The Mediterranean diet has been widely reported to be a model of healthy eating for its contribution to a favourable health status and a better quality of life( Reference Sofi, Abbate and Gensini 1 ). Olive oil, along with fruits, vegetables and fish, is an important constituent of this diet, and is considered a major factor in preserving a healthy and relatively disease-free population( Reference Owen, Giacosa and Hull 2 ). Olive oil contains a high level of MUFA, as well as multiple minor components with biological properties( Reference Covas, Ruiz-Gutiérrez and De La Torre 3 ). The saponiable fraction of olive oil is primarily composed of TAG, partial glycerides, esters of fatty acids or free fatty acids and phosphatides, which represent nearly 98 % of the oil chemical composition, whereas its unsaponifiable fraction is dominated by minor compounds such as tocopherols, phytosterols, carotenoids (β-carotene and lutein), triterpenic alcohols (uvaol and erythrodiol), pentacyclic triterpenes (oleanolic and maslinic acid) and phenolic compounds (i.e. tyrosol, hydroxytyrosol, oleocanthal, oleuropein)( Reference Ghanbari, Anwar and Alkharfy 4 , Reference Sánchez- Quesada, López-Biedma and Warleta 5 ).
A plethora of studies have reported health benefits of olive oil and its minor components in humans, especially in preventing and/or reducing hypercholesterolaemia, serum lipoprotein levels and atherosclerosis, hypertension, CVD and thrombotic risk, oxidation and oxidative stress, obesity and type 2 diabetes, inflammatory processes and cancer( Reference Ghanbari, Anwar and Alkharfy 4 , Reference Omar 6 , Reference Caramia, Gori and Valli 7 ). Although there is information about the use of olive oil as substitute of fish oil in aqua feeds( Reference Naylor, Hardy and Bureau 8 ), published evidence about the use of olive oil and its derivatives as feed additives is scarce, with most of the available information being focused on maslinic acid with contradictory results regarding the potential effects of this triterpenic compound in promoting fish growth performance( Reference Fernández-Navarro, Peragón and Esteban 9 – Reference Matos, Silva and Wulff 11 ).
Supporting fish growth was one of the main objectives of feed producers and fish farmers during the last decades when the efficiency of nutrient conversion and assimilation was one of their main targets of the industry; however, the development of this sector, as well as the intensification of production and strong competition among producers, has forced the sector to reduce production costs for improved economies of scale and find ways to create a competitive edge. In this context, maintaining fish health and welfare is a concern in aquaculture, particularly in the light of the potential effects of climate change and super intensification of the production. Moreover, new management strategies are needed to support growth and health( 12 ). Thus, the use of functional feeds may be regarded as the future of aquaculture; by embracing nutritional strategies to address specific stresses, environmental situations, life stage requirements and pathologies, the industry can optimise animal performance as well as operational efficiency when health improvements lead to reduced production losses. Thus, in the present study, we decided to test the potential benefits of an olive oil bioactive extract (OBE) (unsaponifiable fraction) rich in polyphenols, triterpenic acids, long-chain fatty alcohols, unsaturated hydrocarbons, tocopherols and sterols in terms of growth, feed utilisation, and gut health and integrity in gilthead sea bream (Sparus aurata).
Methods
Experimental diets
A control diet was formulated with high levels of marine-derived protein sources to contain 53 % crude protein, 18 % crude fat and 20·9 MJ/kg gross energy and fulfil the nutritional requirements of juvenile sea bream( Reference Oliva-Teles, Lpatsch and Nengas 13 ). Based on this basal formulation, four additional diets were produced by adding at the expense of fish oil an olive oil containing 9 % triterpenic acids, 2 % polyphenols, 2 % long-chain fatty alcohols and 1 % sterols (OBE, OLEA OS-15 FBP; ProNutra Solutions S.L.) (Table 1). Diets were manufactured by Sparos Lda. Main ingredients were ground (below 250 μm) in a micropulverizer hammer mill (Hosokawa Micron). Powder ingredients and oils were then mixed according to the target formulation in a paddle mixer (RM90; Mainca). All diets were manufactured by temperature controlled extrusion (pellet sizes: 0·8 and 1·5 mm) by means of a low-shear extruder (P55; Italplast). Upon extrusion, all feed batches were dried in a convection oven (OP 750-UF; LTE Scientific) for 4 h at 45°C. Samples of each diet were analysed for proximate composition analysis (Table 1).
Table 1 Ingredient list and proximate chemical composition (in DM) of experimental diets

CP, crude protein; CF, crude fat.
* Peruvian fishmeal LT: 71 % CP, 11 % CF, Exalmar.
† Fair average quality fishmeal: 62 % CP, 12 %CF, COFACO.
‡ Soluble fish-protein concentrate (CPSP 90): 84 % CP, 12 % CF, Sopropêche.
§ Super prime squid meal: 80 % CP, 3·5 % CF, Sopropêche.
|| Soycomil© Soy Protein concentrate: 65 % CP, 1 % CF, ADM.
¶ VITEN: 85·7 % CP, 1·3 % CF, ROQUETTE.
** 61 % CP, 6 % CF, COPAM.
†† Micronised soyabean meal: 51·7 % CP, 2·1 % CF, Sorgal SA.
‡‡ CP 11·6 %, CF 1·4 %, Casa Lanchinha.
§§ CP 0·3 %, CF <0·1 %, Cosucra.
|||| Marine oil omega 3: Henry Lamotte Oils GmbH.
¶¶ PVO40·01 premix for marine fish, PREMIX Lda. Vitamins (mg/kg diet): dl-α tocopherol acetate, 100 mg; sodium menadione bisulphate, 25 mg; retinyl acetate, 6.88 mg; dl-cholecalciferol, 0.050 mg; thiamin, 30 mg; riboflavin, 30 mg; pyridoxine, 20 mg; B12, 0·1 mg; nicotinic acid, 200 mg; folic acid, 15 mg; ascorbic acid, 1000 mg; inositol, 500 mg; biotin, 3 mg; calcium panthotenate, 100 mg; choline chloride, 1000 mg, betaine, 500 mg. Minerals (g or mg/kg diet): cobalt sulphate, 2·5 mg; copper sulphate, 1·1 mg; ferric citrate, 0·2 g; potassium iodide, 5 mg; manganese sulphate, 15 mg; sodium selenite, 0·2 mg; zinc sulphate, 40 mg; magnesium hydroxide, 0·6 g; potassium chloride 1·1 g; sodium chloride, 0·5 g; calcium carbonate, 4 g.
*** LUTAVIT E 50, ENSOL.
††† Paramega PX, Kemin Europe NV.
‡‡‡ Based on analysis of three samples per diet.
§§§ Gross energy content was estimated by using the following: total carbohydrate×17·2 J/kg, fat×39·5 J/kg, and protein×23·5 J/kg.
Animals, experimental conditions and general procedures
Gilthead sea bream fingerlings were obtained from a fish farm (Piscicultura Marina Mediterránea SL), transported by road to the IRTA-SCR facilities and acclimated for 10 d to new husbandry and water conditions in a 2 m3 circular fiberglass tank. During this period, fish were fed twice a day with Microbaq 15 (Dibaq SA) at 2 % of the stocked biomass. Before the onset of the trial, all fish were anaesthetised (tricaine methanesulfonate, MS-222, 150 mg/l), individually weighted (body weight (BW)) and measured for standard length (SL) to the nearest 0·1 g and 1 mm, respectively; and then distributed into twenty fiberglass cylindrical tanks of 400 litres (seventy-five fish per tank, BW i =5·4 (sd 1·2) g).
Water temperature and pH (pH meter 507; Crison Instruments), salinity (MASTER-20T; ATAGO Co. Ltd) and dissolved O2 (OXI330; Crison Instruments) were 22·1 (sd 0·2)°C, 7·8 (sd 0·1), 36 mg/l and 6·8 (sd 0·3) mg/l, respectively. Water flow rate in experimental tanks was maintained at approximately 9·0 l/min via a recirculation system (IRTAmar®) that maintained adequate water quality (total ammonia and nitrite were≤0·10 and 0·4 mg/l, respectively) through UV, biological and mechanical filtration. Photoperiod followed natural changes according to the season of the year (January–April; latitude 40°37'41'N). Each diet was tested with four replicates for 90d. Diets were distributed eight times per d by automatic feeders (ARVO-TEC T Drum 2000; Arvotec) at the rate of 3·3 % of the stocked biomass, which approached apparent satiation.
Sampling to monitor fish growth took place monthly from the onset of the feeding period. For that purpose, all fish in each tank were netted, anaesthetised and their wet BW and SL determined. At the end of the trial (90d), all fish from each tank were measured for their final BW (BW f , g) and final standard length (SL f , cm), as well as for determining final size distribution in BW f . In addition, seventy-two specimens (fasted overnight) per experimental group (eighteen per tank) were killed with an overdose of anaesthetic for assessing the histological organisation of the of the intestinal mucosa, the activity of antioxidative stress enzymes in the intestine and liver (five per tank), proximate carcass composition (five per tank) and gene expression analysis of markers of intestinal integrity and health (eight per tank). Fish growth and feed utilisation from different experimental groups was evaluated by means of the following indices: Fulton’s condition factor (K)=(BW f /SL f 3)×100; specific growth rate in BW (SGR BW , %)=((ln BW f −ln BW i )×100)/time (d); feed conversion ratio (FCR,g/g)=F/(B f −B i ) and apparent specific feed intake (SFI, %)=SGR×FCR, where F was the total feed intake during the experimental period considered (g) and, B i and B f were the initial and final biomass (g).
All animal experimental procedures were conducted in compliance with the experimental research protocol approved by the Committee of Ethics and Animal Experimentation of the Institut de Recerca i Tecnologia Agroalimentàries and in accordance with the Guidelines of the European Union Council (86/609/EU) for the use of laboratory animals.
Proximate composition, lipid peroxidation and antioxidative stress enzymes in liver and intestine
For determining the body proximate composition of fish and feed, samples were homogenised (Ultra-Turrax T25 basic, IKA©; Werke), and small aliquots were dried (120°C for 24 h) to estimate water content. The total fat content in samples was quantified gravimetrically after extraction in chloroform–methanol (2:1) and evaporation of the solvent under a stream of N2 followed by vacuum desiccation overnight( Reference Folch, Lees and Sloane-Stanley 14 ). Protein content was determined according to Lowry et al.( Reference Lowry, Rosebrough and Farr 15 ). Ash contents were determined by keeping the sample at 500–600°C for 24 h in a muffle furnace( 16 ). All chemical analyses were performed in triplicate per fish and feed samples.
Approximately 100 mg of tissue per sample was homogenised for 5 min in eight volumes (v/w) of 0·15 m KCl–KOH, 1 mm-EDTA (pH 7·5) buffer and then subjected to sonication (Vibra-Cell©; Sonics) for 1·5 min at 0–4°C. Homogenised samples were centrifuged at 10 200 g for 5 min at 4°C, and the supernatant collected for analytical determinations. Quantification of lipid peroxidation (LPO) in the intestine and liver was conducted using the thiobarbituric acid reactive substances method described by Solé et al.( Reference Solé, Potrykus and Fernández-Díaz 17 ). In brief, LPO was measured using 200 µl of the homogenate mixed with 650 µl of methanol, 1-methyl-2-phenylindole (solution stock of 10·3 mm) in acetonitrile–methanol (1:3, v/v) and 150 µl of 37 % HCl. This mixture was incubated (40 min, 45°C), cooled on ice and centrifuged at 21000 g for 10 min to remove protein precipitates. Absorbance was read at 586 nm, and the amount of peroxidised lipids (in nmol malondialdehyde/100 g tissue, w/w) was evaluated by means of a calibration curve made of a standard solution (10 mm 1,1,3,3-tetramethoxypropane). Homogenised samples, prepared for the determination of LPO, were used to measure activity of antioxidant enzymes. Catalase (CAT, EC 1.11.1.6) activity was measured by the decrease in absorbance at 240 nm (e=43·6 mm −1×cm−1) using 50 mm-H2O2 as substrate( Reference Aebi 18 ). Glutathione S-transferase (GST, EC 2.5.1.18) was assayed by the formation of glutathione chlorodinitrobenzene adduct at 340 nm (e=9·6 mm −1×cm−1), using 1 mm 1-chloro-2,4-dinitrobenzene and 1 mm glutathione as substrates( Reference Habig, Pabst and Jakoby 19 ). Glutathione reductase (GR, EC 1.8.1.7) activity was determined by measuring the oxidation of NADPH at 340 nm (e=6·22 mm −1×cm−1), using 20 mm glutathione disulphide and 2 mm NADPH as substrates( Reference Carlberg and Mannervik 20 ). Enzyme activities were expressed as specific enzyme activities (nmol/minper mg protein), and soluble protein determined by the Bradford method( Reference Bradford 21 ). All assays were conducted in triplicate at 25°C, and absorbance read using a spectrophotometer (TecanTM Infinite M200; Techan Group Ltd).
Histological organisation of the intestine
For histological purposes, fragments of liver, mid and posterior intestine from twenty fish per dietary treatment were dissected and fixed in 4 % buffered formaldehyde (pH=7·4), dehydrated in a graded series of ethanol, cleared with xylene, embedded in paraffin and cut in serial sections (3–5 µm thick). Sections were stained with Masson’s trichrome stain for general histological descriptions, whereas slides were stained with Periodic Acid Schiff for goblet cell identification (neutral mucins produced in intestinal goblet cells stain in magenta). All sections were observed under a light microscope (Leica DM LB; Leica Microsystems) and photographed (Olympus DP70 Digital Camera; Olympus Imaging Europa GmbH). Digital images were processed and analysed using an image analysis software package (ANALYSIS; Soft Imaging Systems GmbH). Measurements of total goblet cell number (full and empty) and villi height were based on the analysis of eight to ten randomly chosen fields from the intestinal mucosa. Goblet cell counts in intestinal villi were expressed over a contour length of 100 μm, whereas villi height and width were calculated according to Escaffre et al. ( Reference Escaffre, Kaushik and Mambrini 22 ). Size (S) of hepatic fat deposits (unstained vacuoles within hepatocytes that corresponded to lipids dissolved during the embedding process of the tissue in paraffin) was estimated at ×400 magnification according to the formula: S (µm2)=1/4×π×a×b, where a and b were the minimum and maximum diameters of the vacuole( Reference Darias, Gómez and Tello 23 ).
Quantitative PCR and gene expression analyses of intestinal markers
Total RNA was extracted from the mid-posterior intestine of fish using the TRIzol reagent (Invitrogen®) as specified by the manufacturer. The quantity of RNA isolated was determined using a Gene-Quant spectrophotometer (Amersham Biosciences), measuring optical density at 260 nm, its purity was established by the absorbance ratio 260:280 nm, and quality of the RNA was evaluated using 1·2 % agarose gel electrophoresis. A reverse transcription reaction was carried out using equal quantities of total RNA (1 μg) from each sample and Quanti Tect Reverse Transcription Kit (Qiagen®). Electrophoresis using a 1·2 % agarose gel was run to assess the quality of the RT-PCR product used for real-time quantitative PCR analyses of gene expression.
Differences in dietary-induced gene expression patterns in the intestine among groups were only compared between the control group (Diet A) and the experimental group displaying a greater increase in body weight and overall condition (Diet C). In this sense, we used a previously validated specific PCR-array of eighty-eight target genes( Reference Pérez-Sánchez, Benedito-Palos and Estensoro 24 ), this PCR-array was designed to cover different key biological functions: (i) cell differentiation and proliferation, n 14; (ii) intestinal architecture and permeability, n 20; (iii) enterocyte mass and epithelial damage, n 9; (iv) IL and cytokines, n 22; (v) pathogen recognition receptors, n 14; and (vi) mitochondria function and biogenesis, n 11 (Table 2). Real-time quantitative PCR analysis of target genes was performed in eight specimens from Diets A and C using an iCycler IQ Real-time Detection System (Bio-Rad) as described in Pérez-Sánchez et al.( Reference Pérez-Sánchez, Benedito-Palos and Estensoro 24 ). In brief, each PCR-well contained a SYBR Green Master Mix (Bio-Rad) and specific primers were used at a final concentration of 0·9 μm. DNA polymerase was activated and complementary DNA denatured by pre-incubation for 3 min at 95°C, the template was amplified for forty cycles of denaturation for 15s at 95°C and annealing/extension at 60°C for 60s. All pipetting operations were made with a handling robot (Eppendorf epMotion 5070) to minimise technical variability. β-Actin was chosen as a house keeping gene and no differences in gene expression were found between Diet A (control) and Diet C (experimental group) (cycle threshold (C t ) values of β-actin: 19·72 v. 19·83). The efficiency of PCR reactions for all target and reference genes varied between 90 and 98 %. The dynamic range of standard curves (serial dilutions of RT-PCR reactions) spanned five orders of magnitude, and the amount of product in a particular sample was determined by interpolation of the C t value. The specificity of each reaction was verified by analysis of melting curves. Fluorescence data acquired during the extension phase were ultimately normalised to β-actin by the ΔΔC t method( Reference Livak and Schmittgen 25 ).
Table 2 Full list of genes (abbreviation, gene name and accession number) analysed by real-time PCR in intestinal samples of gilthead sea bream (Sparus aurata) fed experimental diets containing different levels of a bioactive extract of olive oil

Statistical analyses
The mean values of BW f , SL f and K were expressed as means and standard deviations. The calculation was based on the values of the individual BW f , SL and K of all the fish belonging to the same treatment (fish from the four tanks/replicate per dietary treatment analysed together, since there were no statistical differences between replicates), and consequently, the sd describes the dispersion of the individual values. In contrast, the mean values of survival, BW f size classes, SGR, FCR, SFI, intestinal goblet cell number, villi width and height, and size of hepatic lipid vacuoles were calculated from each replicate tank (n 4) and expressed as means with their standard errors. Data expressed as percentage were arcsine square root transformed before being analysed. Data were compared by means of one-way ANOVA (data normally distributed, Kolmogorov–Smirnov test) and comparisons between experimental groups after finding statistical significances were performed by a Bonferroni test. C t values between samples obtained from fish fed Diet A and C were compared by means of a t test. Statistically significant differences were indicated by different letters. Data sets were analysed using the SigmaStat 3® software package (Systat Software Inc.).
Results
Survival, somatic growth performance and feed utilisation parameters
Data on survival, somatic growth performance in terms of BW f , SL f and K, and feed utilisation parameters of gilthead sea bream fingerlings fed experimental diets are shown in Table 3. No differences in survival were found among experimental groups with mean values ranging between 96·0 and 97·5 % (ANOVA, P>0·05). At the end of the trial, BW f values were significantly different depending on the tested diet (ANOVA, P<0·05). In this sense, fish fed Diets C and D displayed the best values in BW f , those fed Diet A the lowest growth performance, whereas fish fed Diets B and E showed intermediate values. On average, fish fed Diets C and D were 5 % heavier than those fed the other diets. Results in BW f were supported by those from the distribution of BW f size classes (Fig. 1). In brief, the greatest proportion of larger animals in terms of BW f (61–80 g) was observed among fish fed Diets C and D (51·7 (se 0·9) and 49·4 (se 0·7) %, respectively), whereas it was only 37·6 (se 1·1), 38·3 (se 1·4) and 39·3 (se 1·5) % in fish fed Diets A, B and E, respectively (ANOVA, P<0·05). However, there were no statistically significant differences in SGR values among groups (ANOVA, P>0·05). In addition, SL f and K values were similar among different experimental groups (ANOVA, P>0·05), and no differences in FCR and SFI were found among groups fed different diets (ANOVA, P>0·05).

Fig. 1 Distribution of final body weight (BW f ) of gilthead sea bream (Sparus aurata) fed experimental diets containing different levels of a bioactive extract of olive oil. Values are mean frequencies for each size category calculated, with their standard errors, from all fish from each replicate. a,b,y,z Mean values with unlike letters denote differences among dietary groups (P<0·001).
Table 3 Survival, growth performance in body weight (BW f ), standard length (SL f ), specific growth rate (SGR) and Fulton’s condition factor (K), feed efficiency parameters (feed conversion ratio (FCR) and specific feed intake (SFI)) and carcass chemical composition (in DM) of gilthead sea bream (Sparus aurata) fingerlings fed experimental diets containing different levels of a bioactive extract of olive-oil (Mean values and standard deviations)

a,b Mean values within rows with unlike superscript letters denote significant differences between dietary groups (ANOVA, P<0·05).
* Based on four replicate tanks per diet with seventy-two to seventy-four fish per tank.
† Mean BW f , SL f and K factor values were calculated using the individual values from all fish within the same dietary treatment (four replicate tanks per diet with seventy-two to seventy-four fish per tank).
‡ Based on twenty fish per diet (four replicate tanks per diet with five randomly selected fish per tank).
Carcass proximate composition, hepatic fat content, and lipid peroxidation and antioxidative stress enzymes in liver and intestine
There were no differences in the carcass composition and hepatic fat content among fish fed experimental diets (ANOVA, P>0·05; Table 3). There were no statistically significant differences in LPO levels and activity of CAT, GR and GST in the intestine and liver of gilthead sea bream fed different diets (ANOVA, P>0·05; Table 4).
Table 4 Levels of lipid peroxidation (LPO) and catalase (CAT), glutathione reductase (GR) and glutathione S-transferase (GST) from the intestine and liver of gilthead sea bream (Sparus aurata) fed experimental diets containing different levels of a bioactive extract of olive oil (Mean values with their standard errors; n 4)

MDA, malondialdehyde.
Histological organisation of the intestinal mucosa and liver
In all experimental groups, the intestine was lined by a simple columnar epithelium with basal nuclei, basophilic cytoplasm and prominent microvilli. The organisation of the lamina propria, submucosa and tunica muscularis was normal. No lipid deposits were found either within enterocytes or in the vascular system, indicating that the lipid content in tested diets did not imbalance the absorptive and transporting lipid capacities of the intestine (online Supplementary Fig. S2). No differences in villus size, width and height were found among treatments (Table 4), whereas the number of intestinal goblet cells was significantly affected by the presence of OBE in the diet (ANOVA, P<0·001; Table 5). In this sense, goblet cell density in fish fed Diet A (1·8 (se 0·05) goblet cells in 100 µm of intestinal epithelium) was on average 14·3 % lower than in fish fed diets containing OBE (treatment mean=2·1 (se 0·05) goblet cells in 100 µm of intestinal epithelium).
Table 5 Size of hepatic lipid deposits (µm2), villi size in height and width (µm) and goblet cell density (number of goblet cells in 100 µm of epithelium) in the intestine of gilthead sea bream (Sparus aurata) fed experimental diets with different levels of an olive oil bioactive extract (Mean values with their standard errors; n 4)
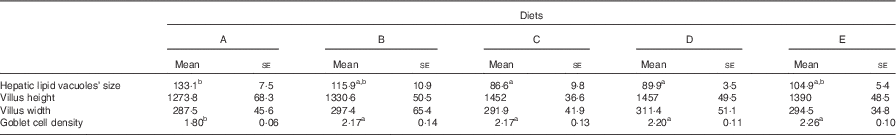
a,b Mean values within rows with unlike superscript letters denote significant differences between dietary treatments (ANOVA, P<0·05).
Under present experimental conditions, the histological organisation of the liver was normal, with hepatocytes arranged along sinusoids. In any case, hepatic steatosis (severe fat accumulation in hepatocytes affecting their integrity and functionality) was not observed in any of the examined fish. Data on the semi-quantitative evaluation of lipid accumulation in hepatocytes of gilthead sea bream fed experimental diets are shown in Table 5. In general terms, fish showed similar levels of hepatic fat accumulation within hepatocytes (online Supplementary Fig. S2); however, the size of lipidic vacuoles and the level of peripheral displacement of nuclei within hepatocytes was slightly lower in fish fed Diets C and D in comparison to the control group (Diet A), whereas the rest of groups showed intermediate values.
Intestinal gene expression profiling
The C t values for all the target genes analysed in this study with regard to the effect of supplementing the diet C with OBE on the integrity and health of the intestine are shown in the online Supplementary Table S1. Among the eighty-eight genes evaluated, twenty-nine were differentially expressed in response to the presence of OBE in the diet (Table 6; t test, P<0·05). Among the thirteen studied genes related to cell differentiation and proliferation, four of them were significantly affected by the diet. In particular, proliferating cell nuclear antigen (Pcna) was the only one down-regulated, whereas bone morphogenetic protein receptor type-1A (Bmpr1a), transcription factor 4 (Tcf4) and Krueppel-like factor 4 (Klf4) were up-regulated in fish fed the OBE diet. Six of the sixteen genes analysed related to intestinal architecture and permeability were up-regulated (occludin (Ocln), Cldm3, claudin 12 (Cldn12), junctional adhesion molecule A (F11r), coxsackievirus and adenovirus receptor homolog (Cxadr) and desmoplakin (Dsp)), whereas the rest of them were not affected by the diet. The expression of calreticulin (Calr) decreased, whereas that of three of the nine genes studied involved in enterocyte function and epithelial damage were up-regulated (intestinal-type alkaline phosphatase (Alpi), liver type fatty acid-binding protein (Fabp1) and Gr). Almost half of the genes coding for IL and cytokines were differentially expressed (10/22); in particular, Il1r1, Il6rb, Il8, Il10ra, Il12b, Tnfα, Csf1r1, Ccr9 and Ccr11 were up-regulated, whereas Il7 was down-regulated. Among the fourteen pathogen recognition receptor genes analysed, four of them (Toll-like receptor 2 (Tlr2), nucleotide-binding protein oligomerisation domain-containing protein 1 (Nod1), CD302 antigen (Cd302) and fucolectin (Fcl)) were up-regulated in the intestinal mucosa of fish fed the OBE diet. Regarding the expression of genes related to mitochondria function and biogenesis, molecular chaperones (mitochondrial 10kDa heat shock protein (mtHsp10), mitochondrial 60kDa heat shock protein (mtHsp60)) and membrane protein translocases (mitochondrial import inner membrane translocase subunit 44 (Tim44), mitochondrial import receptor subunit Tom 22 (Tom22)) were down-regulated in fish fed the OBE diet, whereas an opposite pattern was found for the transcription factor Pgc1.
Table 6 Differentially expressed genes (Student t test, P<0·05) in the intestine of fish fed the control (Diet A) and Diet C containing 1·7 g olive oil bioactive extract per kg feedFootnote * (Mean values with their standard errors; n 8)

Pcna, proliferating cell nuclear antigen; Bmpr1a, bone morphogenetic protein receptor type 1A; Tcf4, transcription factor 4; Klf4, Krueppel-like factor 4; Ocln, occluding; Cldn3, claudin 3; Cldn12, claudin 12; F11r, junctional adhesion molecule A; Cxadr, coxsackievirus and adenovirus receptor homolog; Dsp, desmoplakin; Il1r1, IL 1 receptor type 1; Il6rb, IL 6 receptor subunit β; Il10ra, IL 10 receptor subunit α; Csf1r1, macrophage colony-stimulating factor 1 receptor 1; Ccr9, C–C chemokine receptor type 9; Ccr11, C–C chemokine receptor type 11; Tlr2, toll-like receptor 2; Nod1, nucleotide-binding protein oligomerisation domain-containing protein 1; Cd302, CD302 antigen; Fcl, fucolectin; Mthsp10, mitochondrial 10 kDa heat shock protein; Mthsp60, mitochondrial 60 kDa heat shock protein; Tim44, mitochondrial import inner membrane translocase subunit 44; Tom22, mitochondrial import receptor subunit Tom 22.
* Values>1 are up-regulated genes in fish fed Diet C. Values<1 are down-regulated genes in fish fed Diet C.
Discussion
Previous studies dealing with the inclusion of bioactive compounds derived from olive oil in aqua feeds have been mostly limited to maslinic acid. In particular, it has been shown that a diet supplemented with maslinic acid (250 mg/kg feed) promoted growth in Oncorhynchus mykiss ( Reference Fernández-Navarro, Peragón and Esteban 9 ), whereas similar or lower levels (100 mg/kg feed) of this triterpenic compound did not affect growth performance in gilthead sea bream( Reference Rufino‐Palomares, Reyes‐Zurita and García‐Salguero 10 , Reference Matos, Silva and Wulff 11 ), even though it enhanced hepatic protein-turnover rates( Reference Rufino‐Palomares, Reyes‐Zurita and García‐Salguero 10 ) and induced hypertrophy in muscle fibres were found( Reference Matos, Silva and Wulff 11 ). In addition, no effect on growth performance in Dentex dentex was observed when using higher doses of maslinic acid (20, 40, 80 g/kg feed)( Reference Hidalgo, Skalli and Abellán 26 ). Under present experimental conditions, the inclusion of OBE slightly enhanced growth independently of feed intake without affecting neither feed efficiency parameters nor the proximate composition of the fillet. The effect of OBE on growth in body weight was not-linear, as the highest mean values of BW f were observed in fish fed 0·17 and 0·42 % of OBE and not in those fed the highest inclusion of OBE in diets (0·73 %), which indicated a quadratic growth response to OBE inclusion in diets for gilthead sea bream. In addition, the inclusion of OBE in diets affected the final fish size distribution in BW, resulting in a more homogeneous fish size distribution in those groups fed the OBE diets, although the rationale of these findings with regard to the administered diet needs to be further investigated. However, these findings are of practical importance since the use of OBE in diets might reduce the effort required for size selection during processing of production lots, and also in a reduction of hierarchical dominance situations( Reference Petursdottir 27 , Reference Skalli, Castillo and Andree 28 ). Although these results might be partially attributed to the potential growth-promoting effect of polyphenols( Reference Chakraborty, Horn and Hancz 29 ), our data suggest that they were most likely related to the enhancement of the health condition of the intestinal mucosa as described below.
The intestine is involved not only in digestion and feed absorption but also in water and electrolyte balance, nutrient sensing and immunity( Reference Lallès 30 ). This functional diversity is now starting to be elucidated in fish and different histological and molecular approaches are helping to understand the many vital functions conducted along the gastrointestinal tract( Reference Rønnestad, Yúfera and Ueberschär 31 , Reference Calduch-Giner, Sitjà-Bobadilla and Pérez-Sánchez 32 ). The inclusion of OBE in the diet resulted in an increase of the goblet cell population in the intestinal epithelium. This histological observation was supported by the up-regulated expression of the zinc-finger transcription factor Klf4, a goblet-cell specific intestinal differentiation factor( Reference Katz, Perreault and Goldstein 33 ). The increase in goblet cell number would benefit fish by providing an effective immune barrier against potentially pathogenic gut bacteria( Reference McGuckin, Lindén and Sutton 34 ), which also agrees with an enhanced expression of several pathogen recognition receptors (Tlr2, Nod1, Cd302, Fcl). In higher vertebrates, it has been demonstrated that TLR4- and TLR2-dependent pathways can stimulate β-defensin-2 expression by intestinal epithelial cells that are able to respond to pathogen-associated molecular patterns (PAMP) by secreting antimicrobial peptides, as well as protecting the epithelium from injuries( Reference Abreu, Fukata and Arditi 35 ) and reducing intestinal permeability by protecting tight junctions( Reference Smith, MacDonald and Blumberg 36 ). In addition, NOD1-mediated innate immune responses are critically involved in the intestinal homoeostasis of higher vertebrates( Reference Abreu, Fukata and Arditi 35 ), where epithelial cells remain responsive to invasive bacteria via ligand binding to NOD1 or NOD2( Reference Smith, MacDonald and Blumberg 36 ). Similarly, the CD302 gene encodes for a C-type lectin receptor involved in cell adhesion and migration, as well as in endocytosis and phagocytosis processes( Reference Kato, Khan and Gonzalez 37 ). It is noteworthy that the highest fold change in expression among pathogen recognition receptors was found in fcl, a gene coding for fucolectin that has been described to enhance phagocytosis in in vitro studies with peritoneal macrophages in Dicentrarchus labrax ( Reference Watanabe, Naganuma and Ogawwa 38 ).
The inclusion of the OBE in the diet affected gene expression of approximately half of the IL and cytokines analysed in this study. The up-regulation of a vast array of pathogen recognition receptors and pro-inflammatory IL, IL receptors and cytokines (Il1r1, Il6rβ, Il12, Il8, Tnfα, Csf1r1, Ccr9 and Ccr11) highly supports an immunostimulatory action( Reference Schweickart, Epp and Raport 39 ) of OBE when included at 0·17 % of the diet. Early elimination of potential pathogens has clear implications for improved health, but also for the bioenergenetic balance of the whole animal; much less energy is expended when pathogens are removed before they have opportunities for proliferation. Further, the enhanced mucin production due to increased goblet cell populations provides a physical displacement of potential pathogenic organisms; a more diverse microbiota leads to a thickening of the mucus layer and this improves the microniches of the gut inhabited by these beneficial bacteria( Reference Li, Limenitakis and Fuhrer 40 ). In this sense, further research is needed to evaluate the potential effects of OBE in modulating the microbiota.
The homoeostasis of the constantly renewing intestinal epithelium relies on an integrated control of proliferation, differentiation and apoptosis, as well as on the functional architecture of the epithelial cells. Thus, the down-regulation of pcna in fish fed OBE at 0·17 % of the diet in comparison to the control group might be attributed to lower epithelial turnover rate associated with a better health condition of enterocytes, which was in agreement with the higher transcription of the bmpr1a that codes for the bone morphogenetic protein receptor, type 1 A that plays a major role in cell differentiation. This hypothesis is supported by the up-regulation of Tcf4 in this group of fish, as this transcription factor is a key player in the Wnt pathway signalling and maintaining the homoeostasis of the intestinal epithelium( Reference van Es, Haegebarth and Kujala 41 ). However, BMPR1A inhibits WNT signalling. Thus, although both inhibitory (Bmpr1a) and stimulatory (Tcf4) signals of stem cell proliferation were up-regulated by the OBE diet, the net result would be prone to promote cell differentiation rather than cell proliferation.
Enhanced terminal cell differentiation would have a variety of potentially beneficial consequences, but one possible is the observed increase in goblet cells. Prevention of the entrance of toxic molecules or infectious agents, such as solutes, antigens and micro-organisms, is ensured by the gastrointestinal mucosa. A key structure of the intercellular space is the tight junction, which plays a major role in regulating the paracellular passage of luminal elements. Therefore, proper functioning and regulation of tight junctions is crucial. These junctions are under the influence of intestinal microflora, inflammation and even alimentary components, which can compromise tight junctions( Reference Schneeberger and Lynch 42 ). Three of the main protein families found in tight junctions are occludins, claudins and junction-associated membrane proteins( Reference Turner 43 ). In particular, OBE may reduce intestinal permeability and consequently, the risk of intestinal disorders by increasing the expression of genes coding for mucosal tight junction proteins like occludin, claudin 3 and 12, and F11 receptor, the so-called junctional adhesion molecule A, as well as desmoplakin that is involved in maintaining the desmosome structure.
In addition, the inclusion of OBE at 0·17 % in the diet improved the condition of enterocytes by reducing the expression of several gene markers related to mitochondrial function (mtHsp10, mtHsp60, Tim44, Tom22). In particular, TOM22 and TIM44 proteins are involved in the translocation of transit peptide-containing proteins from the outer and inner mitochondrial membranes( Reference Saeki, Suzuki and Tsuneoka 44 , Reference Wang, Katayama and Terami 45 ), whereas PGC1α is a transcriptional co-activator that is involved in controlling global oxidative metabolism by regulating mitochondrial biogenesis and function( Reference Austin and St-Pierre 46 ). Furthermore, OBE induced the down-regulation of mtHsp60 and mtHsp10, two genes encoding for UPRmt-responsive proteins that are involved in protein homoeostasis in mitochondria( Reference Arnould, Michel and Renard 47 ). Thus, the down-regulation of the above-mentioned genes coupled with the increase in expression of pgc1α may correlate with a lower metabolic rate of mitochondria, protein turnover and potentially lower oxidative stress in the intestinal mucosa of fish fed the OBE diet( Reference Adhihetty, Irrcher and Joseph 48 ). Indeed, this suggestion is in line with the tendency found for fish fed the OBE diets to show lower LPO values (P=0·091) in comparison with the control group.
Regarding the expression of genes involved in enterocytes’ function and epithelial damage, 45 % of the analysed genes were differentially expressed when fish were fed the diet containing OBE at 0·17 % of the diet. In particular, Alpi regulates lipid absorption across the apical membrane of enterocytes, participates in the regulation of bicarbonate secretion and duodenal surface pH, limits bacterial transepithelial passage and finally protects against bacterial endotoxin-induced inflammation by dephosphorylating lipopolysaccharides( Reference Lallès 30 ). Thus, the up-regulation of Alpi may indicate a more mature enterocyte in fish fed the OBE diet( Reference Zambonino-Infante and Cahu 49 ), as well as reinforce the idea of an enhanced intestinal immune function( Reference Skalli, Castillo and Andree 28 ). In addition, the higher expression of Alpi and Fabp1 might be related to their role in fatty acid uptake from the lumen of the intestine and trafficking within the enterocyte( Reference Gajda and Storch 50 ). CALR is reported to play a role in many cellular functions including lectin-like chaperoning, Ca2+ storage and signalling, regulation of gene expression, cell adhesion, wound healing and autoimmunity( Reference Gelebart, Opas and Michalak 51 , Reference Michalak, Groenendyk and Szabo 52 ); thus, the down-regulation of Calr in fish fed the OBE diet is in agreement with the proposed decrease in intestinal epithelial permeability and overall improvement of intestinal health, as it has been recently reported in Ictalurus punctatus ( Reference Liu, Peatman and Wang 53 ).
Regarding the impact of the feed additive on the liver, present results revealed that the inclusion of OBE reduced the size of hepatic deposits within hepatocytes, which may be in agreement with the tendency for lower LPO values in the liver of fish fed diets containing OBE. These results were in agreement with in vitro ( Reference Hou, Xu and Maitland-Toolan 54 ) and in vivo ( Reference Zang, Maitland-Toolan and Zuccollo 55 ) models that have shown that polyphenols reduce hepatocellular lipid accumulation by means of the regulation of the AMP-activated protein kinase signalling and hepatocyte lipid metabolism. In addition, triterpenic acids have been also reported to be involved in regulating hepatic lipid accumulation and reducing hepatic steatosis( Reference Li, Meng and Liao 56 ), stimulating liver protein-synthesis rates( Reference Fernández-Navarro, Peragón and Esteban 9 ), as well as regulating proteins involved in liver metabolism and detoxification processes( Reference Rufino-Palomares, Reyes-Zurita and García-Salguero 57 –59). However, further research is needed in fish model species in order to properly characterise by means of transcriptomic and physiological approaches the impact of OBE in the health condition and metabolism of the liver, especially in fish fed diets with a high level of fish oil substituted by vegetal oil sources.
Conclusions
The inclusion of OBE in the diet had a positive effect on growth performance in gilthead sea bream juveniles. This effect depended on the dose, becoming significant when OBE was included at 0·17 and 0·42 % of the diet. The inclusion of OBE reduced the size of hepatic deposits within hepatocytes, which may be in agreement with the tendency for lower LPO values in the liver of fish fed diets containing OBE, suggesting that OBE may positively affect lipid metabolism in the liver and potentially prevent hepatic steatosis. Results from the transcriptomic profiling of the intestine suggested that OBE enhanced fish growth primarily by improving the condition and defensive role of the intestine. When OBE was fed at 0·17 % of the diet such a response appeared to involve augmented enterocytes’ maturation, increased integrity of the intestinal epithelium and goblet cell density, and improved intestinal innate immune function, as gene-expression data indicated. These results showed that OBE is a promising feed additive for the aquaculture industry with multiple beneficial properties.
Acknowledgements
The authors express their gratitude to S. Molas, M. Sastre and O. Bellot IRTA for their assistance during the trial, and biochemical and histological analyses, respectively. The authors are grateful to M. A. González (CSIC) for assistance in PCR analyses.
This study was funded by the ‘Ministerio de Economía y Competitividad’ (MINECO) from the Government of Spain (project BIOADA, reference no. RTC-2014-2018-2).
Conceived and designed the experiments: J. C. Q., E. G. Analysed the data: J. P.-S., E. G., K. B. A., J. A. C.-G. Fish husbandry and fish sampling: E. G., K. B. A. Manuscript preparation: J. C. Q., J. P.-S., E. G., K. B. A., J. A. C.-G., I. R. I.
The authors declare that there are no conflicts of interest.
Supplementary material
For supplementary material/s referred to in this article, please visit https://doi.org/10.1017/S0007114517000228










