Appropriate nutrition during infancy is important not only for normal growth and development, but also for long-term health outcomes. Breast-feeding is recommended for delivering these short- and long-term outcomes( 1 ). Infant formulas are used to supplement breast milk when breast milk is not sufficient or breast-feeding is not possible. Cow milk infant formulas are widely accepted as the first-line choice for healthy formula-fed infants. These are typically based on cow milk proteins from skimmed milk and have extra whey proteins added to improve the profile of essential and semi-essential amino acids( Reference Raiha, Fazzolari-Nesci and Cajozzo 2 , Reference Hernell 3 ).
There is also consumer demand for goat milk infant formulas as evidenced by widespread reports of the use of raw goat milk and home-made formulas for infants( Reference Ziegler, Russell and Rozenberg 4 – Reference Baur and Allen 7 ). Goat infant formulas are manufactured in several countries. Compositional analysis of an infant formula made from goat milk without added whey proteins suggests that the amino acid profile( Reference Rutherfurd, Moughan and Lowry 8 ) is compatible with international standards for infant formula( Reference Koletzko, Baker and Cleghorn 9 , 10 ). This type of goat milk formula has also been shown in animal studies to have amino acid digestibility and absorption properties similar to those of a cow infant formula with added whey( Reference Rutherfurd, Darragh and Hendriks 11 ). Thus, it was expected that the amino acid delivery to infants would be similar for the two formulas, but this has never been tested.
In addition to meeting compositional criteria, it is important to establish the suitability and nutritional adequacy of infant formulas containing new sources of proteins through clinical trials( Reference Koletzko, Baker and Cleghorn 9 , Reference Koletzko, Ashwell and Beck 12 ). While goat milk has high-quality proteins and fats and has a history of use for human nutrition in many cultures( Reference Silanikove, Leitner and Merin 13 – Reference Haenlein 15 ), there has been only one previous randomised controlled trial of infants fed a goat milk infant formula( Reference Grant, Rotherham and Sharpe 16 ). This study showed that the growth of thirty infants fed a goat milk infant formula was similar to that of thirty-two infants fed a whey-based cow milk infant formula( Reference Grant, Rotherham and Sharpe 16 ). However, the study was insufficient for assessing the safety and nutritional adequacy of goat milk formulas because it was underpowered and lacked blood biochemical data( 17 ).
The primary aim of the present study was to compare the growth and nutritional status of infants fed formulas based on either goat milk or cow milk in a well-powered randomised controlled trial. The secondary aim was to examine a range of health- and allergy-related outcomes, including the incidence and severity of dermatitis.
Subjects and methods
Participants
The study population included two cohorts of infants who were either fed infant formula or breast-fed at the time of recruitment. Infants were eligible for inclusion in the study if the following criteria were met: (1) a healthy term infant with gestation of 37–42 weeks and birth weight ≥ 2·5 kg and ≤ 4·75 kg; (2) aged up to 2 weeks; (3) mother was exclusively feeding infant formula within 2 weeks of birth (for formula cohort) or planned to exclusively breast-feed for at least 4 months (for the breast-fed cohort). Infants were excluded if they were from multiple births or had severe congenital or metabolic disease likely to affect feeding or growth. Infants who were exclusively formula-fed or breast-fed were identified and referred by midwives in the postnatal wards at one of the following three tertiary hospitals: the Women's and Children's Hospital; the Flinders Medical Centre; the Lyell McEwin Hospital in Adelaide, Australia. The study was approved by the relevant Human Research Ethics Committees at all the three study centres. Written informed consent was obtained from all participating families. The trial was registered with the Australian New Zealand Clinical Trials Registry (ACTRN12608000047392).
Nutritional composition of the study formulas
The goat infant formula (GIF) was manufactured by Dairy Goat Co-operative (N.Z.) Limited using whole goat milk without added whey proteins (final whey:casein ratio approximately 20:80) and a blend of approximately 60 % milk fat and 40 % vegetable oils. The control cow infant formula (CIF) contained cow skimmed milk and whey proteins (final whey:casein ratio approximately 60:40) and vegetable oils as the source of fat and was supplied by Nutricia. The protein:energy ratio of both the study formulas was at the lower limit specified by CODEX( 10 ) and similar to that of the low-protein formula suggested to result in a more desired weight gain in infants( Reference Koletzko, von Kries and Closa 18 ). The nutritional composition of both formulas is given in Table 1.
Table 1 Nutritional composition of the two infant formulas used in the study
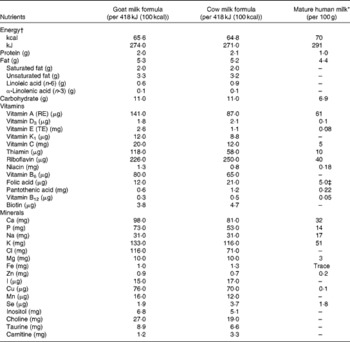
RE, retinol equivalents; TE, α-tocopherol equivalents.
* Wijesinha-Bettoni & Burlingame( Reference Wijesinha-Bettoni, Burlingame, Muehlhoff, Bennett and McMahon 32 ).
† The energy content was calculated based on 14 g powder added to 100 ml water.
‡ Folate.
Study allocation and blinding
Eligible formula-fed infants were randomly assigned to receive either GIF or CIF. Treatment allocation was done through a Web-based randomisation service according to a computer-generated randomisation schedule, which was prepared by an independent statistician. Stratification was by sex and study centre and used variable block sizes of 4 and 8 in equal proportions. The formulas were labelled in four different colours, two of them corresponding to GIF and the other two corresponding to CIF. Cans of both formulas were otherwise identical in appearance to maintain the blind. This ensured that neither the parents nor the research staff were aware whether the formula allocated was GIF or CIF. The blinding index was used to assess the success of blinding( Reference Bang, Ni and Davis 19 ).
Study intervention
The parents and carers of formula-fed infants were asked to feed their infants the allocated study formula from enrolment to at least 4 months of age and thereafter with other complementary foods up to 12 months of age. Study formulas were supplied free of charge until 12 months of age. For breast-feeding infants, mothers were encouraged to continue exclusive breast-feeding for about 4 to 6 months of age in line with current recommendations. Support for breast-feeding was provided by a qualified lactation consultant to mothers free of charge if needed. The timing of introduction of solids about 4 and 6 months was at the discretion of the families for both the formula-fed and breast-fed infants.
Outcome assessments
The primary outcomes were infant weight, length and head circumference, measured at enrolment, 2 weeks and 1, 2, 3, 4, 6 and 12 months. All anthropometric growth data were converted to z-scores using WHO Child Growth Standards (http://www.who.int/childgrowth/en/). Secondary outcomes included nutritional status, general health, tolerance to formula and allergy symptoms.
A small non-fasting blood sample (3–5 ml) was collected to assess blood biomarkers, including Hb, packed cell volume, serum creatinine, urea, albumin, ferritin, and folate, and plasma amino acids, at 4 months of age as indicators of general nutritional status. Fe-deficiency anaemia was defined as Hb concentration < 100 g/l and ferritin concentration < 20 μg/l based on the diagnostic criteria of the test laboratory. Hb concentration was measured spectrophotometrically using a Cell Dyn 4000 analyser (Abbott Laboratories), which has a CV of less than 2 %. Albumin, urea and ferritin concentrations were measured using the Cobas/Hitachi Cobas C System, Cobas 6000 automated analyser (Roche Diagnostics). Albumin concentration was determined spectrophotometrically by an end-point bromocresol green dye-binding method. Urea concentration was measured spectrophotometrically by an enzymatic method. The test method used for measuring ferritin concentration was particle-enhanced immunoturbidmetry. The method used for measuring albumin and urea concentrations has a CV of less than 3 % and that used for ferritin has a CV of less than 4 %. Serum folate concentration was determined with the ARCHITECT i optical system (Abbott) using the Chemiluminescent Microparticle Immunoassay Technology, and this method has a CV of less than 4 %. Amino acid concentrations were measured on the Hitachi L-8900 Amino Acid Analyser. Plasma samples (200 μl) were acidified with 50 μl sulphosalicyclic acid to precipitate intact proteins before analysis. The supernatant was mixed with lithium diluent spiked with S-2-aminoethyl-l-cysteine. The L-8900 Hitachi Analyser utilises a lithium citrate buffer system and ion-exchange (Hitachi column) chromatography to separate amino acids followed by ‘post-column’ ninhydrin reaction detection.
At each growth assessment time point, parents/carers were asked through a structured interview whether their infants had experienced any health problems including respiratory illness, gastrointestinal illness, reflux, eye infection, ear, nose and throat conditions, fever, urinary tract infection and thrush. Serious adverse events, defined as death or hospital admission for more than 24 h during the 12-month study period, were also recorded.
At the same time of growth assessments, the incidence and severity of dermatitis were assessed by trained research staff using SCORAD( 20 ). Food allergy was diagnosed by medical practitioners. Parents/carers were also asked whether their infants had any symptoms related to food allergy and/or gastrointestinal function including hives, swelling of the face or body, wheeze/stridor, vomiting, loose watery stools, blood-stained stools and itchy rash.
Parents/carers were asked to assess stool frequency, consistency and effort as indicators of tolerance to formula using the Bristol Stool Scale( Reference Lewis and Heaton 21 ) as a guide. Sleeping patterns including length of each sleep episode, total number of sleep episodes during the day, and time taken to settle for sleep during the day, in the evening or at night were also assessed by parental report based on the Sleep and Settle Questionnaire( Reference Matthey 22 ).
Other assessments
Demographic and baseline characteristics, including infant sex, weight and length at birth, age at enrolment, and anthropometric measurements at enrolment and maternal age, BMI, parity, and history of smoking and drug and alcohol use during pregnancy, were recorded at trial entry.
Sample size and power calculation
Sample size calculations estimated that sixty-four infants per group were required to detect a 0·5 sd difference (80 % power with α = 0·05) in weight( Reference Koletzko, Ashwell and Beck 12 ). We aimed to enrol 100 infants per feeding group and 100 breast-fed infants to provide reference data. This sample size was also sufficient to detect a clinically important difference of 0·11 (sd 0·26) g/l in serum albumin, an indicator of protein adequacy, with 80 % power (α = 0·05).
Statistical analyses
All analyses were carried out using SAS® software version 9.2 or a later version (SAS Institute, Inc.). Blinded treatment codes were included in the database, and analyses of the primary and secondary outcomes were carried out blinded to treatment group. All analyses were carried out using both intention-to-treat and per-protocol approaches, with infants who did not complete the trial or who had consumed any non-study formula, liquids or solids for more than 12 d between 2 weeks and 4 months of age being excluded from the per-protocol analysis. As the two analysis approaches yielded similar results, only results of the primary intention-to-treat analysis are reported herein.
To minimise bias in the estimation of treatment effects due to missing data, multiple imputation was used to create fifty complete datasets for analysis. The parametric regression method was used to impute continuous variables and the logistic regression method was used for binary variables. In addition to the primary imputed analysis, sensitivity analyses were carried out on the original data and on imputed data created using different seeds and using different imputation models. All approaches yielded similar results; thus, only the results of the primary imputed analysis are reported herein.
Continuous outcomes measured at multiple assessments, including the primary anthropometric outcomes, were compared between the formula-fed and breast-fed groups over time using linear mixed-effects models. Fixed effects for group, time and the interaction between group and time were included in the models, while dependence was accounted for by allowing for correlated residuals within a child. Independent of the statistical significance of the interaction term, differences between the groups were reported separately at each time point, with the effects of treatment group being expressed as mean differences. Continuous outcomes measured at a single time point were compared between the groups using linear regression models, with the effects of group being expressed as mean differences. Binary outcomes were analysed using log binomial regression models, with the effects of group being expressed as relative risks. Rare binary outcomes were analysed using Fisher's exact tests. Both unadjusted and adjusted analyses were carried out, with conclusions on group differences being based on the adjusted analyses. For the primary growth outcomes, comparisons of the two randomised groups were adjusted for centre, while comparisons involving the breast-fed reference group were adjusted for maternal education and the relevant anthropometric z-score at birth. All secondary outcomes were adjusted for the stratification variables centre and sex for comparisons of the randomised groups and maternal education and birth weight for comparisons involving the breast-fed reference group. Due to imbalances in maternal smoking during pregnancy between the randomised groups, sensitivity analyses of the primary growth outcomes adjusting for centre and maternal smoking during pregnancy were also carried out. All tests were two tailed with a significance level of P≤ 0·05.
Results
The participants were recruited between April 2008 and April 2009 from three tertiary hospitals in Adelaide. Of the 1180 families who were approached to participate in the study, 768 were eligible and 301 (39 %) consented. A total of 200 infants were formula-fed and 101 were breast-fed. More details are given in the flow chart of study participant selection in Fig. 1.
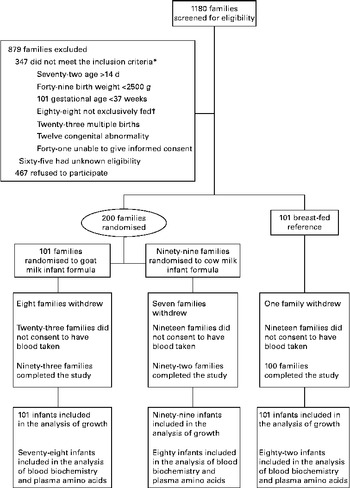
Fig. 1 Flow chart of study participants. * Infants could be ineligible for more than one reason. † Either formula or breast milk.
Maternal characteristics as well as infant anthropometrics at birth and at study entry are summarised in Table 2. The mean age of infants at study entry was 6·2 (sd 3·7) d and 46 % were male. The baseline characteristics of the participants were comparable between the two formula-fed groups, with the exception that the percentage of mothers who smoked during pregnancy was higher in the GIF group (45 %) than in the CIF group (34 %). Compared with the formula-fed infants, the reference group of breast-fed infants had a higher mean birth weight (P= 0·001), a lower mean maternal pre-pregnancy BMI (P< 0·0001), a lower percentage of mothers who smoked (P< 0·0001) during pregnancy and a higher percentage of parents who completed higher education (P< 0·0001). The percentage of mothers who did not know their baby's treatment group was similar between the groups (32 % in the GIF group and 34 % in the CIF group). The blindness index, which indicates the percentage of mothers who guessed their treatment group correctly above chance, was 3·8 % for the GIF group compared with 2·7 % for the CIF group.
Table 2 Characteristics of the participants (Mean values and standard deviations; number of participants and percentages)
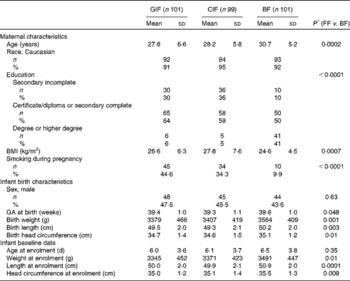
GIF, goat milk infant formula; CIF, cow milk infant formula; BF, breast-fed; FF, formula-fed; GA, gestational age.
* Continuous and categorical characteristics compared using independent-samples t tests and χ2 tests, respectively.
The median daily intake of study formula ranged from 698 (interquartile range 570–825) ml in the first 2 weeks to 1000 (interquartile range 855–1190) ml at 4 and 6 months. Compliance with the definition of exclusive formula feeding or breast-feeding( 23 ) from enrolment to 4 months of age was observed in seventy-six (75 %) of the 101 infants in the breast-fed group, seventy-four (73 %) of the 101 infants in the GIF group and fifty-nine (60 %) of the ninety-nine infants in the CIF group. The level of compliance in the GIF group was significantly different from that in the CIF group (P= 0·02), but not significantly different from that in the breast-fed reference group (P= 0·37).
Growth
There were no differences in the adjusted intention-to-treat analyses of weight, length, head circumference and weight-for-length z-scores between the two formula-fed groups over the 12-month study period (Fig. 2(a)–(d), respectively), with or without adjustment for baseline difference in maternal smoking. Also, gains in weight, length or head circumference from registration to 4 or 6 months did not differ between the two formula-fed groups (data not shown).
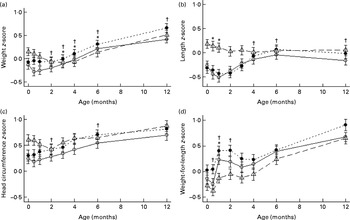
Fig. 2 Weight (a), length (b), head circumference (c) and weight-for-length (d) z-scores of infants fed goat milk formula (○), cow milk formula (●) or breast milk (Δ). Z-score data were based on WHO reference data. Values are means of imputed data, with standard deviations represented by vertical bars. * Mean value of the goat formula-fed group was significantly different from that of the breast milk-fed group (P< 0·05). † Mean value of the cow formula-fed group was significantly different from that of the breast milk-fed group (P< 0·05).
In comparison with the breast-fed infants, infants in the GIF group had higher weight z-scores at 3, 4 and 6 months (mean difference 0·22 (P= 0·04), 0·30 (P= 0·005) and 0·33 (P= 0·003)), while infants in the CIF group had higher weight z-scores from 2 to 12 months of age (mean difference 0·22 (P= 0·04), 0·28 (P= 0·01), 0·39 (P= 0·001), 0·38 (P= 0·001) and 0·36 (P= 0·001)). Infants in the GIF group had lower length z-scores at 2 weeks and 1 month of age compared with the breast-fed infants (mean difference − 0·33 (P= 0·003) and − 0·37 (P= 0·001)), whereas those in the CIF group had higher length z-scores at 4, 6 and 12 months of age (mean difference 0·25 (P= 0·03), 0·35 (P= 0·002) and 0·25 (P= 0·03)). While infants in the GIF and breast-fed groups had similar head circumference z-scores, those in the CIF group had higher z-scores at 2 and 6 months of age compared with the breast-fed infants (mean difference 0·24 (P= 0·04) and 0·3 (P= 0·01)). Infants in the GIF group had higher weight-for-length z-scores compared with the breast-fed infants at 1 month of age only (mean difference 0·40 (P= 0·004)), while those in the CIF group had higher weight-for-length z-scores at 1 and 2 months (mean difference 0·46 (P= 0·001) and 0·39 (P= 0·006)). There were no statistically significant differences between the formula-fed and breast-fed groups at any other time points.
Biomarkers of nutritional status
There were no differences in serum albumin, Hb, packed cell volume and ferritin values between the two formula-fed groups. No infants in either formula-fed group had Fe-deficiency anaemia (defined as Hb concentration < 100 g/l and ferritin concentration < 20 μg/l). Infants in the GIF group had lower mean serum urea, creatinine and folate concentrations compared with those in the CIF group (Table 3). Compared with the breast-fed infants, formula-fed infants had higher mean serum urea concentrations, infants in the GIF group had lower mean serum folate concentrations and infants in the CIF group had higher mean folate concentrations (Table 3). Mean serum folate concentrations in all the three groups of infants were within the normal reference range for infants of this age( Reference Himes, Shulman and Koletzko 24 ).
Table 3 Serum biomarkers at 4 months of age (Mean values and standard deviations; mean differences and 95 % confidence intervals)
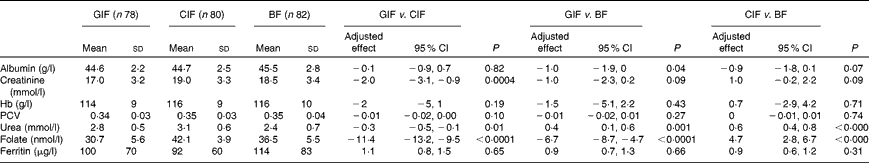
GIF, goat milk infant formula; CIF, cow milk infant formula; BF, breast-fed; PCV, packed cell volume.
The concentrations of essential and semi-essential amino acids in the plasma of infants are presented in Fig. 3. The concentrations of valine and phenylalanine were higher and those of isoleucine and threonine were lower in the plasma of infants fed the GIF than in that of infants fed the CIF. The mean difference for valine was 37 (95 % CI 25, 50) μg/l, phenylalanine 5 (95 % CI 0, 10) μg/l, isoleucine − 9 (95 % CI − 16, − 3) μg/l and threonine − 32 (95 % CI − 45, − 18) μg/l. The concentrations of all the other essential and semi-essential amino acids in the plasma of formula-fed infants did not differ significantly between the groups.
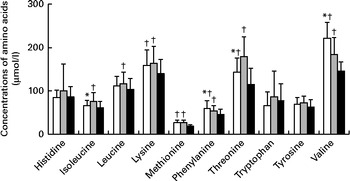
Fig. 3 Concentrations of essential and semi-essential amino acids in the plasma of infants after 4 months of being fed goat milk formula □, cow milk formula ![]() or breast milk ■. Values are means, with standard deviations represented by vertical bars. * Mean value was significantly different from that of the cow milk formula-fed group (P< 0·05). † Mean value was significantly different from that of the breast milk-fed group (P< 0·05).
or breast milk ■. Values are means, with standard deviations represented by vertical bars. * Mean value was significantly different from that of the cow milk formula-fed group (P< 0·05). † Mean value was significantly different from that of the breast milk-fed group (P< 0·05).
Compared with the breast-fed infants, infants fed the GIF had significantly higher concentrations of lysine, methionine, phenylalanine, threonine and valine. Mean differences were 15 (95 % CI 1, 29) μg/l, 6 (95 % CI 4, 9) μg/l, 13 (95 % CI 7, 18) μg/l, 13 (95 % CI 7, 18) μg/l, 19 (95 % CI 4, 34) μg/l and 66 (95 % CI 52, 79) μg/l, respectively. The concentrations of isoleucine, leucine, lysine, methionine, phenylalanine, threonine and valine were higher in the plasma of infants fed the CIF than in that of the breast-fed infants. Mean differences were 13 (95 % CI 7, 20) μg/l, 11 (95 % CI 2, 21) μg/l, 19 (95 % CI 6, 33) μg/l, 6 (95 % CI 3, 8) μg/l, 8 (95 % CI 2, 13) μg/l, 51 (95 % CI 37, 66) μg/l and 29 (95 % CI 15, 44) μg/l, respectively. The concentrations of none of the amino acids were lower in either formula-fed group compared with those in the breast-fed infants.
General health- and allergy-related outcomes
There were no differences in the risk of an adverse health condition, including respiratory illness, gastrointestinal illness, reflux, eye infection, ear, nose and throat conditions, fever, urinary tract infection and thrush, between the two formula-fed groups. There were also no differences in the risk of the above-mentioned health conditions between the formula-fed groups and the breast-fed reference group, with the exception that more infants had oral thrush in the CIF group than in the breast-fed reference group (n 9/86 v. n 2/99, P= 0·02) during the 12-month study period. The proportion of infants who had any serious adverse events during the 12-month study period was similar between the GIF, CIF and breast-fed reference groups: n 15/101 (14·9 %); n 12/99 (12·1 %); n 9/101 (8·9 %), respectively (P= 0·43). The most common serious adverse events were bronchiolitis and other respiratory infections. No infants died.
The proportions of infants with medically diagnosed food allergy (GIF n 2/92 v. CIF n 1/89 v. breast-fed n 5/99) or dermatitis assessed using SCORAD (GIF n 13/91 v. CIF n 20/86 v. breast-fed n 21/99) did not differ between the groups. The mean SCORAD score of infants with dermatitis was 9·9 (sd 6·7) for the GIF group, 11·9 (sd 7·1) for the CIF group and 11·1 (sd 6·3) for the breast-fed group.
There was no difference between the formula-fed groups with regard to the proportion of infants with parentally reported symptoms related to allergy and/or gastrointestinal function, except for parentally reported blood-stained stools (Table 4). Compared with the breast-fed infants, infants in the GIF group had a higher risk of blood-stained stools, while infants in the CIF group had a higher risk of wheeze (Table 4). The proportions of infants with hives (GIF n 5/89 v. CIF n 5/86 v. breast-fed n 6/99) and swelling of the face (GIF n 6/89 v. n 6/86 v. breast-fed n 5/99) did not differ between all the groups in simple unadjusted analyses.
Table 4 Incidence of parentally reported food allergy/gastrointestinal symptoms in the 12-month study period (Relative risk (RR) values and 95 % confidence intervals)
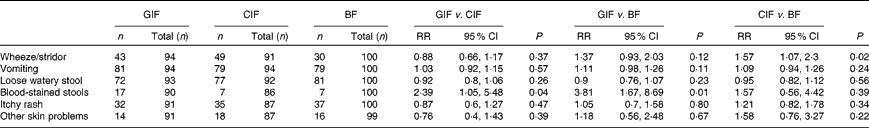
GIF, goat milk infant formula; CIF, cow milk infant formula; BF, breast-fed.
Tolerance to formula
The mean numbers of stool motions per d in infants in the GIF group at 2 weeks, 1 month, 2 months and 3 months of age were 2·5 (sd 1·6), 2·0 (sd 1·3), 1·6 (sd 1·0) and 1·6 (sd 0·9), respectively. These values were not different from those in infants in the CIF group, which were 2·5 (sd 1·4), 2·0 (sd 1·4), 1·5 (sd 0·9) and 1·6 (sd 1·3) at 2 weeks, 1 month, 2 months and 3 months, respectively. However, stool frequency in both the formula-fed groups was significantly lower (P< 0·001) than that in the breast-fed group (6·3 (sd 3·3), 5·0 (sd 2·3), 3·0 (sd 2·2) and 2·4 (sd 1·8) at 2 weeks, 1 month, 2 months and 3 months, respectively). Compared with infants in the CIF group, those in the GIF group had lower mean stool consistency scores at 2 weeks (GIF 4·69 (sd 1·44) v. CIF 5·46 (sd 0·96), P< 0·0001) and 1 month (GIF 4·95 (sd 1·35) v. CIF 5·35 (sd 1·19), P= 0·01). No differences were observed in the stool consistency scores at other assessment time points.
There were no differences in the mean length of each sleep episode or the total number of sleep episodes between the two formula-fed groups, with the exception that infants in the GIF group had a shorter mean length of each sleep episode in the evening (GIF 103 (sd 63) v. CIF 127 (sd 65) min, P= 0·007) and a longer mean length of each sleep episode at night (GIF 317 (sd 96) v. CIF 288 (sd 102) min, P= 0·03) at the 2-month assessment time point. The time taken to settle for sleep during the day, in the evening or at night also did not differ between the GIF and CIF groups. In comparison with the breast-fed infants, there were some differences in sleeping patterns between the formula-fed and breast-fed infants, but the differences were inconsistent (data not shown).
Discussion
The present study is the first to rigorously evaluate in healthy term infants the effect of feeding a goat infant formula up to 12 months of age on growth, nutritional status, tolerance to formula, and a wide range of health- and allergy-related outcomes in a well-conducted randomised controlled trial involving a control group fed a cow milk infant formula and a reference group of breast-fed infants. We could detect no difference in z-scores for infant weight, length, head circumference and weight for length up to 12 months between the two formula-fed groups. The same overall treatment effects were observed in the intention-to-treat or per-protocol analysis that excluded data obtained from infants who consumed any non-study formula, liquids or solids for more than 12 d before 4 months of age. This suggests that it is unlikely that the consumption of non-study foods by some infants within the first 4 months had a significant impact on the outcomes of the study. We did detect some differences in weight and weight-for-length z-scores for both the formula-fed groups compared with the breast-fed group, consistent with the findings of other studies comparing the growth of formula and breast-fed infants( Reference Kramer, Guo and Platt 25 – Reference Agostoni, Grandi and Gianni 27 ). Interestingly, while the differences in weight or weight-for-length z-scores persisted at 12 months between the breast-fed infants and cow milk formula-fed infants in the present study, consistent with the findings of other cow milk-based formula studies( Reference Kramer, Guo and Platt 25 – Reference Agostoni, Grandi and Gianni 27 ), there was no differences between the goat milk formula-fed infants and breast-fed infants. The present study used the same formula with a lower protein content (2 g/418 kJ (100 kcal) and 2·1 g/418 kJ (100 kcal) for goat and cow milk formulas, respectively) through to 12 months rather than switching to a follow-on formula with a higher protein content from 6 months as has been done in the other formula studies( Reference Kramer, Guo and Platt 25 – Reference Agostoni, Grandi and Gianni 27 ). This may partly explain the difference observed between the present study and the other formula studies mentioned above, as it has been shown that weight-for-length z-score at 24 months of infants fed a low-protein formula was not different from that of breast-fed infants, while infants fed a high-protein formula (2·9 g/418 kJ (100 kcal)) had a higher z-score.
There were minor differences in the blood biomarkers between the formula-fed groups, which probably reflected differences in the composition of the two formulas. For instance, the cow infant formula contained added folate close to the recommended maximum, compared with the goat milk formula that had an amount in the mid-range of the recommendations( Reference Koletzko, Baker and Cleghorn 9 , 10 ). Nevertheless, concentrations of blood biomarkers measured at 4 months were within the normal reference range for infants of this age( Reference Himes, Shulman and Koletzko 24 ).
Whey proteins are often added to formulas to help improve the protein quality and availability of essential and semi-essential amino acids( Reference Janas, Picciano and Hatch 28 , Reference Janas, Picciano and Hatch 29 ). Infant formulas made from goat milk without added whey proteins have been shown to have sufficient quantities of all the essential and semi-essential amino acids( Reference Rutherfurd, Moughan and Lowry 8 ) and to have amino acid digestibility and absorption properties similar to a whey-based cow infant formula in an animal model( Reference Rutherfurd, Darragh and Hendriks 11 ). The present study demonstrates some differences in plasma amino acid profile between the formula-fed groups as well as in comparison with the breast-fed infants, but there were large inter-individual variations. Although the differences were statistically significant, they are unlikely to be clinically important, as the mean plasma amino acid concentrations in infants in both the formula-fed groups are similar to those reported in other studies( Reference Lonnerdal and Hernell 30 , Reference Sandstrom, Lonnerdal and Graverholt 31 ).
The present study is the first to record a wide range of outcomes related to general health, gastrointestinal function and allergy when infants were exposed to a goat infant formula using a combination of objective clinical assessments and subjective parental reports. There were no differences in the objective assessments of allergy-related outcomes including dermatitis and medically diagnosed food allergy.
The only statistically significant finding between the formula-fed groups was a greater number of parental reports of blood-stained stools in infants fed the goat infant formula than in those fed the cow infant formula. We are unsure about the significance of this finding. First, the number of reports of blood-stained stools was low overall, and second, there was no indication of other gastrointestinal disorders, differences in stool characteristics, crying and sleeping patterns, general health or other allergy-related symptoms. Furthermore, none of the infants in the study had Fe-deficiency anaemia, which would indicate no significant blood loss over time. Finally, the outcomes related to allergy and gastrointestinal function were secondary outcomes, which the study did not have adequate power to rigorously assess, and thus they need to be interpreted with caution, as it is possible that this may be due to chance. A much larger, adequately powered randomised controlled trial with objective assessment of clinical outcomes and biomarkers of allergy is needed to rigorously evaluate the effects of goat milk infant formula on allergy and gastrointestinal function.
In conclusion, the growth and blood biomarkers of nutritional status of infants fed a whole-goat milk-based infant formula did not differ from those of infants fed a standard cow infant formula with added whey. The lack of a significant difference between the formula-fed groups for an extensive range of health-related outcomes and for the occurrence of serious adverse events supports the safety of using goat milk in infant formula.
Acknowledgements
The authors thank the families who participated in the study, the medical, nursing and research staff at each participating centre, the staff of the Child Nutrition Research Centre, and the staff of the Data Management and Analysis Centre, University of Adelaide and University of California, Davis, USA. M. M. and R. A. G. were supported by the National Health and Medical Research Council Senior Research Fellowship (ID: 565000 for M. M. and ID: 519324 for R. A. G.). Infrastructure support was provided by the Women's and Children's Health Research Institute, the University of Adelaide, the Women's and Children's Hospital Adelaide, the Flinders Medical Centre Adelaide and the Lyell McEwin Hospital Adelaide.
Dairy Goat Co-operative (N.Z.) Limited, New Zealand, provided the funding to conduct the study. The funder contributed to the design of the study, interpretation of the findings and preparation of the manuscript. Data collection, management and analysis were conducted independently of the funder.
The authors' contributions were as follows: M. M., S. J. Z., R. A. G., T. S., C. G. P. and D. J. L. designed the research; M. M., S. J. Z., R. A. G. and B. L. conducted the research; T. S., S. J. Z. and M. M. analysed the data and carried out the statistical analyses; S. J. Z. drafted the manuscript with contributions from all authors; M. M. and S. J. Z. had primary responsibility for the final content. All authors reviewed and approved the final manuscript.
M. M. serves on scientific advisory boards for Nestlé, Fonterra and Nutricia. R. A. G. serves on scientific advisory board for Fonterra. Associated honoraria for M. M. and R. A. G. are paid to their institutions to support conference travel and continuing education for postgraduate students and early career researchers. C. G. P. and D. J. L. work for the Dairy Goat Co-operative (N.Z.) Limited, which manufactured the goat milk formula used in the study. None of the other authors has any conflicts of interest to declare.








