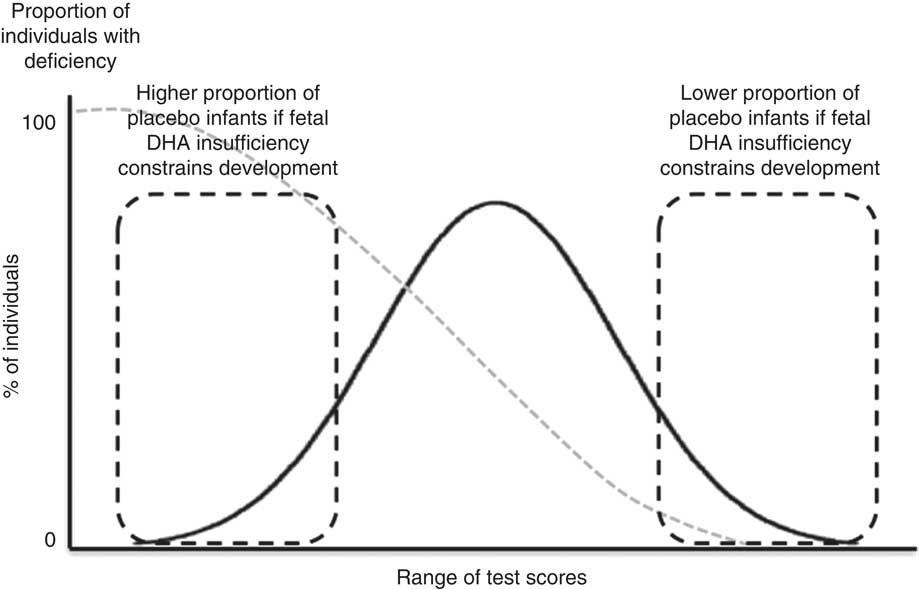
Fig. 1 Illustration to highlight our assumption that children with high neurodevelopment test scores were unlikely to be nutrient deficient in utero. The potential long-term effects of fetal DHA insufficiency were assessed as the risk that a child from the maternal placebo group would fail to achieve a test score in the highest quartile of neurodevelopment test scores.
Methods
Subjects and study design
This was a follow-up of children born to mothers who participated in a randomised, double-blind, placebo-controlled study to assess whether DHA deficiency during pregnancy occurs and is sufficient to limit infant central nervous system (CNS) development up to 18 months of age( Reference Mulder, King and Innis 34 ). In brief, pregnant women were randomised to 400 mg/d DHA or a placebo from 16 weeks of gestation until delivery, with follow-up only of singleton, term-gestation infants, with no congenital or metabolic diseases likely to affect infant neurological development. A total of 200 infants, ninety-six and 104 for maternal DHA or placebo supplementation groups, respectively, were invited for follow-up. Of these, ninety-eight parents agreed to participate in this follow-up study that assessed dietary intake, DHA status and CNS development in children at 5·75 years. New computer-generated codes were made for all participants to avoid knowledge of the gestational intervention group and infant outcome data to 18 months.
The present study was conducted according to guidelines laid down in the Declaration of Helsinki, with written informed consent obtained from a parent or guardian for each child before any assessment or data collection. All procedures involving human subjects in this study were approved by the Committee for Ethical Review of Research Involving Human Subjects at the University of British Columbia and the British Columbia Children’s and Women’s Hospital.
Subject characteristics, dietary assessment and biochemical analysis
Family and socio-demographic information, including income, education and number of adults and children in the home, was updated by questionnaire. Parental intelligence quotient (IQ) was assessed with the Test of Nonverbal Intelligence-3rd edition, which assesses aptitude, abstract reasoning and problem-solving, without dependence on language or formal education( Reference Brown 35 ). Each child weight and height was measured, and then weight, height and BMI z scores calculated using the WHO Anthroplus anthropometric calculator (version 1.0.4).
Dietary intake was assessed using an FFQ by face-to-face interview with the parent, using food models and measuring utensils, including all foods and beverages consumed by the child over the previous 4 weeks. Dietary intake of macronutrients and fatty acids were quantified using nutrient analysis software (ESHA Food Processor SQL, version 10.10.0.0; ESHA Research), ensuring that the database had complete and accurate fatty acid compositions based on product labels for our location, and where necessary our laboratory analysed the food fatty acids.
Child venous blood (6 ml) was collected with EDTA, centrifuged (2500 g , 10 min, 4°C), the plasma and buffy coat separated, aliquoted and frozen at −80°C within 30 min of blood collection. The erythrocytes were washed by re-suspension with saline and EDTA, centrifuged twice and then the erythrocytes were frozen at −80°C until analysis. Erythrocyte total lipids were extracted, then fatty acids quantified by routine GLC( Reference Liou, King and Zibrik 36 ). Preparation of blood and analysis of erythrocytes was led by technician D. J. King, an expert with clinical samples for fatty acids.
Child neural development
Child neural development was assessed using age-appropriate standardised tests, in a dedicated room free of distractions, with a one-way mirror to enable the parent to view their child. The tests included the Kaufman Assessment Battery for Children, 2nd edition (KABC), which enables scaled subset scores to assess cognitive ability. The sequential processing scale measures short-term memory; the learning ability scale measures long-term storage and retrieval; and the simultaneous processing scale measures visual processing. General mental processing ability is also measured by combining all three scales to give a composite Mental Performance Index (MPI) score( Reference Kaufman and Kaufman 37 ). The KABC delayed recall was also done, which measures long-term memory. Language development using the Peabody Picture Vocabulary Test-4 (PPVT)( Reference Dunn and Dunn 38 ) and visual–motor integration using the Beery-Buktenica Developmental Test of Visual-Motor Integration (Beery)( Reference Beery, Buktenica and Beery 39 ) were also assessed. Finally, the Test of Variables of Attention (TOVA)( Reference Leark, Kindschi and Dupuy 40 ) was used to assess attention and impulsivity, measured by the scores of errors of commission and omission, respectively, and also response time, a measure of processing speed, and response time variability, a measure of response time consistency.
Data analysis
We used descriptive statistics to summarise subject characteristics, and compared variables between the DHA supplement and placebo groups using independent t tests, Mann–Whitney U tests or χ 2 tests, as appropriate. Family and child characteristics including infant birth weight, sex and human milk-feeding duration, maternal IQ, number of children and adults in the home were screened for potential associations with child neurodevelopment test scores.
The potential impact of gestation was assessed using contingency tables and Fisher exact tests to determine the OR and 95 % CI that a child from the placebo group would fail to score in the top quartile of children for each test. Where children could not be separated into an exact upper quartile owing to several children with the same score, a cut-off point ‘quartile’ giving the lowest deviation from 25 % of the group was used. Our previous report showed a considerable overlap in maternal DHA status, measured as erythrocyte phosphatidylethanolamine (PE) and phosphatidylcholine (PC) DHA, between the DHA and placebo groups after approximately 20 weeks of supplementation during pregnancy( Reference Mulder, King and Innis 34 ). Thus, Pearson’s correlation or Spearman’s rank correlation coefficient, as appropriate, were used to determine the potential relationship between maternal DHA status at 16 and 36 weeks of gestation and child performance on cognitive tests.
The potential association between the mother’s prenatal dietary DHA intake and DHA status with the children’s DHA intake and DHA status at 5·75 years was assessed using Pearson’s correlation or Spearman’s rank correlation coefficient, as appropriate. Owing to the 400 mg/d DHA supplementation from 16 weeks of gestation to term infant birth, the relationship between the maternal erythrocyte DHA, 22 : 4n-6, 22 : 5n-6 and the DHA:22 : 4n-6+22 : 5n-6 ratio was addressed for all children at 16 weeks of gestation, but not at 36 weeks of gestation. Data analysis was done using IBM SPSS Statistics (version 20.0.0, 2011), with significance set at P<0·05.
Results
A total of ninety-eight children, aged 5·75 years, from the study on DHA deficiency during gestation( Reference Mulder, King and Innis 34 ) participated in this follow-up study from 2010 to 2014. No significant differences were found in maternal or infant characteristics at birth or through the 18-month infant follow-up between children who did not participate in this study (n 102) and those who did (n 98). The child and family characteristics are shown in Table 1. For the group, 74·5 % of the children were Caucasian, 12·2 % were Chinese and 13·3 % were of other ethnicities; of the children, 53·1 % were girls, 83·5 % of the children lived with two adults, 55·1 % had one sibling, 89·8 % were human milk-fed at least 3 months and had a BMI z score mean 0·06 (sd 1·01) at 5·75 years. No differences were found in maternal IQ measures, family characteristics or the child sex, length of human milk-feeding, weight, height or BMI assessment, P>0·05, between children in the placebo (n 52) and DHA (n 46) groups. Cognitive tests were completed for >90 % of children, with incomplete tests for PPVT, n 1; Kaufman ABC sequential, n 2; learning, n 4; simultaneous, n 1; MPI, n 5; delayed recall, n 1; Beery, n 0; TOVA, n 9. The FFQ was analysed for all children, and blood samples sufficient for fatty acid analysis were obtained from seventy-three children.
Table 1 Family and child characteristics (Mean values and standard deviations; percentages)
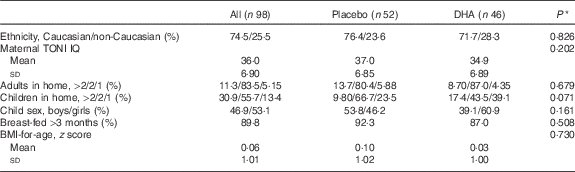
TONI, Test of Nonverbal Intelligence.
* Statistical comparison between the prenatal maternal placebo and DHA groups.
Potential variables associated with child neural development were screened for all cognitive tests and showed a lower reaction time (P=0·003) and higher percent errors of commission (P=0·004) in boys than girls on the TOVA, and a higher PPVT scores for Caucasian than non-Caucasian children (P=0·001). Infant human milk-feeding duration showed no association with child development test results; however, only 10·2 % of the children were human milk-fed <3 months. There was no effect of any other collected or measured child or family characteristic on child cognitive scores at 5·75 years.
No significant differences were found in mean test performance between children in the maternal gestation DHA and placebo groups (Table 2). We also found no differences between children in the two maternal groups in achieving performance in the upper quartile of test performance (Table 3); PPVT (P=1·00), Beery (P=1·00), the KABC sequential processing (P=0·808), learning ability (P=0·628), simultaneous processing (P=0·818), MPI (P=0·476) and delayed recall (P=0·825), or on the TOVA response time variability (P=0·739), response time (P=0·717), errors of commission (P=0·645) and errors of omission (P=0·111).
Table 2 Cognitive scores for all children and by maternal DHA supplement or placebo groupFootnote * (Mean values and standard deviations; medians and 2·5–97·5 percentiles)
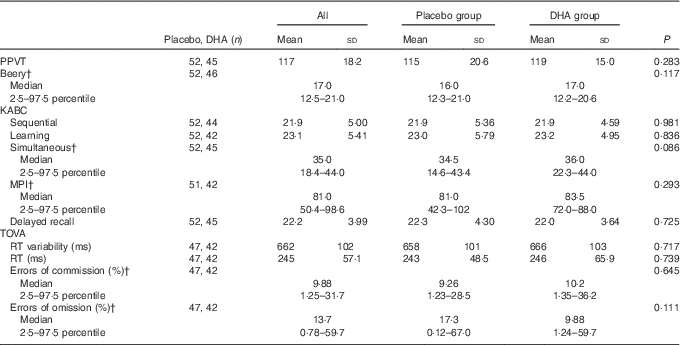
PPVT, Peabody Picture Vocabulary Test; Beery VMI, Beery-Buktenica Developmental Test of Visual-Motor Integration; KABC, Kaufman Assessment Battery for Children, 2nd edition; MPI, Mental Performance Index; TOVA, Test of Variables of Attention; RT, response time.
* Compared by Student’s t test or Mann–Whitney U test.
† Data are not normally distributed.
Table 3 Risk that a child in the placebo group would be in the lowest quartile rather than the highest quartile of cognitive development test scores (Odds ratios and 95 % confidence intervals)
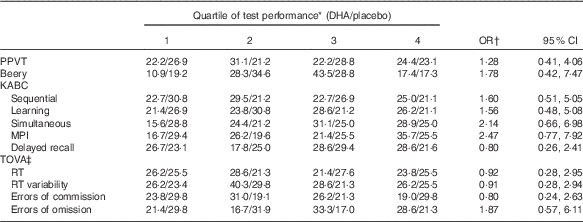
PPVT, Peabody Picture Vocabulary Test; Beery VMI, Beery-Buktenica Developmental Test of Visual-Motor Integration; KABC, Kaufman Assessment Battery for Children, 2nd edition; MPI, Mental Performance Index; TOVA, Test of Variables of Attention; RT, response time.
* Data are % of children from maternal DHA group/% of children from maternal placebo group. The number of children for each test as n DHA/placebo are: PPVT, n 45/52; Beery, n 46/52; KABC sequential, n 44/52; learning, n 42/52; simultaneous, n 45/52; MPI, 42/51; delayed recall, n 45/52; TOVA scores, n 42/47.
† P>0·10 for each test.
‡ For TOVA scores, better performance is the achievement of a lower score; data were analysed as the lowest scores in quartile 4, and highest scores in quartile 1.
There were no associations between the maternal prenatal dietary DHA and child cognitive test scores (online Supplementary Table S1). The assessment of maternal erythrocyte DHA status during gestation and child cognitive performance are shown in the online Supplementary Tables S2 and S3. The children’s Beery score was positively associated with maternal DHA/22 : 4n-6+22 : 5n-6 in erythrocyte PE at 16 weeks of gestation (ρ=0·247, P=0·016) and 36 weeks of gestation (ρ=0·221, P=0·032), and inversely associated with maternal 36-week erythrocyte PE 22 : 4n-6 (ρ=−0·247, P=0·016) (online Supplementary Table S2). The children’s Beery scores were also associated with maternal erythrocyte PC fatty acids of interest at 16 weeks of gestation and are shown in the online Supplementary Table S2.
As previous studies have shown associations between the maternal and child diet( Reference Brion, Ness and Rogers 41 – Reference Zuercher, Wagstaff and Kranz 44 ), we addressed the relationship of child DHA intake and erythrocyte DHA to that of the mother’s. DHA intake among the children was skewed, with a median (2·5–97·5 %) of 43·2 mg/d (0−300 mg/d) (online Supplementary Table S4). There was no difference in DHA intake between boys and girls (P=0·524), or between children in the prenatal placebo and DHA groups (P=0·603). The child DHA intake at 5·75 years was significantly related to their mother’s DHA intake at both 16 weeks and 36 weeks of gestation, ρ=0·317, P=0·002 and ρ=0·256, P=0·013, respectively (Table 4). In addition, the children’s DHA intake was related to maternal erythrocyte PE and PC DHA/22 : 4n-6+22 : 5n-6 at 16 weeks of gestation, ρ=0·297, P=0·004 and ρ=0·264, P=0·010, respectively, (n 94) (Table 4).
Table 4 Associations of child dietary DHA with maternal dietary and erythrocyte markers of DHA sufficiency during gestation
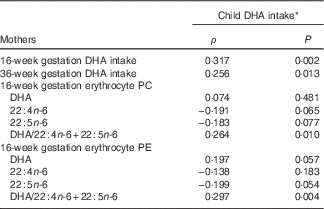
PC, phosphatidylcholine; PE, phosphatidylethanolamine.
* Data are Spearman’s rank correlation coefficients, for all mother–child pairs with available dietary DHA and erythrocyte fatty acids, n 69.
Child erythrocyte DHA mean was 5·20 (sd 1·43) %TFA, with 3·31 (sd 0·68) and 0·63 (sd 0·16) 22 : 4n-6 and 22 : 5n-6, respectively, and erythrocyte DHA/22 : 4n-6+22 : 5n-6 of median (2·5–97·5 %) 1·31 (0·60–3·34) %TFA. Child erythrocyte DHA was significantly related to child DHA intake (ρ=0·396, P<0·001). The relationship between child erythrocyte DHA at 5·75 years and maternal DHA intake and erythrocyte DHA at 16 weeks of gestation is shown in Table 5. Child erythrocyte DHA was related to maternal DHA intake at 36 weeks of gestation (ρ=0·277, P=0·021), but not at 16 weeks of gestation (ρ=0·195, P=0·109). The child erythrocyte DHA was related to maternal 16-week erythrocyte PE DHA (ρ=0·239, P=0·048), erythrocyte PE 22 : 5n-6 (ρ=−0·286, P=0·017) and erythrocyte PE DHA/22 : 4n-6+22 : 5n-6 (ρ=0·313, P=0·009), but not erythrocyte PE 22 : 4n-6 (r 0·124, P=0·312). Child erythrocyte DHA was also related to erythrocyte PC DHA/22 : 4n-6+22 : 5n-6 (ρ=0·306, P=0·010), but not erythrocyte PC DHA (r 0·219, P=0·375), erythrocyte PC 22 : 4n-6 (ρ=−0·080, P=0·512) or erythrocyte PC 22 : 5n-6 (ρ=−0·120, P=0·323) at 16 weeks of gestation.
Table 5 Associations of maternal dietary and erythrocyte DHA during gestation with the children’s erythrocyte markers of DHA sufficiency
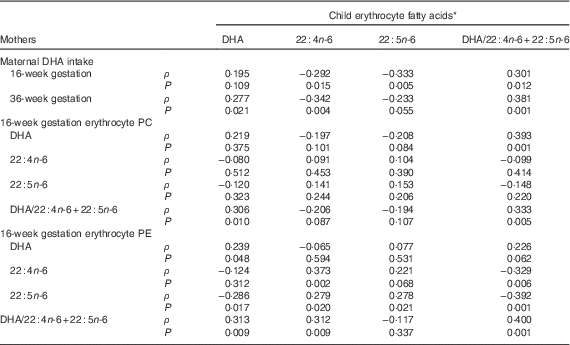
PC, phosphatidylcholine; PE, phosphatidylethanolamine.
* Data are Spearman’s rank correlation coefficients, for all mother–child pairs with available dietary DHA and erythrocyte fatty acids, n 69.
Discussion
We used a risk-reduction model to address the potential long-term effect of DHA during gestation on cognitive performance when assessed at 5·75 years (Fig. 1). Although we found that DHA insufficiency during gestation was associated with a greater risk of failure to achieve a high score in infant neurodevelopment tests to 18 months( Reference Mulder, King and Innis 34 ), evidence of lasting effects in children up to 5·75 years was not detected. The apparent effects of DHA insufficiency during gestation on CNS development up to 18 months of age may have been lost or masked by postnatal dietary or other variables in children at 5·75 years. The potential importance of the child’s postnatal diet to continued brain development and the association between the children’s dietary habits and that of the mother raises the question of the implications of epidemiological pregnancy studies that consider only the maternal diet or status on long-term child outcome.
The primary purpose of the present study was to determine whether DHA insufficiency during gestation had long-term effects on neurological development when assessed in a prospective group of children at 5·75 years. In our previous report, we found that infants from the placebo group were at an increased risk of not achieving a high visual acuity score at 2 months, although this was not detected at 12 months( Reference Mulder, King and Innis 34 ). Infants from the placebo group were also at increased risk of not achieving a score in the highest quartile for tests of language development at 14 and 18 months( Reference Mulder, King and Innis 34 ). At 5·75 years, there was no difference in risk of failure to achieve a high score between maternal DHA and placebo groups on the language test (PPVT) (P=1·00), or any other cognitive test assessed (P>0·05). Although studies of the effect of DHA supplementation during pregnancy on infant outcome have reported mixed results, the majority of studies on long-term outcome from 2 to 7 years have reported no benefit of maternal DHA supplementation during pregnancy on tests of child attention( Reference Gould, Makrides and Colombo 45 ), working memory and inhibitory control( Reference Gould, Makrides and Colombo 45 ), general mental processing ability (KABC MPI), short-term memory (KABC Sequential) and visual processing (KABC Simultaneous)( Reference Helland, Smith and Blomen 46 ), general cognitive function( Reference Gould, Treyvaud and Yelland 11 ) or the Hempel and Touwen neurological examinations( Reference Escolano-Margarit, Ramos and Beyer 47 ). In contrast, one study found higher mean scores for hand–eye coordination in children aged 2·5 years (n 72) born to mothers taking fish oil during pregnancy compared with a placebo( Reference Dunstan, Simmer and Dixon 7 ). One other study reported a slightly higher mean score on general mental processing ability (KABC MPI) in a subset of children at 4 years (n 84) whose mothers had taken cod liver oil compared with a placebo during pregnancy (P=0·049)( Reference Helland, Smith and Saarem 6 ), although this was not seen when the children were assessed at 7 years (n 143)( Reference Helland, Smith and Blomen 46 ).
In most DHA interventions, the placebo groups do not have ‘non-exposure,’ which leads to overlap of nutrient intake and status with the DHA supplementation group. Although a higher amount of supplemental DHA may mitigate the overlap to some extent, nutrient intake exceeding individual requirements should not provide additional benefit. Thus, our study differs from efficacy studies in that it was designed to detect whether fetal DHA deficiency extended to 5·75 years, and not to show that DHA supplements during gestation enhance neurodevelopment assessed in childhood. As both the DHA and placebo groups in our study would likely contain individuals who were not deficient in gestation and hence unable to respond to intervention, we did not expect to find differences in mean scores between the groups. It is possible that an intervention in a population with a high prevalence of DHA deficiency may enable the detection of an efficacious response to increased DHA intake. However, a biomarker of DHA insufficiency is currently unavailable.
In the present study, visual–motor integration (Beery) scores were negatively associated with maternal prenatal erythrocytes 22 : 4n-6 and 22 : 5n-6. Both 22 : 4n-6 and 22 : 5n-6 have been shown to accumulate in the brain when brain DHA is low( Reference Farquharson, Jamieson and Abbasi 2 , Reference Makrides, Neumann and Byard 48 ). In animals, increased brain 22 : 4n-6 and 22 : 5n-6 is associated with functional consequences( Reference Lamptey and Walker 1 ), but it is unknown whether the negative effects are attributable to low brain DHA or the high long-chain n-6 fatty acids. A SNP in the fatty acid desaturase (FADS) and elongase (ELOVL) genes may also limit endogenous synthesis of DHA; however, 20 : 4n-6, 22 : 4n-6 and 22 : 5n-6 may depend on the same enzymes for synthesis( Reference Xie and Innis 49 , Reference Koletzko, Lattka and Zeilinger 50 ). Whether or not SNPs in FADS and ELOVL genes may protect the brain from accumulating 22 : 4n-6 and 22 : 5n-6 in the absence of DHA is unclear, and thus further work is required to determine whether the effects of low brain DHA are observed and whether levels of 22 : 4n-6 and 22 : 5n-6 are proportionately low.
The brain of a healthy term-born infant doubles its birth weight (about 370 g) in the first six postnatal months( Reference Dekaban 25 ), and DHA to support continuing brain growth must be provided by n-3 fatty acids in human milk or infant formula. Accretion of DHA in the infant brain continues rapidly after birth for about 2 years, with brain PL DHA increasing until approximately 8 years( Reference Martinez 22 , Reference Martinez and Mougan 51 ). During this period, the brain undergoes morphological and functional changes including synaptic proliferation, remodelling and pruning( Reference Tau and Peterson 52 ), but whether prenatal DHA insufficiency alone is sufficient to alter these processes is unknown. The DHA intake of the present group of children was highly skewed, with a median intake of 43·2 mg/d and 2·5–97·5 % intake of 0–300 mg/d. The DHA intake of children in this study is within the range of international estimates for this age group, but the interquartile range (IQR) of 17·4–97·4 mg/d is higher than the population IQR for DHA intake of US children aged 1–5 years of 10·8–26·2 mg/d (n 2496)( Reference Keim and Branum 53 ). Although it is not known whether the level of intake is sufficient for continued brain development of the young child, positive associations have been found between neurodevelopment and both n-3 intake and DHA status in children up to 10 years( Reference Ryan and Nelson 28 , Reference Kirby, Woodward and Jackson 31 , Reference Baym, Khan and Monti 32 , Reference van der Wurff, Bakker and Hornstra 54 ). A recent study showed positive associations between plasma DHA at age 7 years and assessments of reading (β=0·158, P<0·001) and spelling (β=0·146, P=0·001)( Reference van der Wurff, Bakker and Hornstra 54 ). The children’s reading and spelling scores were not significantly related to cord blood plasma DHA or maternal plasma DHA in gestation.
The present study highlights the challenge in understanding whether long-term effects on outcomes, particularly those with a developmental time course extending beyond infancy, are explained by dietary intake during pregnancy or by the child’s own diet, if children’s diets reflect the diet of the mother. The associations between maternal prenatal and child DHA intake and status suggest dietary and potentially genetic similarities between mothers and children. Maternal DHA intake at 16 weeks and 36 weeks of gestation was positively associated with child DHA intake, and maternal DHA intake at 36 weeks of gestation was positively associated with child erythrocyte DHA at 5·75 years. Although the association between the maternal and child DHA intakes have not previously been published, several studies have reported a relationship between the maternal and child diet( Reference Brion, Ness and Rogers 41 – Reference Deroma, Valent and Parpinel 43 ), with associations stronger for the maternal-child diet than the paternal-child diet( Reference Brion, Ness and Rogers 41 , Reference Oliveria, Ellison and Moore 42 ). The Framingham Children’s study reported that at 3–5 years the child’s polyunsaturated fat intake was positively associated with the mother’s (r 0·33, P≤0·01, n 87) but not the father’s intake (r 0·10, P>0·05, n 83), with similar findings for several other nutrients( Reference Oliveria, Ellison and Moore 42 ). Similarly, the ALSPAC study reported a positive association between the child’s intake at 10 years and the maternal prenatal (n 5717) and postnatal (n 5593) diet for protein, carbohydrate and fat, but only child protein intake was associated with the paternal diet (n 3009)( Reference Brion, Ness and Rogers 41 ). Notably, the relationship between child and maternal prenatal total fat intake was stronger than the postnatal intake( Reference Brion, Ness and Rogers 41 ). For a subsample of a prospective cohort study in Italy, fish and seafood intake was lower in the prenatal diet than the postnatal diet, but there was no difference in fish intake between mother’s postnatal diet and fish intake of the children at 8–11 years (n 37)( Reference Deroma, Valent and Parpinel 43 ).
In conclusion, the results of the present study have shown that the effects of maternal DHA during gestation observed in infants to 18 months were not identified at 5·75 years. The potential long-term effects of DHA insufficiency may be too small to detect with our experimental design, or it is possible that the DHA intake of our population was not low enough during gestation to have lasting effects. Human brain development begins as early as the third week of gestation, and while considerable DHA accretion has been reported to occur during the brain growth spurt beginning in the third trimester it is unknown whether a low DHA supply early during gestation compromises embryonic brain development. Most intervention studies of prenatal DHA and infant neurodevelopment began supplementation in the second trimester, and thus our intervention at 16 weeks of gestation may have been too late if DHA is important for early structural brain development. However, the inverse association of child Beery scores with maternal erythrocytes 22 : 4n-6 and 22 : 5n-6 may suggest that visual–motor integration development is sensitive to low prenatal DHA, consistent with the time course of brain maturation, with maturation occurring in the visual cortex before the prefrontal cortex( Reference Tau and Peterson 52 ). The association between the maternal and child diet may explain the discrepancy between epidemiologic and intervention studies of prenatal DHA on child outcome, as it seems reasonable to assume that mothers with low DHA intakes during pregnancy may have children with low DHA intakes. Thus, with results of several studies also suggesting that the DHA supply in childhood is associated with brain development after birth, separating the prenatal and postnatal effects of DHA supplies on brain development is a challenge. Regardless, evidence that even short-term DHA supplementation in children improved multiple areas of neural function( Reference Dalton, Wolmarans and Witthuhn 27 , Reference Sinn, Cooper and O’Dea 29 , Reference Richardson, Burton and Sewell 30 , Reference McNamara, Able and Jandacek 33 ) raises the possibility that the child’s DHA intake and DHA status at 5·75 years may affect neurological performance.
Acknowledgements
The authors would like to acknowledge Dr Sheila Innis, whose infectious passion for research and discovery led to numerous advances in our understanding of early-life nutrition. She was a dedicated mentor and will be missed dearly by her trainees and colleagues. The authors would also like to offer the gratitude to the parents and children who dedicated their time and effort to participate in the study. Finally, the authors would like to acknowledge Roger Dyer, Janette King for their assistance with sample analysis and Kelly Richardson for conducting the developmental assessments of the children.
This research was funded by the Canadian Institutes for Health Research grant no. MOP 84248. The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript.
K. A. M. and S. M. I. designed the research questions and the study; K. A. M. and S. M. I. conducted the research; K. A. M., S. M. I. and R. E. analysed the data and wrote the manuscript, and S. M. I. passed away before the completion of the final draft of the manuscript. K. A. M. and R. E. read and approved the final manuscript and take responsibility for the published paper.
None of the authors has any conflicts of interest to declare.
Supplementary Material
For supplementary material/s referred to in this article, please visit https://doi.org/10.1017/S0007114517003531









