Vitamin A deficiency (VAD) is a public health problem of paramount relevance. Its growing prevalence has been warned since the 1990s(Reference Ferraz, Daneluzzi and Annucchi1–Reference Sommer5).
Vitamin A is vital during the initial stages of life. Its role goes beyond embryonic development, tissue homeostasis, lipid metabolism and cellular differentiation and proliferation. Human placentae express factors for the nuclear transcription of retinoic acid receptors and retinoic X receptors. Modulation of these factors by retinoic acid is capable of modulating the expression of several genes such as: chorionic gonadotrophic hormone; placental lactogenic hormone; leptin; epidermal growth factor receptor; triiodothyronine; oestrogen; progesterone; cortisol; aldosterone; testosterone; vitamin D; cholesterol; fatty acids(Reference Sarni, Kochi and Ramalho7–Reference Burri9).
In 1996, the WHO underscored the need for proposed guidelines on proper selection, use and interpretation of indicators, not just to map deficiency but also to propose programmes to assess the impact of interventions to control VAD.
The placenta is the only organ composed of cells from two distinct individuals(Reference Iyengar and Rapp10). So far, no studies have been done to evaluate retinol and carotenoid concentration in the placenta and its relation with the nutritional state of the mother and the child. Some authors describe the presence of receptors for the vitamin in the brush border membrane of the placenta, implying that the placenta may have a regulatory mechanism(Reference Barnes11–Reference Sundaram, Sivaprasadarao and De Sousa13).
In this scenario, the objective of the present study was to evaluate the association between serum and placental concentration of vitamin A and to propose values of placental retinol representing VAD.
Methodology
Population and sample
The population studied was made up of low-risk puerperal women, who received antenatal care services at the maternity hospital of the Universidade Federal do Rio de Janeiro, being 262 women chosen according to the following criteria: single-child pregnancy; absence of clinically proven pathologies identified before gestation (diabetes mellitus and liver, heart or kidney diseases) or no use of vitamin–mineral supplementation containing vitamin A during gestation.
Collection and analysis of placenta samples
Obtaining the placentae as well as their weighing were performed immediately postpartum after separation of the newborn(Reference Thomson, Billewicz and Hytten14, Reference Saunders, Leal and Flores15). Before obtaining placentae samples, the amniochorionic membrane and the umbilical cord were separated. The collection was carried out by using a surgical scalpel in a dimly lit environment(Reference Saunders, Leal and Flores15, Reference Barreto-Lins, Campos and Azevedo16). Treatment, storage and transportation of the samples were carried out according to procedures described by Saunders et al. (Reference Saunders, Leal and Flores15).
Biochemical evaluation of vitamin A nutritional status
To determine the concentration of maternal and cord blood retinol and total carotenoids, 5-ml samples of blood were collected intravenously from the puerperal women fasting for 8 h, as well as from the newborns' umbilical cord immediately postpartum(Reference Saunders, Leal and Flores15, Reference Ramalho, Anjos and Flores17). The blood samples obtained were centrifuged (3000 rpm) to separate and extract the serum and were immediately frozen at a temperature of − 20°C at the laboratory of the ME/UFRJ. Thereafter, all the samples were packaged in order to guarantee that the temperature was maintained during transportation to the INJC/UFRJ, where they were kept frozen until the moment the retinol and carotenoids concentration was analysed at the Institution's Biochemical Laboratory.
Biochemical quantification
Determination of serum retinol and carotenoid concentration was performed through spectrophotometric analysis based on the Bessey et al. (Reference Bessey, Lowry and Brock18) method modified by Araujo & Flores(Reference Araújo and Flores19) and in accordance with procedures adopted by Flores et al. (Reference Flores, Ramalho and Ribeiro20) for dosing the hepatic vitamin A. All the samples were analysed in duplicate, following the precautionary measures recommended by the International Vitamin A Consultative Group, in order to assure sample quality before analysis(Reference Barreto-Lins, Campos and Azevedo16, Reference Arroyave, Chichester and Flores21). For a sample of nine placental portions, vitamin A concentration was also determined by HPLC(Reference Hess, Keller and Oberlin22).
Cut-off points of 0·7 and 1·05 μmol/l were adopted to indicate VAD(Reference Christian, West and Khatry23–26). To indicate carotenoid insufficiency, cut-off points of < 800 μg/l for the puerperal women(Reference Sauberlich, Apud Oliveira and Marchini27) and < 400 μg/l for the newborns(Reference Sauberlich, Apud Oliveira and Marchini27, Reference Robles-Sardin, Astiazarán-Gracia and Dávalos-Navarro28) were adopted.
Treatment of statistics
Outlier retinol values (defined as mean ± 3 sd) were identified in two blood and seven placenta samples. All the samples originated from the blood and placentae in which these extreme values detected were excluded from the final analysis.
The Student t test was used to compare means. The log transformation was used to approximate variables to the normal distribution. The paired t test was used to compare biochemical methods. The receiver operating characteristic curve was used to establish the placental retinol and carotenoid concentration representative of their serum concentration through sensitivity and specificity evaluation for each cut-off point. The best optimal point was determined to be the one, which maximised the sensitivity and specificity values. The level of significance established was P < 0·05. Statistical analysis was performed using the statistical program SPSS for Windows version 15.0 (SPSS, Chicago, IL, USA).
Ethical issues
The study was carried out through an institutional accord between the Nucleus of Micronutrient Research of Josué de Castro Institute of Federal University of Rio de Janeiro (NPqM/INJC/UFRJ) and the maternity hospital (ME/UFRJ). Data collection took place after approval by the ethics commission of the said maternity school and the ethics committee of the Escola Nacional de Saúde Pública of Fundação Oswaldo Cruz, Rio de Janeiro, Brazil.
Results
The puerperal participants in the study were on average of 26 (sd 5·8) years old, presented an average pre-pregnancy weight of 55·2 (sd 9) kg and total weight gain of 12·9 (sd 5·7) kg. Their newborns presented birth weights of 3·27 (sd 0·45) kg and the placentae weighed on average of 0·640 (sd 0·144) kg. Gestational duration was 39 (sd 1·6) weeks.
According to the results shown in Tables 1 and 2, a decrease in concentration in placental retinol within the VAD margins was observed for both the mother and the newborn, regardless of the cut-off point adopted.
Table 1 Placental retinol and total carotenoid averages according to maternal and newborn vitamin A nutritional state
(Mean values and standard deviations)
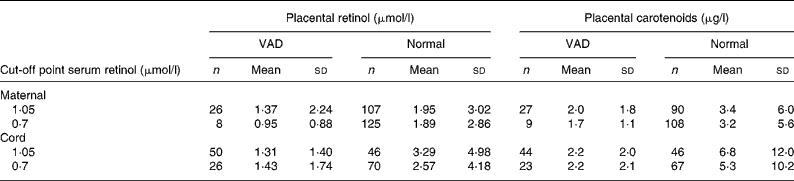
VAD, vitamin A deficiency.
Placental retinol and total carotenoids means were compared according to vitamin A status classified by serum retinol cut-off points (1·05 and 0·70 μmol/l) for mother and newborn. Vitamin A status was defined as VAD and normal according to each cut-off point. Placental retinol and total carotenoids means were then calculated for each group.
Table 2 Comparison of placental retinol and total carotenoid averages after logn transformation according to maternal and newborn vitamin A nutritional state
(Mean values and standard deviations are presented as logn transformation)
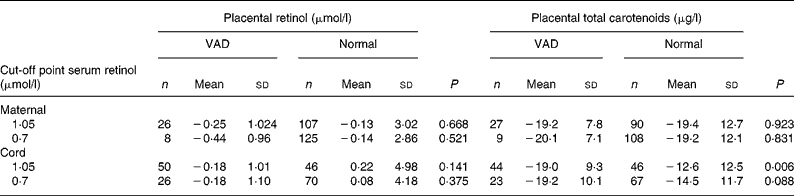
VAD, vitamin A deficiency.
Placental retinol and total carotenoids means were compared according to vitamin A status classified by serum retinol cut-off points (1·05 and 0·70 μmol/l) for mother and newborn. Vitamin A status was defined as VAD and normal according to each cut-off point. Placental retinol and total carotenoids means were then calculated for each group.
Regarding carotenoids, the drop was also observed in newborns as there is a statistically significant difference between the placental carotenoid averages regardless of the cut-off point.
Analysis of the receiver operating characteristic curve was carried out for the placental concentrations of retinol according to the two cut-off points for classifying VAD both for the mother and the newborn. Values for the placental concentrations of retinol of < 0·80 μmol/l were adopted as predictors of inadequate serum concentration according to values of specificity, sensitivity and the area under the curve (accuracy) (Table 3) presented. It was observed that sensitivity increases as the cut-off point for serum concentrations is lowered, in other words, as the VAD is aggravated. Additionally, regardless of the cut-off point adopted to classify serum concentration of retinol, the sensitivity and specificity results show increases in the newborn when compared with the puerperal woman. The best accuracy value (65 %) was found for the curve made from the second 0·70 μmol/l cut-off point to identify puerperal deficiency.
Table 3 Sensitivity and specificity results according to serum cut-off points for vitamin A deficiency adopting the placental cut-off point 0·80 μmol/l according to analysis of the receiver operating characteristic curve
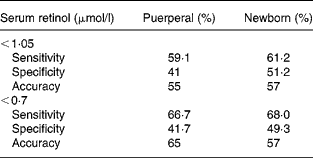
A receiver operating characteristic curve taken from the placental concentrations of carotenoids did not permit the adoption of any value that could represent their serum inadequacy.
No difference was found between the values obtained in retinol concentration with the spectrophotometric and with the HPLC analytical methods (P = 0·318). The spectrophotometric method may be an alternative when HPLC is not available.
Discussion
The placenta is able to esterify retinoid and produce active retinoid by means of retinol, thus allowing it to produce the active metabolites it needs(Reference Marceau, Gallot and Borel6). The present study aims to evaluate the association between serum and placental concentration of vitamin A and propose a placental retinol value representing VAD.
An association between average concentrations of total placental carotenoids according to fetal nutritional states of vitamin A was found. Although the analysis of the receiver operating characteristic curve from the placental retinol concentration has shown not to predict sub-clinical deficiency, it was noted that sensitivity and specificity values increased when the cut-off point was lowered from 1·05 to 0·70 μmol/l. This fact may be interpreted as the placental vitamin A content being more related to a severer state of VAD.
In this sense, evaluation of the curve with the cut-off points at different stages of severity of the deficiency illness in question is necessary. Such an approach was not carried out in the present study, due to the fact that there were not a large enough number of grave VAD cases (according to the WHO's cut-off points, 1996)(26) to create the curve. The same phenomenon was also noted for sensitivity and specificity values when comparing puerperal women and newborns, the results tend to be more expressive in the newborns.
In states of privation, retinol is the priority ahead of provitamin A carotenoids, being the latter converted to vitamin A as needed. It is known that the enzyme β-carotene 15,15′-monooxygenase, responsible for splitting the β-carotene molecules into two retinal molecules, is present in the fetal part of the amniotic membrane of the human placenta(Reference Marceau, Gallot and Borel6, Reference Morriss-Kay and Sokolova29). This fact may account for the better association of placental concentrations with the serum concentrations of newborns, besides justifying the difficulty in finding placental concentrations of carotenoids to represent both the maternal and the newborn serum concentrations.
The placenta appears to be a possible indicator of vitamin A status for women and their newborns and could be used to determine the prevalence of VAD. On the other hand, during the puerperal period, the greatest transfer of vitamin A to the neonate takes place through breastfeeding. Thus, this organ may also contribute to the development of treatment strategies to prevent transmission of the afore-mentioned deficiency.
The results of the present study point to an association between vitamin A nutritional state and the placental concentrations of retinol and carotenoids. The present study using the placenta as a marker for VAD suggests the need for further studies to assess additional cut-off points for severe privation and to define cut-off points for the placental concentrations.
Although spectrophotometric method is not the best for vitamin A dosing, the present study analysed a sub-sample with both the spectrophotometric and the HPLC methods. Spectrophotometrics seemed to be an alternative method when HPLC is not available. Unfortunately this analysis could not cater for all the cases studied. So we recommend further studies on this topic.
Acknowledgements
The authors want to express their gratitude to: the researchers and volunteers who participated in the present study; the Board of directors of the maternity hospital of Universidade Federal do Rio de Janeiro that made the study possible; the Conselho Nacional de Desenvolvimento Científico e Tecnológico – CNPq; the Fundação de Amparo à Pesquisa do Estado do Rio de Janeiro Carlos Chagas Filho – FAPERJ; the Secretaria Estadual de Saúde do Rio de Janeiro for financial support. M. M. G. participated in data collection and analyses; C. S. supervised the fieldwork and data collection and participated in study design; A. R. participated in study design. All the authors participated in manuscript preparation. None of the authors had a personal or financial interest to declare.




