Elevated total plasma homocysteine (tHcy) levels are associated with increased oxidative stress and endothelial damage (Welch & Loscalzo, Reference Welch and Loscalzo1998). tHcy has been suggested to be an independent risk factor for several multi-system diseases (Virtanen et al. Reference Virtanen, Voutilainen, Alfthan, Korhonen, Rissanen, Mursu, Kaplan and Salonen2005), including CHD (Homocysteine Studies Collaboration, 2002; Vrentzos et al. Reference Vrentzos, Papadakis, Malliaraki, Zacharis, Mazokopakis, Margioris, Ganotakis and Kafatos2004), stroke (Ford et al. Reference Ford, Smith, Stroup, Steinberg, Mueller and Thacker2002), dementia and Alzheimer's disease (Seshadri et al. Reference Seshadri, Beiser, Selhub, Jacques, Rosenberg, D'Agostino, Wilson and Wolf2002), risk of hip fracture (McLean et al. Reference McLean, Jacques, Selhub, Tucker, Samelson, Broe, Hannan, Cupples and Kiel2004) and pregnancy complications (Hague, Reference Hague2003).
Lifestyle factors such as smoking, lack of physical activity, excessive alcohol intake, obesity, high coffee consumption as well as nutritional deficiencies in folate and vitamin B12 are associated with elevated levels of tHcy (Jacques et al. Reference Jacques, Bostom, Wilson, Rich, Rosenberg and Selhub2001; Bates et al. Reference Bates, Mansoor, Gregory, Pentiev and Prentice2002; Silaste et al. Reference Silaste, Rantala, Alfthan, Aro and Kesaniemi2003; Chrysohoou et al. Reference Chrysohoou, Panagiotakos, Pitsavos, Zeimbekis, Zampelas, Papademetriou, Masoura and Stefanadis2004; van Beynum et al. Reference van Beynum, den Heijer, Thomas, Afman, Oppenraay-van Emmerzaal and Blom2005). In addition, a major genetic determinant of tHcy levels has been identified, the methylenetetrahydrofolate reductase (MTHFR) polymorphism 677C>T (Frosst et al. Reference Frosst, Blom and Milos1995). Age and gender seem to play a specific role. Levels of tHcy are higher in men than in women and this gender effect seems to be enhanced during and after puberty (De Laet et al. Reference De Laet, Wautrecht, Brasseur, Dramaix, Boeynaems, Decuyper and Kahn1999; Bates et al. Reference Bates, Mansoor, Gregory, Pentiev and Prentice2002; van Beynum et al. Reference van Beynum, den Heijer, Thomas, Afman, Oppenraay-van Emmerzaal and Blom2005).
Objectively measured physical activity has been negatively associated with traditional cardiovascular risk factors in children and adolescents (Strong et al. Reference Strong, Malina and Blimkie2005). Poor cardiorespiratory fitness is another important health problem (Myers et al. Reference Myers, Prakash, Froelicher, Do, Partington and Atwood2002; Carnethon et al. Reference Carnethon, Gidding, Nehgme, Sidney, Jacobs and Liu2003). Recently, cardiorespiratory fitness has been negatively associated with features of metabolic syndrome in children and adolescents (Mesa et al. Reference Mesa, Ruiz, Ortega, Warnberg, González-Lamuño, Moreno, Gutiérrez and Castillo2006; Ruiz et al. Reference Ruiz, Ortega, Meusel, Harro, Oja and Sjöström2006a) and it has been negatively associated with tHcy in adult women (Kuo et al. Reference Kuo, Yen and Bean2005). Studies examining the possible interplay between physical activity, cardiorespiratory fitness and fatness with tHcy levels in children and adolescents are lacking. For public health strategies and preventive purposes, it is of interest to understand the relative influence of modifiable factors on tHcy levels on early ages. Therefore, we examined the associations of tHcy with physical activity, cardiorespiratory fitness and fatness, after controlling for potential confounders including the MTHFR 677C>T genotype, in children and adolescents.
Methods
Subjects
The subjects were participants in the Swedish part of the European Youth Heart Study, a school-based, cross-sectional study of risk factors for future CVD in a random sample of healthy children (9–10 years old) and of adolescents (15–16 years old) (Poortvliet et al. Reference Poortvliet, Yngve, Ekelund, Hurtig-Wennlöf, Nilsson, Hagströmer and Sjöström2003). Data collection took place from September 1998 to May 1999 in thirty-seven schools in central Sweden. The present report is based on the 680 subjects who had both tHcy and MTHFR genotypes measured (301 children, 379 adolescents). Study design, sampling procedure and participation rates have been reported elsewhere (Wennlöf et al. Reference Wennlöf, Yngve and Sjöström2003). The study was approved by the Research Ethics Committees of Örebro County Council and Huddinge University Hospital. Written informed consent was obtained from the parents of the children and from both the parents of the adolescents and the adolescents themselves.
Before any testing was performed, the parents completed a questionnaire in which part of the questions addressed the child's previous and current health status. Socioeconomic status was also assessed via the questionnaire and defined by the maternal educational status (coded as 0 = below university education and 1 = university education). Using the maternal educational status as socioeconomic status indicator has the advantage of having a high response rate and relatively unbiased responses in comparison with questions regarding income (Kaplan & Keil, Reference Kaplan and Keil1993).
Physical examination
Height and weight were measured by standardized procedures. BMI was calculated as weight/height2 (kg/m2). Skinfold thicknesses were measured with a Harpenden caliper (Baty International, Burgess Hill, UK) at the biceps, triceps, subscapular, suprailiaca and triceps surae areas on the left side of the body according to the criteria described by Lohman et al. (Reference Lohman, Roche and Martorell1991). These measures have been shown to correlate highly with dual-energy X-ray absorptiometry-measured body fat percentage in children of similar ages (Gutin et al. Reference Gutin, Litaker, Islam, Manos, Smith and Treiber1996; Rodriguez et al. Reference Rodriguez, Moreno, Blay, Blay, Fleta, Sarria and Bueno2005). All measurements were taken twice and in rotation and the mean value was calculated. If the difference between the two measurements was >2 mm, a third measurement was taken and the two closest measurements were averaged. The sum of the five skinfold thicknesses (hereafter referred to as ‘skinfold thickness’) was used as an indicator of body fat rather than BMI, because BMI has been suggested to be a less valid measurement of body fatness in children (Rennie et al. Reference Rennie, Livingstone, Wells, McGloin, Coward, Prentice and Jebb2005) and because fatness rather than weight has been shown to be associated with poor health (Allison et al. Reference Allison, Zhu, Plankey, Faith and Heo2002). However, for the purpose of comparing the results with previous publications, the subjects were also categorized as normal-weight or overweight-obese following the International Obesity Task Force proposed gender- and age-adjusted BMI cut-off points (Cole et al. Reference Cole, Bellizzi, Flegal and Dietz2000).
Identification of pubertal development was assessed according to Tanner & Whitehouse (Reference Tanner and Whitehouse1976). Pubertal stage was recorded by a researcher of the same gender as the child, after brief observation. Breast development in girls and genital development in boys were used for pubertal classification.
Measurement of physical activity
Physical activity was measured during four consecutive days (two weekdays and at least one weekend day) with an activity monitor (MTI model WAM 7164; Manufacturing Technology Inc., Shalimar, FL, USA) worn at the lower back. At least 3 d of recording, with a minimum of 10 h registration per d, was set as an inclusion criterion. Total physical activity was expressed as total counts recorded, divided by total daily registered time (counts/min). The time engaged in moderate physical activity and vigorous physical activity was calculated and presented as the average time per d during the complete registration. Moderate physical activity (3–6 metabolic equivalents) and vigorous physical activity (>6 metabolic equivalents) intensities were also calculated and were based upon the cut-off limits published elsewhere (Trost et al. Reference Trost, Pate, Sallis, Freedson, Taylor, Dowda and Sirard2002). The time spent in at least moderate intensity level (>3 metabolic equivalents) was calculated as the sum of time spent in moderate or vigorous physical activity. Each minute over the specific cut-off was summarized in the corresponding intensity level group. Validation studies examining the accelerometer used in the present study and the construction of summary variables for intensity of movement suggest that it is a valid and reliable measure of children's and adolescent's physical activity (Trost et al. Reference Trost, Ward, Moorehead, Watson, Riner and Burke1998; Puyau et al. Reference Puyau, Adolph, Vohra, Zakeri and Butte2004). The precision of objective assessment of physical activity in children is superior to subjective methods (Kohl et al. Reference Kohl, Fulton and Caspersen2000); however, there are some limitations that should be highlighted. The accelerometer must be removed during swimming, contact sports, showering and bathing. Any activity involving minimal vertical displacement of the body (i.e. cycling) is also underestimated. A period of 4–5 d of activity monitoring has been proposed as a suitable duration for accurate and reliable estimates of usual physical activity behaviour in children (Trost et al. Reference Trost, Pate, Freedson, Sallis and Taylor2000). Data for 4 d were available for most of the participants in the present study.
Measurement of cardiorespiratory fitness
Cardiorespiratory fitness was determined by a maximum cycle-ergometer test, as described elsewhere (Hansen et al. Reference Hansen, Froberg, Nielsen and Hyldebrandt1989). Briefly, the workload was pre-programmed on a computerized cycle ergometer (Monark 829E; Ergomedic, Vansbro, Sweden) to increase every third minute until exhaustion. Heart rate was registered continuously by telemetry (Polar Sport Tester; Polar Electro Oy, Kempele, Finland). The criteria for exhaustion were a heart rate ≥ 185 beats per min, failure to maintain a pedalling frequency of at least 30 rpm and a subjective judgement by the test leader that the child could no longer keep up, even after vocal encouragement. The power output was calculated as = W1+ (W2· t/180), where W1 is a work rate at fully completed stage, W2 is the work rate increment at final incomplete stage and t is time in seconds at final incomplete stage. The ‘Hansen formula’ for calculated VO2max (ml/min) was = 12 × calculated power output +5 × body weight (kg) (Hansen et al. Reference Hansen, Froberg, Nielsen and Hyldebrandt1989). Cardiorespiratory fitness was expressed as VO2max per kg body mass (ml/kg per min) because of the homogeneity in stature and obesity grade of the subjects, because it is well established and for ease of comparison with the results of previous studies. The test used has been previously validated in children of the same age (Riddoch et al. Reference Riddoch, Edwards and Page2005) and is highly correlated in children with directly measured VO2max (r 0·95 and r 0·90, in girls and boys, respectively) (Hansen et al. Reference Hansen, Froberg, Nielsen and Hyldebrandt1989).
Dietary assessment
Dietary intake was assessed by an interviewer-mediated 24-h recall. For the 9-year-olds, a qualitative food record, completed the day before the interview, acted as a checklist once the 24-h recall was obtained. A food atlas was used to estimate portion sizes. Dietary data were entered into StorMats (version 4.02; Rudans Lättdata, Västerås, Sweden) and analysed using the Swedish National Food Database, version 99.1 (www.s/v.se). Folate and vitamin B12 intakes were calculated in μg/d.
Homocysteine assay and methylenetetrahydrofolate reductase genotyping
With the subject in the supine position, blood samples were taken by venipuncture after an overnight fast using vacuum tubes (Vacuette; Greiner Bio-One GmbH, Essen, Germany). The fasting state was verbally confirmed by the subject before blood sampling. Plasma was separated and stored at − 80°C until analysis. Homocysteine in acidified citrated plasma (Willems et al. Reference Willems, Bos, Gerrits, den Heijer, Vloet and Blom1998) was assayed using a fluorescence polarization immunoassay on an IMx® unit (Abbott Laboratories, Abbott Park, IL, USA). All CV were under 7·5 %. Total blood DNA was extracted and purified from 200 μl whole blood anticoagulated with EDTA, using the QIAamp DNA Blood Mini Kit by the spin procedure, according to the instructions of the manufacturer (QIAGEN Inc., Valencia, CA, USA). Genotyping of the 677C>T variant in the MTHFR gene was performed using the Pyrosequencing platform (Biotage AB, Uppsala, Sweden), as described recently (Börjel et al. Reference Börjel, Yngve, Sjöström and Nilsson2006).
Statistical analysis
The data are presented as means and standard deviations unless otherwise stated. All variables were checked for normality of distribution before analysis. Skinfold thickness, tHcy, folate and vitamin B12 were normalized by transformation to the natural logarithm, and for the total physical activity the square root was calculated.
Gender and age differences were assessed by two-way ANOVA. The levels of tHcy according to MTHFR 677C>T and age group were analysed by two-way ANOVA and mean tHcy levels between MTHFR 677C>T subgroups were compared by Tukey's test.
The tHcy mean values did not differ between the CC and CT groups; therefore, the MTHFR 677C>T genotype was analysed as a recessive trait, i.e. dichotomized into TT and CC + CT groups.
Multiple regressions were used to study the relationship between tHcy and physical activity, cardiorespiratory fitness and body fat, after controlling for potential confounders. Model 1 examined the influence of total physical activity on tHcy after controlling for gender, pubertal development, socioeconomic status, folate and vitamin B12 intake and MTHFR 677C>T genotype. Model 2 examined the influence of cardiorespiratory fitness on tHcy after controlling for the same potential confounders included in Model 1. Model 3 examined the influence of body fat (expressed as skinfold thickness or as BMI) on tHcy after controlling for the same potential confounders included in Models 1 and 2. Semipartial correlation (sr) was used as a measure of the relationship between tHcy and physical activity, cardiorespiratory fitness and body fat, after controlling for the effect that one or more additional variables (e.g. pubertal development, socioeconomic status, etc.) had on one of those variables. The analyses were performed using the Statistical Package for Social Sciences (SPSS, v. 14·0 for Windows; SPSS Inc, Chicago, IL, USA) and the level of significance was set to = 0·05.
Results
Data completeness and baseline characteristics
None of the analysed variables showed a statistically significant difference between included subjects (i.e. those who had both tHcy and MTHFR genotype measurements) and excluded subjects (i.e. those who did not have both). Valid physical activity data were obtained in 71 % of the studied subjects and 84 % had valid cardiorespiratory fitness data, as determined by the criteria explained earlier (see p. 00). Pubertal development status was obtained from 88 %, and 91 % had skinfold thickness measured. Socioeconomic status and dietary intake data were available for 95 and 99 % of the subjects, respectively.
The descriptive characteristics of the study sample are shown in Table 1. The levels of tHcy did not differ between boys and girls in either age group, but significant differences were observed between age groups in both girls and boys. In both children and adolescents, girls had significantly lower values of physical activity related-variables and cardiorespiratory fitness than boys (Table 1). In boys, physical activity related-variables, cardiorespiratory fitness and body fat were significantly different between children and adolescents. Mean values of total physical activity, cardiorespiratory fitness and body fat were not significantly different across tHcy quartiles in both children and adolescents (Fig. 1).
Table 1 Descriptive characteristics of the subjects§ (Values are means and standard deviations unless otherwise stated)

Gender and age differences were assessed by two-way ANOVA.
*P for gender differences.
† P for age differences in girls.
‡ P for age differences in boys.
§ For details of subjects and procedures, see p. 256.

Fig. 1 Mean values of total physical activity (a), cardiorespiratory fitness (b) and sum of five skinfolds stratified by quartiles of homocysteine for children (—●—) and adolescents (—□—). Errors bars represent 95 % CI. For details of subjects and procedures, see p. 256.
Mean values of tHcy were significantly higher in the TT subgroup compared with CC and CT subgroups in children (P < 0·001 and P = 0·019, respectively) and adolescents (both P < 0·001). No statistically significant differences were found between CC and CT subgroups either in children or in adolescents. The TT genotype was present in 8·6 % of children and 8·7 % of adolescents (Table 2).
Table 2 Total plasma homocysteine (tHcy) levels according to methylenetetrahydrofolate reductase (MTHFR) 677C>T genotype and age group‡ (Values are means and standard deviations)
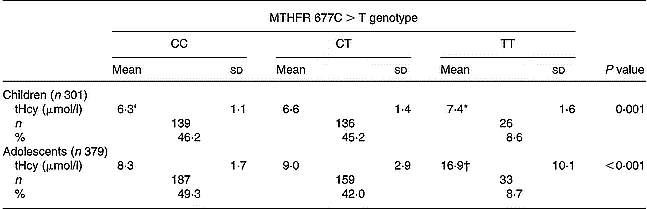
* Mean values were significantly different from CC (P = 0·001) and CT (P = 0·019) by Tukey's test.
† Mean values were significantly different from CC (P < 0·001) and CT (P < 0·001) by Tukey's test.
‡ For details of subjects and procedures, see p. 256.
Relationship between total plasma homocysteine and physical activity, cardiorespiratory fitness and body fat after controlling for different confounders and separation by age
The results of the regression models using tHcy as the outcome variable are shown in Table 3. Variation in tHcy levels was not significantly explained by total physical activity, cardiorespiratory fitness or body fat (expressed as skinfold thickness) in any age group. The results did not change when body fat was expressed as BMI. Subsequent analysis examining tHcy levels among BMI categories did not show any significant influence (data not shown). Further analysis examining the association of physical activity intensity levels (i.e. moderate, vigorous and moderate-vigorous) with tHcy did not change the results. The results remained the same when the regression models were performed separately for the MTHFR 677TT and MTHFR 677CC+CT subgroups.
Table 3 Standardized multiple regression coefficients (β), se, semipartial correlations (sr) and standardized coefficient of determination (R2) examining association of level of plasma total homocysteine with total physical activity, cardiorespiratory fitness and body fat (expressed as skinfold thickness) after controlling for gender, pubertal development, socioeconomic status, folate and vitamin B12 intake, and MTHFR 677C>T genotype*

* For details of subject and procedures, see p. 256.
Discussion
The results suggest that tHcy levels are not influenced by the studied modifiable factors in young subjects as other well-established cardiovascular risk factors in children and adolescents have been shown to be (Brage et al. Reference Brage, Wedderkopp, Ekelund, Franks, Wareham, Andersen and Froberg2004; Mesa et al. Reference Mesa, Ruiz, Ortega, Warnberg, González-Lamuño, Moreno, Gutiérrez and Castillo2006; Ruiz et al. Reference Ruiz, Ortega, Meusel, Harro, Oja and Sjöström2006a, Reference Ruiz, Rizzo, Wennlöf, Ortega, Harro and Sjöströmb). However, tHcy levels were significantly higher in the MTHFR 677TT subgroup compared with the MTHFR 677CC and MTHFR 677CT subgroups in both children and adolescents. Differences in tHcy levels were also observed between age but not gender groups, which is in concordance with previous studies showing that tHcy increases with age (Bates et al. Reference Bates, Mansoor, Gregory, Pentiev and Prentice2002). The levels of tHcy seen in the present study were within the normal ranges for these ages (Ueland & Monsen, Reference Ueland and Monsen2003). Similar tHcy levels have been reported in Belgian (De Laet et al. Reference De Laet, Wautrecht, Brasseur, Dramaix, Boeynaems, Decuyper and Kahn1999), Dutch (van Beynum et al. Reference van Beynum, den Heijer, Thomas, Afman, Oppenraay-van Emmerzaal and Blom2005) and Greek (Papoutsakis et al. Reference Papoutsakis, Yiannakouris, Manios, Papaconstantinou, Magkos, Schulpis, Zampelas and Matalas2005) children and adolescents. To the best of our knowledge, there are no other data available on the association of tHcy levels with objectively measured physical activity, fitness and fatness after controlling for several potential confounders, including MTHFR 677C>T genotype, in children and adolescents.
Physical activity and homocysteine
The association between physical activity and tHcy has been evaluated in few studies. Randeva et al. (Reference Randeva, Lewandowski, Drzewoski, Brooke-Wavell, O'Callaghan, Czupryniak, Hillhouse and Prelevic2002) showed that 6 months of sustained brisk walking for 20–60 min for 3 d/week significantly decreased tHcy levels in young overweight and obese women with polycystic ovary syndrome, a group at increased risk of premature atherosclerosis. Similarly, a weight-reduction programme including physical activities had a positive effect on the tHcy of obese children (Gallistl et al. Reference Gallistl, Sudi, Erwa, Aigner and Borkenstein2001). We did not find any association between total physical activity and tHcy levels, even when the influence of physical activity intensity levels on tHcy levels was examined. When the analyses were performed separately for normal-weight and overweight-obese children and adolescents, no associations were found. Studies examining the influence of objectively measured physical activity and physical activity intensity levels on tHcy in children and adolescents are lacking. Intervention programmes studying the effect of physical activity amount and the influences of different physical activity intensities on tHcy in children and adolescents are needed.
Cardiorespiratory fitness and homocysteine
Cardiorespiratory fitness is a direct marker of physiological status and recent data suggest that fitness is one of the strongest predictors of health outcomes (Myers et al. Reference Myers, Prakash, Froelicher, Do, Partington and Atwood2002; Carnethon et al. Reference Carnethon, Gidding, Nehgme, Sidney, Jacobs and Liu2003). Cardiorespiratory fitness represents the ability of active skeletal muscle to utilize O2 during exercise. Theoretically, poor cardiorespiratory capacity may be the consequence of pathological changes peripherally affecting the tissues and the associated vasculature or centrally perturbing the heart and coronary arteries. These pathological changes, to some extent, may be attributed to elevated levels of tHcy and the related tissue toxicity in adults. In fact, elevated tHcy levels have been associated with an increased risk of decline in physical function in elderly people (Kado et al. Reference Kado, Bucur, Selhub, Rowe and Seeman2002). Moreover, high tHcy levels have been negatively associated with estimated cardiorespiratory fitness in adult women (Kuo et al. Reference Kuo, Yen and Bean2005).
High cardiorespiratory fitness during childhood and adolescence has been associated with a healthier metabolic profile during these years (Brage et al. Reference Brage, Wedderkopp, Ekelund, Franks, Wareham, Andersen and Froberg2004; Mesa et al. Reference Mesa, Ruiz, Ortega, Warnberg, González-Lamuño, Moreno, Gutiérrez and Castillo2006; Ruiz et al. Reference Ruiz, Ortega, Meusel, Harro, Oja and Sjöström2006a). Results from the Alimentación y Valoración del Estado Nutritional en Adolescentes (AVENA) (Food and Assessment of the Nutritional Status of Spanish Adolescents) study showed significant associations between increased cardiorespiratory fitness and a favourable lipid profile and fasting glycaemia in both overweight and non-overweight adolescents aged 13–18 years (Mesa et al. Reference Mesa, Ruiz, Ortega, Warnberg, González-Lamuño, Moreno, Gutiérrez and Castillo2006). Results from the Swedish and Estonian part of the European Youth Heart Study revealed negative associations between cardiorespiratory fitness and body fat (expressed as skinfold thickness) (Ruiz et al. Reference Ruiz, Rizzo, Wennlöf, Ortega, Harro and Sjöström2006b). The same relationship was noted between cardiorespiratory fitness and other features of metabolic syndrome (e.g. insulin resistance, raised TAG, total cholesterol:HDL-cholesterol ratio, etc.) in children aged 9–10 years (Ruiz et al. Reference Ruiz, Ortega, Meusel, Harro, Oja and Sjöström2006a). However, we did not observe any association between cardiorespiratory fitness and tHcy in children and adolescents. It must be borne in mind that the subjects involved in the present study were healthy children and adolescents with no existing cardiovascular pathologies and it may be that tHcy is not as sensitive to cardiorespiratory fitness as are other traditional cardiovascular risk factors.
Body fat and homocysteine
The amount of body fat has been associated with tHcy levels in obese children and adolescents (Gallistl et al. Reference Gallistl, Sudi, Mangge, Erwa and Borkenstein2000). Insulin resistance has been implicated in the relationship, since insulin levels are strongly correlated with body fat (Gallistl et al. Reference Gallistl, Sudi, Mangge, Erwa and Borkenstein2000). We did not find an association between body fatness (as expressed as skinfold thickness or as BMI) and tHcy levels, even when the analyses were performed separately for normal-weight or overweight-obese categories. This may be due to the low number of children and adolescents in the obese BMI category participating in the present study.
The results from the present study should be interpreted with caution due to the limitations of the cross-sectional design that cannot support evidence for causality. Another limitation of the study is the lack of information on other factors that have been shown to influence tHcy levels, such as serum levels of folate and vitamin B12 (Jacques et al. Reference Jacques, Bostom, Wilson, Rich, Rosenberg and Selhub2001; Papoutsakis et al. Reference Papoutsakis, Yiannakouris, Manios, Papaconstantinou, Magkos, Schulpis, Zampelas and Matalas2005; van Beynum et al. Reference van Beynum, den Heijer, Thomas, Afman, Oppenraay-van Emmerzaal and Blom2005) and endothelial NO synthase G894T polymorphism (Brown et al. Reference Brown, Kluijtmans and Young2003). The inclusion of available intake data on folate and vitamin B12 was an attempt to overcome this. The inclusion of a large number of subjects and several potential confounders including the MTHFR 677C>T genotype are notable strengths of the present study.
Results from cross-sectional studies have shown strong associations between tHcy and lifestyle-related factors (Jacques et al. Reference Jacques, Bostom, Wilson, Rich, Rosenberg and Selhub2001; Bates et al. Reference Bates, Mansoor, Gregory, Pentiev and Prentice2002; Silaste et al. Reference Silaste, Rantala, Alfthan, Aro and Kesaniemi2003; Chrysohoou et al. Reference Chrysohoou, Panagiotakos, Pitsavos, Zeimbekis, Zampelas, Papademetriou, Masoura and Stefanadis2004; van Beynum et al. Reference van Beynum, den Heijer, Thomas, Afman, Oppenraay-van Emmerzaal and Blom2005). However, findings are different when analysed prospectively (Husemoen et al. Reference Husemoen, Thomsen, Fenger and Jorgensen2006). The effect of lifestyle intervention based on smoking cessation, increased physical activity and healthy dietary habits on changes in tHcy after 1 year of follow-up was studied in a population-based sample of 915 men and women aged 30–60 years. The results suggested that none of the studied lifestyle changes was associated with changes in tHcy levels, indicating that tHcy may not be reduced by general lifestyle interventions.
One potential explanation for the discrepancy may be that the tHcy-lifestyle associations observed in cross-sectional studies are perhaps not causal. Furthermore, lifestyle-related data are usually obtained by means of questionnaires, which may lead to misreporting. The relationships between tHcy and objectively measured modifiable factors in children and adolescents should be studied prospectively.
In conclusion, the results of the present cross-sectional study suggest that objectively measured physical activity, cardiorespiratory fitness and body fat are not associated with tHcy levels in children and adolescents, even after controlling for presence of the MTHFR 677C>T genotype, the main influence on tHcy levels in these subjects.
Acknowledgements
The study was supported by grants from the Stockholm County Council (M.S.) and Nyckelfonden, Örebro (T.K.N.). J.R.R. and F.B.O. were supported by a grant from Ministerio de Educación y Ciencia de España (AP2003-2128, AP2004-2745).






