Medical efforts towards fracture prevention have been aimed at retarding the rate of age-related bone loss in the elderly. However, there is evidence suggesting that the risk of osteoporotic fractures in later life may be associated with the quantity of bone mass acquired in childhood and adolescence. Thus, osteoporosis may be caused by inadequate bone mass accretion rather than by just excessive bone loss in old ageReference Heaney, Abrams, Dawson-Hughes, Looker, Marcus, Matkovic and Weaver1. As life expectancy increases in Asian populations, the prevalence of osteoporosis is expected to rise; making it a significant health challenge in Asia in the next few decadesReference Cooper, Campion and Melton2.
Peak bone mass is defined as the maximum amount of bone when skeletal maturation is complete, with most skeletal accretion occurring between the ages of 10 and 20 yearsReference Heaney, Abrams, Dawson-Hughes, Looker, Marcus, Matkovic and Weaver1. Although peak bone mass is mainly determined by genetics, other factors, such as nutrition and physical exercise, can modify the level of bone mineral deposition during adolescence. There has been much research in Caucasian children and adolescents, which relates body composition and environmental factors to bone mass accretion, but there have been relatively few studies on Chinese post-menarcheal adolescent girlsReference Afghani, Xie, Wiswell, Gong, Li and Johnson3. An understanding of the factors that influence bone mass accretion could help develop strategies to maximise bone mineral deposition during childhood and adolescence, which could help prevent osteoporosis-related fractures later in life. Intervention is likely to be more effective with children and adolescents, because later in life when fractures are likely, the loss of bone mass is very difficult to reverse completely. Therefore, the aim of the present study was to examine whether there is association between body composition, handgrip muscle strength, dietary intake and physical exercise with bone mineral content (BMC) and bone area (BA) and then to identify any interdependence and predictive strength of these variables on total body and forearm skeletal bone mass in Chinese adolescent girls at 15 years of age in Beijing, China.
Materials and methods
Subjects
A cohort of 504 adolescent girls in Beijing was investigated to determine whether there was any relationship between genetic, pubertal and nutritional factors and physical activity with bone mass accretion. This cohort had been recruited previously as part of a 5-year dietary intervention study. Details of the recruitment procedures have been described elsewhereReference Du, Zhu, Trube, Zhang, Ma, Hu, Fraser and Greenfield4, Reference Zhu, Zhang, Foo, Trube, Ma, Hu, Du, Cowell, Fraser and Greenfield5. It was found that 3 years after completing a 2-year milk supplementation study, all the benefits derived from the milk supplement on bone mass and body size had disappearedReference Zhu, Zhang, Foo, Trube, Ma, Hu, Du, Cowell, Fraser and Greenfield5. A random subset of subjects (n 283) had their total body bone mineral measurements taken. In addition, there were no significant differences found in body composition parameters, handgrip muscle strength, stage of pubertal development, dietary intakes and physical activity level between subjects who had completed their total body bone measurements and those who had not. Therefore, all the 283 girls were included in this cross-sectional analysis to determine whether any independent factors were associated with bone mass quantity. None of the subjects had clinical signs of bone disease that could potentially prevent them from being physically active, nor were they taking any medications known to influence bone metabolism. The study was approved jointly by the Human Ethics Review Committee of The University of Sydney and that of the National Institute for Nutrition and Food Safety, China Centers of Disease Control and Prevention. Written informed consent was also obtained from both subjects and their parents or guardians prior to examination.
Body composition, handgrip muscle strength and Tanner pubertal stage measurements
Anthropometric measurements such as body weight and height were performed using standardised procedures. Subjects were weighed (wearing light clothing and without shoes) to the nearest 0·1 kg with an electronic digital scale (Thinner, Fairfield, WI, USA). Height of subjects with bare feet was measured to the nearest 0·1 cm, using a body height measuring stadiometer (TG-III Type; No. 6 Machinery Plant, Beijing, China) at the hospital where the dual-energy X-ray absorptiometry scans were carried out. All anthropometric measurements were taken twice and an average value was calculated. BMI was calculated as body weight (kg) divided by height (m2). Handgrip strength was measured on both non-dominant and dominant hands using a hand-held dynamometer with adjustable widths (model 78 010; Lafayette Instrument Company, Lafayette, IN, USA). Subjects were instructed to squeeze the dynamometer as hard as possible for 3 s, without pressing the instrument against their body or bending at the elbow. Two measurements were taken at 1- to 2-min intervals and the greater of the two handgrip values was used in the final analysis. The CV for repeated measurements (n 10) was 1·7 %. Breast development and pubic hair development were assessed according to Tanner's definitions of the five stages of pubertyReference Tanner6. When there were discrepancies between breast and pubic hair assessment, breast development was used as the determining criterion for stage of pubertal development. Age at menarche was also ascertained and recorded.
Bone age and bone mass measurements
Bone age was determined using the Greulich and Pyle Atlas methodReference Greulich and Pyle7. Radiological examination of the left hand and wrist of each subject was carried out using the standard protocol of the Department of Radiology (304 Military Hospital of Beijing, China). Skeletal age to the nearest 3 months was determined from the stage of growth of the metacarpals, phalanges and carpal bones in reference to a set of CHN method-China Population Skeletal Development Standard photographs). Total body and distal and proximal forearm bone masses were determined using dual-energy X-ray absorptiometry (XR-36; Norland Medical Systems Inc., Fort Atkinson, WI, USA). All bone measurements were performed by the same two trained technicians, who were unaware of into which earlier treatment groups the subjects had been placed. To minimise technical variation, all bone data were analysed by the same person, using the Norland enhanced software version 3.9.4. Quality control scans were performed each day. Prior to each scan, the densitometer was calibrated according to the manufacturer's recommendations. During the course of the study (2–6 months), the precision of repeated measurements (CV) using a manufacturer-supplied phantom was < 1 %, indicating satisfactory long-term stability of the instrument with no sign of drift. Additionally, a short-term precision measurement was made each day using the same phantom, which gave CV 0·31 %. To minimise radiation exposure, no repeat scans were done on any child. In the present study, BMC and BA of the total body and of the distal and proximal forearm were used as primary dependent variables. It is now apparent that that areal bone mineral density (BMD) should not be used as a measure of bone mineral status during growthReference Prentice, Parsons and Cole8. Lean body mass (LBM) and fat body mass (FBM) were also obtained from the bone densitometer data, from which percentage body fat was also calculated.
Assessment of physical activity and dietary intake
Habitual physical activity over the previous 12 months was assessed using a structured and detailed Modifiable Activity Questionnaire for AdolescentsReference Aaron, Kriska, Dearwater, Cauley, Metz and LaPorte9, which was modified to include physical activities commonly practised by Chinese school children. This physical activity has been proven to give reliable and valid estimates of physical activity in Beijing school childrenReference Ma, Liu, Zhang and Hu10. Both qualitative and quantitative information was collected over a 12-month time interval to cover seasonal and variation in the type, frequency, intensity and duration of activities and participation in organised sport, either at school or during leisure time. Furthermore, the questionnaire also solicited information on the pattern of physical inactivity during weekdays and at the weekend, such as the time spent in reading, watching television and playing with a computer. Four levels of physical impact were used, namely, none, low, moderate or high impact based on the classification used in the EPIC Norfolk studyReference Jakes, Khaw, Day, Bingham, Welch, Oakes, Luben, Dalzell, Reeve and Wareham11 and expressed as total hours per week.
Dietary intakes were assessed from two 3-day food records, which included two week days and one weekend day. Chinese measures of bowls, plates and spoons of standard size were used to quantify food items with the assistance of a set of food measure models and a trained interviewer. Because data on the content of vitamin D in Chinese food were not available, vitamin D food values were obtained from the UK food composition tablesReference Holland, Welch, Unwin, Buss, Paul and Southgate12, with an adjusted decrease in the values for vitamin D in eggs and in fortified fresh milk based on local analyses of these foods in ChinaReference Du, Zhu, Trube, Zhang, Ma, Hu, Fraser and Greenfield4.
Statistical analysis
Descriptive statistics are reported as mean values and standard deviations and any differences as means with their standard errors for all variables, unless otherwise indicated. Analysis using bivariate correlation coefficients examined the direction and magnitude of the relationship between the bone mass measurements and other explanatory continuous variables. Analysis of covariance was used, with post-hoc Bonferroni's correction for multiple comparisons to assess the relationships between physical activity tertiles and different body composition measures and handgrip muscle strength, after adjusting for potential confounding factors. FBM and LBM were entered instead of body weight, as the sum of these parameters represents total body weight. A multiple regression model with stepwise elimination was used to assess the influence of differences in body composition, pubertal stage development, muscle strength, dietary intakes and physical activity on BMC and BA. Since body size indicators, such as body weight, height and BA were strong predictors of BMC, these factors were given higher priority in the regression models to minimise the size-artefacts on bone mass in growing adolescentsReference Prentice, Parsons and Cole8. Where appropriate, interaction between physical activity and body composition, handgrip muscle strength and dietary intake factors was also tested. A similar approach was followed to determine whether there was any association between body composition, physical activity and nutrition on bone size, where BA was then treated as a dependent variable. Possible inter-associations between all independent variables were also sought using collinearity diagnostics tests. Results from all separate models were expressed as regression coefficients (β) and standard errors. The relative importance of all explanatory variables included in all the models and the adjusted R 2 for each regression model as well as the partial coefficient of adjusted R 2 for each significant explanatory variable was also determined. Statistical analysis was carried out using SPSS version 12.0 for Windows (SPSS Inc., Chicago, IL, USA) and statistical significance for all the tests was defined by a P value < 0·05.
Results
General characteristics
General characteristics, body composition, handgrip muscle strength, stage of pubertal development, physical activity levels and nutrient intake of the subjects are presented in Table 1. Mean age of the subjects was 15·0 (sd 0·5) years and average bone age was 16·0 (sd 0·9) years. The mean BMI of all subjects was 21·1 (sd 3·5) kg/m2 with approximately 80·6 % being in the normal range. Only a small number were classified as at risk of obesity (3·9 %) or were overweight (15·5 %) based on the recent international cut-off points recommended by the International Obesity Task ForceReference Cole, Bellizzi, Flegal and Dietz13.
Table 1 General characteristics, body composition, pubertal status, physical activity and dietary factors measures of Chinese adolescents girls*
(Mean values and standard deviations for 283 subjects)
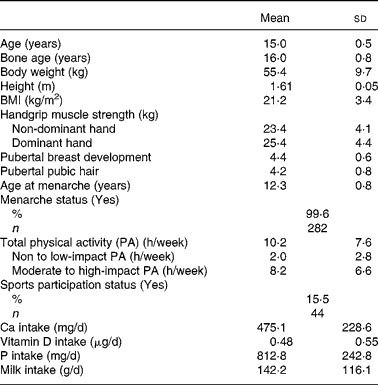
* For details of subjects and procedures, see Materials and methods.
Mean handgrip muscle strength of the non-dominant and dominant hands was 23·4 (sd 4·1) kg and 25·3 (sd 4·3) kg, respectively, with that of the dominant hand being significantly higher than the non-dominant hand (Paired t test 18·513; P < 0·001). Most of the girls had reached pubertal Tanner breast and pubic hair stages IV and V (96·5 % and 84·7 %, respectively), indicating that most were post-pubertal at the time of assessment.
Analysis of physical activity by intensity of impact, as defined by Jakes et al. Reference Jakes, Khaw, Day, Bingham, Welch, Oakes, Luben, Dalzell, Reeve and Wareham11, showed that the time spent in none-to-low impact and in moderate-to-high impact physical activity were respectively 2·0 (sd 2·8) and 8·2 (sd 6·6) h/week. Only 15·5 % of the subjects (n 44) reported participation in organised sports training either inside or outside school. The average intakes of dietary Ca, vitamin D and milk were 475·1 (sd 228·6) mg/d, 0·48 (sd 0·55) μg/d and 142·2 (sd 116·1) g/d, respectively.
Multivariate analysis
Table 2 shows the results of multivariate regression analyses for total body and distal and proximal forearm BMC. Similar analyses for independent predictor variables of BA at all the skeletal sites are also shown (Table 3). When body composition, handgrip muscle strength, stage of puberty, dietary intake and physical activity were included in the multivariate regression models, the significant independent predictors of BMC of the total body were LBM (P < 0·0001), BA (P < 0·0001), FBM (P < 0·0001) and milk intake (P < 0·0001). All these factors together accounted for 77 % of the variance for total body BMC (Table 2). In the distal forearm BMC model, BA (P < 0·0001), LBM (P < 0·0001), milk intake (P < 0·05) and height (P < 0·05) emerged as the main independent variables for BMC of the distal forearm, accounting for about 43 % of the variance for the distal forearm BMC. Only BA (P < 0·0001), LBM (P < 0·0001), handgrip muscle strength (P < 0·0001) and milk intake (P < 0·0001) emerged as positive independent predictors for proximal forearm BMC. Total non-impact physical activity was a significant inverse predictor of BMC (P < 0·01), accounting for 68 % of the total variance for proximal forearm BMC. BA accounted for a variance range of 8·6–54·7 % for BMC of all skeletal sites. The relationship between LBM and BMC was much stronger than the relationship between FBM and BMC at all skeletal sites. The overall R 2 of BMC that could be explained by LBM was 8·8–64·5 %, whereas the contribution of FBM to BMC for the total body accounted for only 2·9 % of the variance for BMC.
Table 2 Multivariate regression model predicting total body and forearm bone mineral content (BMC) in Chinese adolescent girls*
(Mean values with their standard errors)

* For details of subjects and procedures, see Materials and methods.
TB, total body; LBM, lean body mass; BA, bone area; FBM, fat body mass; DF, distal forearm; PF, proximal forearm; PA, physical activity.
Table 3 Multivariate regression model predicting total body and forearm bone area (BA) in Chinese adolescent girls*
(Mean values with their standard errors)
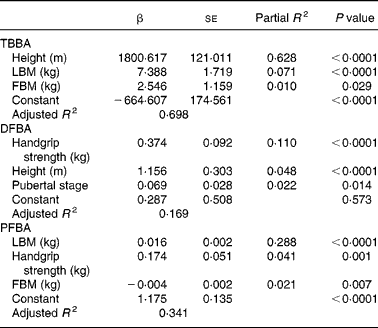
* For details of subjects and procedures, see Materials and methods.
TB, total body; LBM, lean body mass; FBM, fat body mass; DF, distal forearm; PF, proximal forearm.
Height (P < 0·0001), LBM (P < 0·0001) and FBM (P < 0·05) were significant independent determinants for total body BA (Table 3). All these body composition parameters contributed about 70 % of the variance for total body BA. In contrast, neither dietary Ca nor physical activity had any influence as independent predictors of total body BA. Handgrip muscle strength (P < 0·0001), total height (P < 0·0001) and pubertal breast stage (P < 0·05) were strong independent predictors of the distal forearm BA, accounting for 17 % of the variance for distal forearm BA, while LBM (P < 0·0001), handgrip strength (P < 0·01) and FBM (P < 0·01) were significant independent predictors of proximal forearm BA. All these variables accounted for 34 % of the total BA variance at the proximal forearm. The strength of the positive associations for physical activity levels on body mass measurements at all skeletal sites by univariate analysis (L.H. Foo, unpublished results) were mostly attenuated after further adjusting for body composition parameters and handgrip muscle strength in the full multivariate regression models, except for proximal forearm BMC. Comparisons between subjects across three tertiles of physical activity levels and body composition measures of LBM and percentage body fat and handgrip muscle strength showed that subjects in the highest tertile of physical activity had significantly greater LBM (P < 0·0001) and handgrip muscle strength (P < 0·0001) than those in the lowest tertile of physical activity level (Table 4). In contrast, there were no significant differences in percentage body fat between subjects in the highest or lowest level of physical activity, indicating that subjects in the highest level of physical activity were taller, heavier, leaner and with greater handgrip muscle strength than those with the lowest physical activity levels. There were no significant interactions between dietary Ca and physical activity on total body and forearm bone mass measurements and no significant independent predictor was found between pubertal stage and any of the bone variables at skeletal sites, except for distal forearm BA.
Table 4 Relationship between body composition measures and handgrip muscle strength and physical activity levels in Chinese girls†
(Mean values and standard deviations for 283 subjects)
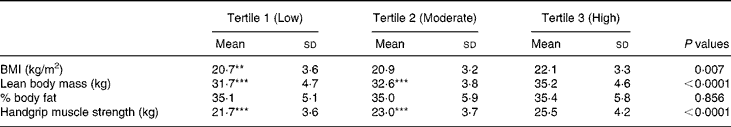
Mean values were significantly different from the highest tertile of physical activity group; **P < 0·01; ***P < 0·0001 (one-factor ANOVA post hoc Bonferroni's test).
† For details of subjects and procedures, see Materials and methods.
Discussion
There is little information for China, comparable to other populations, about whether variations in body composition and the level of physical activity influence skeletal growth in older adolescent girls. If such associations were found, this could help develop strategies to optimise bone mass accumulation during adolescence.
In this investigation, LBM and FBM were statistically significant predictors of BMC and BA at all skeletal sites measured. Body size and bone size emerged as the greatest independent determinants of BMC at all sites and accounted for 41 % to 76 %, respectively, of the total variance in BMC. The present findings show that both LBM and FBM contributed the greatest variance to BMC and BA of the total body and the distal and proximal forearm, in line with other reports of growing children and adolescentsReference Young, Hopper, Nowson, Green, Sherwin, Kaymakci, Smid, Guest, Larkins and Wark14–Reference McKay, Petit, Khan and Schutz16. However, in the present study, LBM was found to be a much stronger independent determinant than FBM of BMC and BA at all of the skeletal sites measured. About 84 % of the variance in total body BMC and 85 % of that for BMD was explained by LBM, whereas fat mass accounted for only 3–11 % total variance for either BMC or BA. This association remained highly significant after adjusting for other known confounding variables. A study of twin pre- and post-menarcheal adolescent girls also found that LBM was a stronger determinant than FBM of total body BMCReference Young, Hopper, Nowson, Green, Sherwin, Kaymakci, Smid, Guest, Larkins and Wark14. Similarly in CaucasianReference Faulkner, Bailey, Drinkwater, Wilkinson, Houston and McKay17, Reference Valdimarsson, Kristinsson, Stefansson, Valdimarsson and Sigurdsson18 and Chinese adolescent boys and girls aged 12 to 16 yearsReference Afghani, Xie, Wiswell, Gong, Li and Johnson3, there was a positive association between LBM and BMC and BMD.
Handgrip muscle strength also emerged as a strong independent determinant of BMC of the proximal forearm and of BA measured at both the distal and proximal forearm. This association remained significant when adjusted in multivariate analysis for other known confounding factors. In contrast, there was no significant association between handgrip muscle strength and any of the bone variables for the total body, suggesting that the influence of muscle strength on bone mass is more site specific rather than systemic. The present results are in line with other studies in young women, which reported a positive correlation between handgrip strength and radius BMD, but not BMD at other skeletal sitesReference Beverly, Rider, Evans and Smith19, Reference Snow-Harter, Bouxsein, Lewis, Charette, Weinstein and Marcus20. A mechanism to explain the relationship between muscle strength as related to bone mass development has not yet been found. However, studies in female twin pairs and in elderly women have suggested that interactions between muscle strength and bone mass may be under genetic controlReference Seeman, Hopper, Young, Formica, Goss and Tsalamandris21. Although the present study cannot rule out a possible genetic influence, the findings do support the mechanostat model of bone development, which proposes that muscle and bone form a functional unit in which greater muscular strength leads to increased bone strength and massReference Burr22, Reference Frost23.
Of all the dietary components examined, only milk intake was found to be a significant determinant of BMC for the total body and the distal and proximal forearm, in agreement with earlier studiesReference Ilich, Skugor, Hangartner, Boashe and Matkovic15, Reference Du, Greenfield, Fraser, Ge, Liu and He24. Despite the obvious link between milk Ca intake and bone mineralisation, other components of milk, such as insulin-like growth factor I and basic milk protein, have also been suggested to influence actual bone growthReference Philipps, Dvorak, Kling, Grille and Koldovsky25, Reference Toba, Takada and Matsuoka26. Milk may also have contributed to the overall nutritional quality of the diet and thus had a growth-promoting effect on bone mass. In contrast, total dietary Ca intake was not a predictor of any of the bone measurements, perhaps because for most subjects total Ca intake was below that recommended (1000 mg per d) for maximum Ca accretionReference Matkovic and Heaney27, 28.
Regular participation in physical activity with mechanical loading causes an osteogenic response in bone, reducing the rate of bone turnover and stimulating bone formation at cortical and trabecular surfaces and thus leading to an increase in bone massReference Turner and Robling29. In the previous study of the same cohort of girls, at the age of 9 to 11 years, the physical activity score was a positive predictor of BMC and BMD at the distal and proximal forearm as well as of BMD of the total bodyReference Zhu, Du, Greenfield, Zhang, Ma, Hu and Fraser30. Similar findings have also been reported for Caucasian adolescentsReference Vatanparast, Baxter-Jones, Faulkner, Bailey and Whiting31. However, exercise assessment in that study did not distinguish between the type, frequency and intensity of mechanical forces in the different physical activities. Furthermore, most other studies did not distinguish between exercise at school and that in leisure time. Different physical activities vary in their effects on bone mass accretion and developmentReference Turner and Robling29, Reference Rubin and Lanyon32. Therefore, our questionnaire gathered information on the type, frequency and impact intensity of physical activities. After adjusting for body composition and muscle strength, no significant association was found between physical activity levels and any bone mass measurements, other than a link between non-impact physical activity and proximal forearm BMC. This result is similar to that found with Chinese adolescents in Hong Kong, where neither the physical activity level nor participation in sport had any influence on bone mass of the lumbar spine and forearmReference Cheng, Maffulli, Leung, Lee, Lau and Chan33. This contrasts with observations of Caucasian children and adolescents, where the nature, magnitude and duration of weight-bearing physical activity and/or strength resistance training have been found to be positively related to bone mass acquisitionReference Welten, Kemper, Bertheke Post, van Mechelen, Twisk, Lips and Teule34, Reference Boot, de Ridder, Pols, Krenning and de Muinck Keizer-Schrama35. These differences between studies of Chinese and Caucasian children may be related to the duration and magnitude of mechanical forces as well as the intensity of the physical activities, which were all lower in the Chinese girls and may not have been of sufficient force to induce osteogenic responses.
Most of the subjects had a more sedentary lifestyle compared with Caucasian subjects in the other investigations. Only a small part of the total time for exercise was spent in activities of high impact intensity (27 %) and only 16 % of the subjects participated in organised sports, both of which were much lower than found with Caucasian girls of comparable age in Western countriesReference McKay, Petit, Khan and Schutz16. This low participation rate in physical activity by the present subjects was mostly related to the considerable time spent in study because of social pressure to achieve scholastic success in China. This finding is similar to that previously reported in the national survey of Chinese adolescents throughout all provinces of ChinaReference Tudor-Locke, Ainsworth, Adair, Du and Popkin36. Nevertheless, significant associations were found in the present subjects between physical activity levels and both the LBM and handgrip muscle strength, which suggests that these respond to physical activity independent of the intensity of that exercise.
Because this was a cross-sectional study, no clear cause and effect association between body composition, muscle strength and physical activity and bone mass status can be established. The dual-energy X-ray absorptiometry scan technique for assessing bone mass growth and development in children and adolescents does not measure bone geometry and structural properties of the skeleton and provides only a two-dimensional rather than three-dimensional analysis. It is therefore possible that lifestyle or dietary factors, which might have affected bone strength and geometry, might not have been identified. Therefore, future longitudinal studies need to use more advanced bone densitometry techniques including peripheral quantitative computed tomography to determine whether modifiable lifestyle factors affect bone strength and its geometric properties in growing children and adolescents.
Conclusion
In conclusion, the present findings demonstrate that LBM, FBM, handgrip muscle strength and milk intake were all significant independent determinants of BMC and BA of the total body and/or forearm sites. However, LBM was found to be a much stronger independent determinant than FBM of BMC and BA at all skeletal sites. The only significant determinant found between handgrip muscle strength and bone mineral was between handgrip muscle strength and forearm BMC and BA. This suggested that the influence of muscle strength on bone mass is more site specific rather than systemic. Furthermore, there were significant associations between physical activity levels and handgrip muscle strength and LBM, indicating that greater muscle strength and LBM may be the result of a higher level of physical activity. Therefore, promoting healthy lifestyle practices, such as an adequate intake of high Ca foods and continuous participation in physical activity, should be encouraged throughout adolescence to optimise bone growth during this period.
Acknowledgements
This study was supported by the Nestlé Foundation and Danone China. The authors are grateful to all personnel involved from the National Institute for Nutrition and Food Safety, China Centers for Disease Control, Department of School Health, Xichen District of Beijing, Department of Nuclear Medicine, Hospital No. 304, school headmasters and school nurses for their full cooperation throughout the fieldwork of data collection and biochemical analyses. L.H. Foo is the recipient of a PhD scholarship from the University Science Malaysia through its Academic Staff Training Scheme (ASTS) Fellowship programme.






