Biotin is a water-soluble vitamin that serves as a coenzyme for five human carboxylases, namely acetyl-CoA carboxylases (ACC) 1 and 2, pyruvate carboxylase, propionyl-CoA carboxylase (PCC) and 3-methylcrotonyl-CoA carboxylase (MCC)(Reference Zempleni, Wijeratne and Hassan1). These enzymes play an important role in the metabolism of fatty acids, glucose and amino acids. Histones H3 and H4 are also modified by covalent attachment of biotin(Reference Camporeale, Shubert and Sarath2–Reference Rios-Avila, Pestinger and Zempleni5). Biotinylation of histones is a rare event, and less than 0·001 % of histones H3 and H4 are biotinylated(Reference Stanley, Griffin and Zempleni6–Reference Kuroishi, Rios-Avila and Pestinger8). Biotinylated histones are over-represented in repressed loci(Reference Pestinger, Wijeratne and Rodriguez-Melendez4, Reference Rios-Avila, Pestinger and Zempleni5, Reference Camporeale, Oommen and Griffin9) and, despite their rarity, biotinylation marks contribute to chromatin condensation(Reference Filenko, Kolar and West10). The binding of biotin to both carboxylases and histones is catalysed by holocarboxylase synthetase(Reference Zempleni, Wijeratne and Hassan1, Reference Bao, Pestinger and HY11).
Despite the essential roles of biotin in intermediary metabolism and gene regulation, human biotin requirements are unknown. Consequently, only recommendations for adequate intake, but no RDA, are available for biotin(12). Recommendations for adequate intake are based solely on biotin intake in the general, apparently healthy, population(12). This approach is flawed in the case of biotin, where dietary intake data are only crude estimates. Currently, no studies are available quantifying biotin in foods using chemically specific assays(Reference Zempleni, Mock, Song and Beecher13), and it is unclear whether intake estimates exceed or underestimate the true biotin intake. Also, the ‘normal state’ is defined using biotin-dependent enzymes or urinary biotin metabolites as markers, while ignoring the changes occurring at the chromatin level.
As of today, no comprehensive study has been published that investigated markers of human biotin status from various possible angles, including carboxylase biotinylation, metabolome and transcriptome in healthy adults. The identification of sensitive and robust markers of biotin status would represent a major advance in our pursuit of the quantification of human biotin requirements. In the present study, we devised a biotin feeding protocol to create states of biotin depletion, biotin sufficiency and biotin supplementation in healthy women and men. We assessed the suitability of twenty distinct markers to identify those that reliably discriminate among the subjects in the three treatment groups. Only two markers met our stringent requirements and can be considered as reliable markers of biotin status. Some of the other markers might be useful for use in future population studies, but only if used in combination with additional markers.
Subjects and methods
Study principle
The present study is a randomised cross-over outpatient study. Defined states of biotin supply were established by supplementing the study subjects with drinks containing spray-dried egg-white. Egg-white contains the protein avidin, which binds biotin tightly and makes the vitamin unavailable for intestinal absorption(Reference Green14, Reference Mock, Malik and Stumbo15). Graded levels of chemically pure biotin were added back to the egg-white shakes, thereby inducing states of biotin depletion, biotin sufficiency and biotin supplementation (given later).
Study subjects
A total of seventeen apparently healthy adults (seven men and ten women) aged 21–45 years completed the present study. Exclusion criteria included a recent history of smoking(Reference Sealey, Teague and Stratton16), inborn errors of biotin metabolism (carboxylase deficiency, holocarboxylase synthetase deficiency and biotinidase deficiency)(Reference Weiner, Grier and Wolf17, Reference Wolf, Heard and Weissbecker18), use of vitamin supplements, use of anticonvulsants(Reference Mock and Dyken19, Reference Rathman, Eisenschenk and McMahon20), pregnancy(Reference Mock and Stadler21, Reference Mock, Stadler and Stratton22) and lactation(12, Reference Mock, Mock and Dankle23). Women were tested for pregnancy prior to each period of egg-white feeding by using a commercial pregnancy test. Subjects were allowed to engage in normal daily activities. After the end of the study, one male subject (21 years old) admitted that he did not comply with the study protocol and his data were excluded from the analysis. During the biotin-deficient phase, none of the subjects had frank signs of severe biotin deficiency such as dermatitis, rash and alopecia. The present study was conducted according to the guidelines laid down in the Declaration of Helsinki, and all procedures involving human subjects were approved by the Institutional Review Board of the University of Nebraska-Lincoln, USA. Subjects signed an informed consent prior to enrolling in the study.
Study protocol
The entire study took each subject 15 weeks to complete (Fig. 1). During the first 2 weeks, subjects consumed a regular, self-selected diet without the use of any vitamin supplements. The objective of this adjustment period was to eliminate excess vitamins from inadvertent use of vitamin supplements before the study began. Subjects were instructed to avoid biotin-rich foods such as yeast, livers, meats, cereals, mayonnaise and eggs(Reference Camporeale, Zempleni, Bowman and Russell24), and were provided with a list of foods to avoid. After completion of the adjustment period, each subject completed the following three treatment phases (3 weeks each)(Reference Stratton, Henrich and Matthews25) in randomised order: biotin depletion (zero dietary biotin, representing levels seen in individuals consuming diets containing raw egg-white and individuals with undiagnosed deficiencies of biotinidase)(Reference Baugh, Malone and Butterworth26, Reference Wolf, Heard, Barness and Oski27), biotin sufficiency (30 μg/d, representing recommendations for adequate intake of biotin)(12) and biotin supplementation (600 μg/d, representing the level of biotin in typical over-the-counter supplement users)(Reference Zempleni, Helm and Mock28, Reference Manthey, Griffin and Zempleni29). In the second week of each treatment, participants filled out 3-d dietary records in order to estimate biotin intake. Periods of biotin-defined diets were interrupted by 2 weeks with no biotin treatment. These periods are sufficiently long for biotin levels to return to baseline level(Reference Mock, Malik and Stumbo15, Reference Stratton, Henrich and Matthews25, Reference Kaur Mall, Chew and Zempleni30). Subjects consumed a regular, self-selected diet (given earlier), modified only by the amounts of biotin during treatment phases.
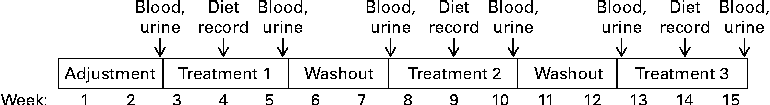
Fig. 1 Study protocol.
During treatment, dietary biotin was adjusted by consuming an egg-white drink (200 ml) with every major meal (breakfast, lunch and dinner) of the day. The amount of egg-white in the drinks was chosen as follows. First, the amount of avidin in spray-dried egg-white (Barry Farm, Cridersville, OH, USA) was titrated based on the 2-(4′-hydroxyazobenzene)-benzoic acid (HABA) assay (n 8)(Reference Green31, Reference Durance32). Avidin activity (μg/ml dried egg-white) was calculated based on the method of Green(Reference Green31, Reference Durance32) using the following equation:
where AA is avidin activity (μg/ml dried egg-white), 3·1 is the sample volume (ml) after the addition of HABA, 68 000 molecular weight of avidin, OD is optical density of the supernatant of the egg-white solution plus HABA at 500 nm corrected by the optical density before HABA, 3 is the sample volume (ml) before the addition of HABA and 4 is the number of avidin molecules in the avidin tetramer.
A measure of 1 g of egg-white contained approximately 600 μg of avidin. Second, we calculated the amount of egg-white needed to bind the biotin in an average diet (30–70 μg/d)(12), including an allowance for microbial biotin synthesis and diets that were inadvertently high in biotin. Assuming that 1 μg of avidin binds 0·014 μg biotin(Reference Durance32), we prepared egg-white drinks containing 7·1 mg of avidin in 200 ml (one serving), which is sufficient to bind about 100 μg of biotin. Spray-dried egg-white was dissolved in orange juice to improve palatability; the juices were stored at 4°C for up to 2 d before consumption. This drink was used for depleting subjects of biotin. For biotin-sufficient subjects, biotin was added back to the drinks to saturate all the avidin plus provide an additional 30 μg/d. For biotin-supplemented subjects, the amount of available biotin was adjusted to 600 μg/d. Our estimates were confirmed by quantifying the content of free biotin in egg-white drinks by using the avidin-binding assay(Reference Manthey, Griffin and Zempleni29).
Sample collection
Blood samples were collected after an overnight fast by an experienced phlebotomist at the University Health Center of Nebraska-Lincoln. A volume of 180 ml of fasting blood was collected after the initial 2-week adjustment period and on the last day of each treatment period. In addition, 30 ml of fasting blood were collected after the 2-week washout period of each treatment. Lymphocytes were isolated by density gradient centrifugation and counted using a haemocytometer(Reference Zempleni and Mock33). Aliquots of twenty million and two million cells were frozen ( − 80°C) for subsequent carboxylase analysis and for isolation of total RNA, respectively. Spot urine samples were collected after an overnight fast at the end of each washout and treatment phase. Urine samples were sub-aliquoted to avoid repeated freeze–thaw cycles and frozen at − 20°C for the subsequent analyses of organic acids, biotin and creatinine.
Sample analysis
The abundance of biotinylated (holo-)carboxylases was quantified in lymphocyte extracts as described(Reference Kaur Mall, Chew and Zempleni30), using 20 μg of protein and 3–8 % Tris–acetate gels (Invitrogen). Holocarboxylase-bound biotin in transblots was probed with fluorophore-labelled streptavidin; glyceraldehyde-3-phosphate dehydrogenase was used as a loading control(Reference Pestinger, Wijeratne and Rodriguez-Melendez4). The membrane was scanned using the 800CW channel in an IR imaging system (Odyssey LI-COR) and band intensities were quantified by gel densitometry(Reference Kaur Mall, Chew and Zempleni30).
Total RNA was extracted from lymphocytes using the ZR RNA MicroPrep (Zymo Research) following the manufacturer's instructions. RNA was reverse transcribed into complementary DNA using the High Capacity RNA-to-cDNA Kit (Applied Biosystems), following the manufacturer's instructions. The abundance of mRNA coding for the Na-dependent multivitamin transporter, monocarboxylate transporter 1, holocarboxylase synthetase, ACC1 and MCC (β bchain) were quantified by quantitative real-time PCR using Quanta Perfecta Power SYBR Green and the cycle threshold method(Reference Pestinger, Wijeratne and Rodriguez-Melendez4, Reference Livak and Schmittgen34). The abundance of GAPDH mRNA was used to normalise data for the efficiency of PCR. PCR primers were the same as reported previously(Reference Kaur Mall, Chew and Zempleni30). Our rationale for focusing on these five genes was that they play a role in biotin metabolism(Reference Zempleni, Wijeratne and Hassan1) and because their expression depended on biotin in previous studies in human cell cultures(Reference Kaur Mall, Chew and Zempleni30).
Concentrations of biotin in urine samples were quantified by the avidin-binding assay, as described(Reference Manthey, Griffin and Zempleni29). As urine was collected as a spot urine sample (as opposed to 24-h collections), urinary biotin was normalised by the excretion of creatinine to account for possible incomplete collections and dilution effects. Creatinine was quantified using the standard picric acid method of Jaffe(Reference O'Brien, Ibbott and Rodgerson35). Concentrations of urinary organic acids were determined by a capillary electrophoresis method in our laboratory. Briefly, urine samples were thawed and centrifuged at 16 100 g for 1 min. A volume of 100 μl of supernatant was lyophilised and weights of the freeze-dried samples were recorded for data normalisation. After being re-dissolved in 50 μl of nanopure water, samples were injected into a 50 cm bare fused-silica capillary (inner diameter 50 μm) under the pressure of 0·5 psi (pounds per square inch) for 5 s. Various organic acids were resolved at 15 kV and 25°C using a Beckman P/ACE MDQ capillary electrophoresis system (Beckman Coulter) equipped with a 200 nm UV filter using 250 mm-phosphate buffer (pH 6·0) with 0·1 mm-hexadecyltrimethylammonium bromide and 5 % methanol (by vol.). Authentic standards of organic acid were purchased from Sigma-Aldrich. The following nine organic acids were quantified by a standard curve method and their urinary excretion was normalised by the excretion of creatinine: acetate, α-ketoglutarate, butyrate, citrate, fumarate, malate, oxalate, succinate and 3-hydroxyisovaleric acid (3-HIA). Our rationale for focusing on these organic acids was that they are implicated in biotin-dependent pathways in intermediary metabolism and that, for some of them, links have been established with biotin deficiency(Reference Zempleni, Wijeratne and Hassan1, Reference Mock, Henrich and Carnell36).
Statistical analysis
To test for homogeneity of variances, Barlett's test or Levene's test was performed(37). Data were log transformed if variances were heterogeneous; log transformation resulted in homogeneous variances. The significance of differences among groups was tested with one-way ANOVA and the Bonferroni method was used to adjust for multiple comparisons where appropriate(37). StatView 5.0.1 (SAS Institute) was used to perform all calculations. Differences are considered significant if P< 0·05. Data are expressed as mean and standard deviations, where sd represents sample standard deviation in all cases.
Results
Holocarboxylases
Biotinylated holo-MCC (molecular mass = 83 kDa) and holo-PCC (molecular mass = 80 kDa) from lymphocyte extracts were easily detectable in streptavidin blots and appear to be reliable markers for biotin status (Fig. 2(A)). Note that streptavidin blotting detects the biotinylated α-chains of these carboxylases, but not the non-biotinylated β-chains. While the abundance of holo-pyruvate carboxylase also appeared to respond to dietary biotin, the signal produced by holo-pyruvate carboxylase was faint and hard to detect, suggesting that pyruvate carboxylase might not be a good marker for biotin status. Holo-ACC1 and ACC2 were not detectable in streptavidin blots of lymphocyte extracts, which is consistent with previous studies in human lymphoid cell cultures(Reference Manthey, Griffin and Zempleni29). Gels were equally loaded, judged by the abundance of the housekeeping protein glyceraldehyde-3-phosphate dehydrogenase.

Fig. 2 (A) Abundance of biotinylated (holo-)pyruvate carboxylase (PC), 3-methylcrotonyl-CoA carboxylase (MCC) and propionyl-CoA carboxylase (PCC) in lymphocytes from biotin-deficient (DEF), biotin-sufficient (SUF) and biotin-supplemented (SUP) healthy adults (top gel). Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as the loading control (bottom gel). The image was cropped from an original full-size scanned picture. (B) Gel densitometry analysis of holo-MCC (n 16, 0·0003 < P< 0·0012 with Bonferroni correction for all possible comparisons). (C) Gel densitometry analysis of holo-PCC (n 16, P< 0·0003 with Bonferroni correction for all possiblecomparisons). Values are means, with standard deviations represented by vertical bars.
Gel densitometry analysis of holo-MCC and holo-PCC is consistent with the notion that these proteins are good markers for biotin status. The abundance of holo-MCC (arbitrary units) was 4·1 (sd 2·6) in biotin-depleted subjects, 8·2 (sd 3·5) in biotin-sufficient subjects and 15·7 (sd 7·5) in biotin-supplemented subjects (0·0003 < P< 0·0012 with Bonferroni correction for all possible comparisons, n 16, Fig. 2(B)). Likewise, the abundance of holo-PCC (arbitrary units) was 4·1 (sd 1·9) in biotin-depleted subjects, 9·1 (sd 3·3) in biotin-sufficient subjects and 17·0 (sd 5·7) in biotin-supplemented subjects (P< 0·0003 with Bonferroni correction for all possible comparisons, n 16, Fig. 2(C)).
Real-time PCR
The abundance of mRNA coding for the biotin transporters, Na-dependent multivitamin transporter and monocarboxylate transporter 1; the biotin-dependent carboxylases, ACC1 and MCC (β-chain); and holocarboxylase synthetase was not significantly different among the treatment groups (Fig. 3), contrary to previous observations in human cell cultures(Reference Kaur Mall, Chew and Zempleni30). This apparent absence of effect can largely be explained by the large inter-individual variations among subjects. When men and women were analysed separately, the results were the same as for the pooled dataset (data not shown). We conclude that the abundance of mRNA coding for genes in biotin metabolism is not a good marker of biotin status in outpatient studies.

Fig. 3 Abundance of mRNA coding for (A) sodium-dependent multivitamin transporter (SMVT), (B) monocarboxylate transporter 1 (MCT1), (C) 3-methylcrotonyl-CoA carboxylase β (MCCβ), (D) acetyl-CoA carboxylase 1 (ACC1) and (E) holocarboxylase synthetase (HLCS) in lymphocytes from biotin-deficient (DEF), biotin-sufficient (SUF) and biotin-supplemented (SUP) healthy adults. Values are means, with standard deviations represented by vertical bars (n 16, 0·0784 < P< 0·9823).
Excretion of biotin in urine
The urinary excretion of biotin was up to five times higher in biotin-supplemented subjects compared with the other treatment groups (Table 1). There was a trend towards higher urinary biotin in biotin-sufficient individuals compared with biotin-depleted individuals, but the difference was not statistically significant. This apparent absence of effect can largely be explained by the large inter-individual variations among subjects. When men and women were analysed separately, the results were the same as for the pooled dataset (data not shown). We conclude that the urinary excretion of biotin is a good marker for identifying supplement users, but is not a good marker to distinguish deficient from sufficient individuals in outpatient studies.
Table 1 Urinary excretion of biotin in the three treatment groups (n 16) (Mean values and standard deviations)
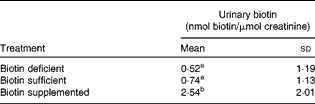
a,bMean values with unlike superscript letters were significantly different (0·0001 < P< 0·16).
Excretion of organic acids in urine
The urinary excretion of 3-HIA was, on average, two times higher in biotin-deficient subjects compared with the other treatment groups (Fig. 4(A)). The use of 3-HIA as a marker of biotin status is limited by the following characteristics. First, the excretion of 3-HIA does not distinguish biotin-sufficient and biotin-supplemented subjects. Second, for eight of the sixteen subjects, the urinary excretion of 3-HIA did not increase during biotin depletion compared with other phases (Fig. 4(B)), suggesting that the urinary excretion of 3-HIA has a tendency to produce false negatives in biotin-deficient subjects. For clarity, we show values only for those eight subjects for whom urinary 3-HIA did not respond to dietary biotin; for comparison, the figure also includes the data for one subject that showed the expected pattern of a higher excretion of 3-HIA during biotin depletion compared with other phases. When men and women were analysed separately, the results were the same as for the pooled dataset (data not shown).

Fig. 4 (A) Average urinary excretion of 3-hydroxyisovaleric acid (3-HIA) in biotin-deficient (DEF), biotin-sufficient (SUF) and biotin-supplemented (SUP) healthy adults. Values are means, with standard deviations represented by vertical bars (n 16). a,bMean values with unlike letters were significantly different (0·04 < P< 0·98). (B) Individual patterns of the eight subjects in whom urinary 3-HIA did not respond to dietary biotin and in one of the subjects who exhibited the expected pattern of high urinary 3-HIA during biotin depletion (denoted ‘Responder’).
Two of the remaining organic acids produced potentially interesting trends regarding their urinary patterns, although effects did not reach a statistically significant level. Urinary citrate was positively linked with biotin intake (Fig. 5(A)), while urinary malate was negatively linked with biotin intake (Fig. 5(B)), but none of these organic acids produced statistically significant effects due to large inter-individual variation. Results were the same when men and women were analysed separately. The urinary excretion of acetate, α-ketoglutarate, butyrate, fumarate, oxalate and succinate did not depend on biotin intake.
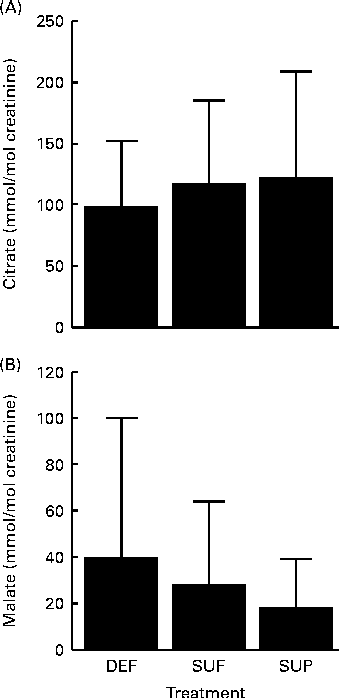
Fig. 5 Urinary excretion of (A) citrate and (B) malate in biotin-deficient (DEF), biotin-sufficient (SUF) and biotin-supplemented (SUP) healthy adults. Values are means, with standard deviations represented by vertical bars (0·4620 < P< 0·6943, n 16).
Dietary records
The dietary intake of biotin was of the expected magnitude and well within the amount that can be bound by the avidin in the egg-white drinks. The intake of biotin over a 3-d period was 37·9 (sd 23·5) μg in biotin-depleted subjects, 65·2 (sd 71·4) μg in biotin-sufficient subjects and 49·3 (sd 29·2) μg in biotin-supplemented subjects (n 16), excluding the biotin that was added back to drinks. These values should be considered as crude estimates because there is currently no database that accurately quantifies biotin in foods(Reference Staggs, Sealey and McCabe38, 39). Despite the limited accuracy of existing databases, the intake estimates reported here are consistent with the notion that subjects avoided foods rich in biotin throughout the study.
Efficacy of washout
Adjustment and washout periods were chosen to be sufficiently long to allow for biotin levels to return to baseline levels. When carboxylase-bound biotin in lymphocyte extracts was probed with streptavidin, no apparent difference among the various phases was detected for any of the study subjects. Fig. 6 depicts the raw data from one subject as a representative example. The abundance of glyceraldehyde-3-phosphate dehydrogenase was used as a loading control. Likewise, the urinary excretion of biotin returned to baseline levels by the end of each washout period (approximately 1 nmol biotin/μmol creatinine, assayed in randomly selected samples).

Fig. 6 Abundance of biotinylated (holo-)pyruvate carboxylase (PC), 3-methylcrotonyl-CoA carboxylase (MCC) and propionyl-CoA carboxylase (PCC) in lymphocytes from biotin-deficient, biotin-sufficient and biotin-supplemented subjects at the end of adjustment and washout phases (top gel). Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as the loading control (bottom gel). The image was cropped from an original full-size scanned picture.
Discussion
The present paper is the first study that uses an outpatient feeding protocol in normal healthy adults to assess the effects of deficiency, sufficiency and supplementation of biotin on a comprehensive set of twenty variables from proteomics, transcriptomics and metabolomics. The study provides compelling evidence that the abundance of biotinylated (holo-)MCC and PCC in lymphocytes are the only markers that can reliably discriminate between states of moderate biotin deficiency and biotin sufficiency. This observation is consistent with previous studies in human lymphoid cell cultures, which also suggest that biotinylation of MCC and PCC is responsive to biotin intake(Reference Manthey, Griffin and Zempleni29, Reference Kaur Mall, Chew and Zempleni30). Greater than 50 % of PCC in lymphocytes from biotin-depleted individuals is present in the biotin-free apo-form, based on ex vitro cultures of lymphocytes from patients on biotin-free nutrition(Reference Velazquez, Zamudio and Baez40). Previous studies in healthy adults(Reference Mock, Henrich and Carnell41) and rats(Reference Mock and Mock42) also led to the conclusion that the activity of PCC is a good marker of biotin status in the population. Those studies used the incorporation of radiolabelled bicarbonate for assessing carboxylase activity, while in the present study we quantified the binding of biotin to carboxylases. In previous studies, we demonstrated that both carboxylase activity and carboxylase biotinylation correlate well(Reference Manthey, Griffin and Zempleni29). However, we believe that the dependence on radiolabelled compounds is a meaningful limitation when using carboxylases as markers for biotin status. We also believe that streptavidin blots of carboxylases has the additional advantage of assessing multiple carboxylases in one single run, while activity assays require distinct analytical procedures for each of these enzymes(Reference Zempleni, Trusty and Mock43).
When biotin is insufficient during leucine metabolism, it shunts the pathway to an alternative pathway, leading to the formation of 3-HIA(Reference Zempleni, Wijeratne and Hassan1). Urinary 3-HIA is generally considered a useful marker for biotin status(Reference Mock, Henrich and Carnell36, Reference Mock, Mock and Malik44, Reference Stratton, Horvath and Bogusiewicz45). Based on the findings in the present study, the usefulness of 3-HIA as a marker of biotin status needs to be re-evaluated because of the following concerns. First, urinary 3-HIA does not permit discriminating biotin-sufficient and biotin-supplemented individuals. Second, the average urinary excretion of 3-HIA was greater in biotin-deficient subjects compared with other treatment groups, but produced a meaningful number of false-negative results when looking at the individuals in the biotin-deficient group. More recently, carnitine conjugates of 3-HIA have been recommended as markers of biotin status(Reference Stratton, Horvath and Bogusiewicz45–Reference Mock, Stratton and Horvath47). Carnitine conjugates of 3-HIA were not tested in the present study, but might be a useful alternative to free 3-HIA. When validating carnitine conjugates in future studies, great care should be taken (a) to document inter-individual variation, (b) to test for false negatives in a population of biotin-depleted individuals and (c) to determine whether these conjugates discriminate between sufficient and supplemented individuals.
Some variables such as organic acids produced borderline significant changes of some markers in response to biotin intake. One could argue that these variables may produce statistically significant results if tested in a sample larger than sixteen subjects. Such concerns are formally correct. However, concerns would remain as to whether such variables are useful markers when trying to single out individuals with slightly lower and slightly higher biotin intake compared with the average intake in the sample.
One could consider using urinary biotin as a marker of biotin status. Our studies suggest that the urinary excretion of biotin reliably identifies biotin-supplemented subjects, but does not distinguish between biotin-depleted and biotin-sufficient individuals. Also, urine contains biotin metabolites such as bisnorbiotin, biotin-d,l-sulphoxides, biotin sulphone, bisnorbiotin methyl ketone and tetranorbiotin-l-sulphoxide(Reference Mock, Lankford and Cazin48, Reference Zempleni, McCormick and Mock49). Typical biotin assays do not quantify these metabolites accurately, and their analysis is reserved for specialty laboratories.
Serum biotin concentration and biotinidase activity were not measured in the present study, based on the following lines of reasoning. Previous studies suggest that serum concentrations of biotin and biotin metabolites do not decrease in biotin-deficient individuals(Reference Mock and Mock50) and in patients on biotin-free total parenteral nutrition(Reference Velazquez, Zamudio and Baez40) during reasonable periods of observation. Previous studies also suggest that the expression of biotinidase does not depend on biotin in human cell cultures(Reference Kaur Mall, Chew and Zempleni30).
Of the twenty potential markers of biotin status that were tested in the present study, quantification of mRNA abundance returned the least compelling results. None of the five genes from biotin turnover was linked in any way to biotin intake. This can be attributed to large inter-individual variations in the expression of these genes. This observation is in striking contrast to studies in cell cultures and laboratory animals, where the expression of these genes depended on biotin(Reference Kaur Mall, Chew and Zempleni30, Reference Rodriguez-Melendez, Cano and Mendez51, Reference Solorzano-Vargas, Pacheco-Alvarez and Leon-Del-Rio52). We propose that the genetic diversity in human subjects and environmental factors create a unique scenario, and the studies in cell cultures and inbred animals need to be interpreted with care.
Collectively, we conclude that the number of reliable markers of biotin status is limited and that more groundwork needs to be done before human biotin requirements can be quantified with a reasonable level of certainty. No single marker is currently known that reliably distinguishes moderately biotin-deficient, biotin-sufficient and moderately biotin-supplemented individuals.
Acknowledgements
The present study was a contribution of the University of Nebraska Agricultural Research Division, supported in part by funds provided through the Hatch Act. Additional support was provided by National Institutes of Health grants (grant nos. DK063945, DK077816 and DK082476). The contributions of authors were as follows: J. Z., V. L. S., D. W. and D. G. designed the research; D. G. conducted the preliminary research; D. G. and W. K. E. conducted the research; B. H. L. conducted the capillary electrophoresis analyses; J. Z., D. W., D. G. and W. K. E. analysed the data; W. K. E. and J. Z. prepared the manuscript. All authors read, edited and approved the final manuscript. The authors declare that there is no conflict of interests.









