A plant-based diet has been recommended for preventing obesity, diabetes, CVD, cancer and other chronic diseases(Reference Fraser1–Reference Martinez-Gonzalez, Sanchez-Tainta and Corella3). While a plant-based diet refers to a diet rich in vegetables, fruits and whole grains and low in red and processed meats in dietary recommendations(4), it may also be interpreted as the reduction or elimination of animal foods to various degrees. For example, a vegan diet refers to the complete elimination of animal foods from the diet, a lacto-ovo-vegetarian diet refers to the elimination of all animal foods except for dairy products and/or eggs, a pesco-vegetarian diet refers to the elimination of meat but not fish (e.g. fish eaters) and a semi-vegetarian diet refers to the reduction of meat consumption (e.g. occasional or low meat eaters)(Reference Clarys, Deliens and Huybrechts5).
Whether eliminating or reducing animal foods from diet confers health benefits remains controversial. The Adventist Health Study 2, where over half of the participants had no or low consumption of animal foods from their diet (7·6 % vegan, 28·9 % lacto-ovo-vegetarian, 9·8 % fish eaters and 5·5 % occasional meat eaters), found a 12 % lower risk of all-cause mortality in those who had no or low consumption of animal foods from their diet compared with those who consumed animal foods(Reference Orlich, Singh and Sabate6). The European Prospective Investigation into Cancer and Nutrition-Oxford Cohort and the Oxford Vegetarian Study, however, found no difference in all-cause mortality between those who consumed no meat or fish and those who consumed meat and/or fish(Reference Appleby, Crowe and Bradbury7). When vegetarian diets were compared by subcategories, Adventists who were fish eaters had 19 % lower all-cause mortality compared with non-vegetarian Adventists, and fish eaters in the two UK cohorts also had a 19 % lower risk of cancer mortality(Reference Orlich, Singh and Sabate6). These findings suggest that the quality of animal food may play a more important role in health outcomes than simply reducing or eliminating animal foods from the diet.
Similarly, not all plant-based foods are equal in their nutrient contents and associations with health. For example, consumption of healthful plant-based foods (e.g. vegetables, fruits, whole grains, nuts/seeds and legumes) has been associated with a lower risk of CHD, diabetes and all-cause mortality, whereas consumption of less healthful plant-based foods (e.g. refined grains, white potatoes and sugar-sweetened beverages) has been associated with a higher risk(Reference Jenkins, Kendall and Marchie8–Reference Malik, Popkin and Bray12).
Previous studies that assessed the quality of plant-based foods did not jointly distinguish the quality of animal foods. Among a nationally representative sample of US adults, we evaluated whether the quality of plant-based foods, animal foods or both is associated with mortality by using a Comprehensive Dietary Quality Index (cDQI) that distinguishes the quality of both plant-based and animal foods. We further explored whether associations between the cDQI and mortality differ by age, sex, income, weight status, levels of physical activity and co-morbidity conditions at baseline.
Materials and methods
Participants
The present study utilised data from the National Health and Nutrition Examination Survey (NHANES) from 1999 to 2014 and included 36 825 individuals aged 20+ years who completed at least one valid 24-h diet recall. NHANES is conducted biannually by the National Center for Health Statistics and is designed to assess the health and nutritional status of adults and children in the USA. Written informed consent was obtained from all participants at the time of enrollment in the study to allow for their data to be used for research purposes. Ethical approval for NHANES was granted by the National Center for Health Statistics research ethic review board.
The average response rate of NHANES survey during the study period was 70·6 %, ranging from 56·3 % in the 2015–2016 cycle to 78·3 % in the 2001–2002 cycle(13). Individuals with at least one valid diet recall were included in the present study. For this analysis, we excluded pregnant and lactating women (n 1550), individuals with potentially unreliable dietary intake, defined as total energy intake exceeding three standard deviations above and below the mean value of the natural log-transformed energy intake (n 209), individuals with no linked mortality data (n 48) and those who died within 12 months of dietary assessment (n 349). These exclusions left 36 825 individuals aged 20+ years as the final study population. We used NHANES sampling weights in all analyses, which account for the complex survey design, oversampling of minorities and survey nonresponses. The dietary sampling weights additionally account for the dietary interview-specific nonresponse and day of the week for dietary intake interviews(14).
Dietary intake
In-person 24-h recalls conducted by trained interviewers at a Mobile Examination Center are used to determine intake in NHANES participants. From 1999 to 2002, one dietary recall was conducted with participants (in-person at the Mobile Examination Center). From 2003 onwards, a second recall was included, which was carried out by telephone 3–10 d after the initial in-person recall. About 68 % of the participants who provided the first diet recall also provided the second recall during the study period (1999–2014), with the percentage being 92, 90, 85, 86, 89 and 87 % from 2003 and onwards. Both of these recalls employed the Automated Multiple Pass Method and used standard measuring guides to ensure that all food and beverage consumed on the previous day were recorded. These dietary records were then coded. The United States Department of Agriculture Food Patterns Equivalents Database and MyPyramid Equivalents Database, which disaggregate mixed foods into their component parts, were harmonised and used to assess intake of major food groups. Food groups were further disaggregated into subgroups to evaluate subtype (e.g. vegetables were further disaggregated into dark green vegetables v. white potatoes). Nutrients were estimated based on cycle-specific versions of the United States Department of Agriculture Food and Nutrition Database for Dietary Studies(15). To correct for measurement error associated with the use of 1- or 2-d diet recalls to estimate usual intake, we used the National Cancer Institute method to adjust for usual intake estimates. The method also uses regression calibration to correct for bias due to the measurement error in evaluating associations between usual intake and health outcomes(Reference Dodd, Guenther and Freedman16–Reference Herrick, Rossen and Parsons18).
Comprehensive Diet Quality Index
To assess the quality of both plant- and animal-based food components of the diet, we developed a de novo cDQI score based on a previously validated plant-based dietary index(Reference Satija, Bhupathiraju and Rimm11,Reference Satija, Bhupathiraju and Spiegelman19) . The plant-based dietary index distinguishes the quality of plant-based foods in its scoring but scores all animal foods reversely. The new cDQI additionally assesses the quality of animal foods by scoring positively for healthful animal foods and reversely for unhealthful animal foods. The selection and scoring of animal foods is based on meta-analyses of prospective cohort studies and randomised intervention trials with strong evidence base, including the Third Expert Report of the World Cancer Research Fund/American Institute for Cancer Research(20) and the evidence review of the Global Burden of Disease Nutrition and Chronic Disease Expert Group(Reference Micha, Shulkin and Penalvo21). For example, processed meats and red meats were included as unhealthful animal foods and scored reversely, whereas fish/seafood, dairy products and poultry are included as healthful animal foods and scored positively. Egg was included as an unhealthful animal food based on the most recent evidence(Reference Zhong, Van Horn and Cornelis22).
The cDQI has seventeen components, including eleven plant-based foods and six animal foods (online Supplementary Table S1). Dietary intake of each food component was adjusted for total energy intake using the density method. For healthful plant-based foods (whole grains, vegetables excluding white potatoes, whole fruits, nuts/seeds/legumes, vegetable oils and coffee/tea) or animal foods (fish/seafood, dairy products and poultry), a score of 0 is assigned for no intake or lowest quintile, and the scores increase proportionately as intakes increase. For unhealthful plant-based (refined grains, fruit juices, sugar-sweetened beverages and sweets/desserts) or animal foods (processed meats, unprocessed red meats and eggs), levels of intakes at the recommended level or lowest quintile are assigned the maximum score, and the scores decrease proportionally as intakes increase. The scoring standards were adapted from those used in the Health Eating Index-2015(Reference Krebs-Smith, Pannucci and Subar23), Alternative Healthy Eating Index(Reference Chiuve, Fung and Rimm24) and the American Heart Association score based on the 2020 Strategic Impact Goals for Health(Reference Rehm, Penalvo and Afshin25). For food components not included in Health Eating Index-2015, Alternative Healthy Eating Index and American Heart Association scores, quintiles were used as the scorning standards, similar to those used to score the plant-based dietary index(Reference Satija, Bhupathiraju and Spiegelman19). Separately, the total score for plant-based Diet Quality Index (pDQI) is the sum of the eleven plant-based food components, ranging from 0 to 55, and the total score for animal-based Diet Quality Index (aDQI) is the sum of the six animal components, ranging from 0 to 30. The total cDQI total score, combining both plant- and animal-based components, ranges from 0 to 85. A higher pDQI, aDQI and cDQI score indicates a higher quality of plant-based foods, animal foods and both foods, respectively (Table 1).
Table 1. Components, mean intake, scoring standards and mean score for the comprehensive, plant-based and animal-based Diet Quality Index among US adults aged 20 + years, National Health and Nutrition Examination Survey (NHANES) 1999–2014 (Mean values and standard deviations; 95% confidence intervals)
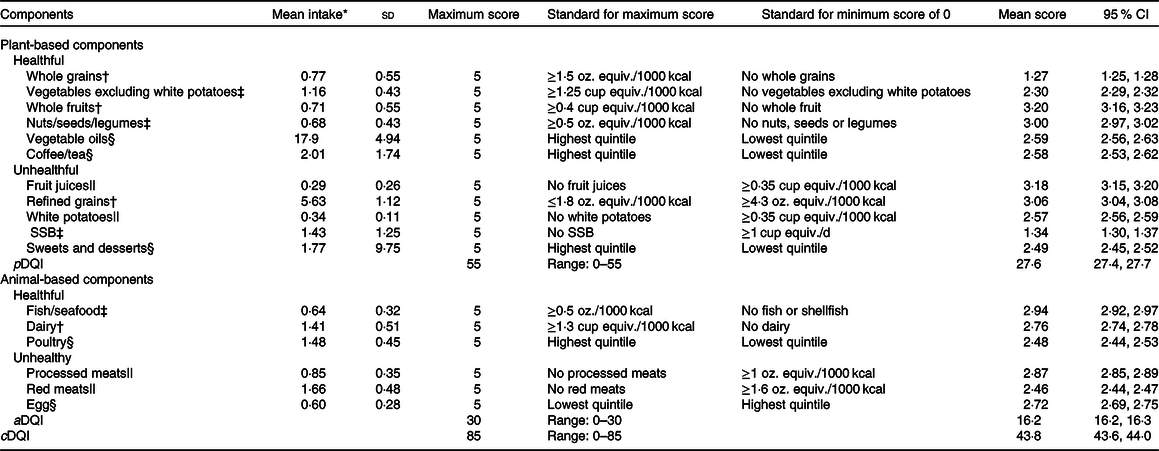
SSB, sugar-sweetened beverages; pDQI, plant-based Diet Quality Index; aDQI, animal-based Diet Quality Index; cDQI, Comprehensive Diet Quality Index.
* Units are oz. equiv. for mean intake of whole grains, nuts/seeds/legumes, refined grains, fish/seafoods, poultry, processed meats, red meats and eggs; cup equiv. for mean intake of whole fruits, fruit juices, vegetables excluding white potatoes, white potatoes and dairy products; 8-fluid oz. cup for mean intake of coffee/tea and SSB; and serving for mean intake of sweets and desserts; gram for vegetable oils. The conversion factors for conventional unit to metric unit vary by foods and food groups(27). The approximate conversion factors are one cup of equivalent fruits or vegetables is 100 g; one cup of equivalent legumes is 175 g; 1 oz. of equivalent whole or refine grains is 30 g; 1 oz. of equivalent fish/seafood, poultry, processed meat, unprocessed red meat or nuts/seeds is 28·35 g; one cup of 8-fluid oz. sugar-sweetened beverages, coffee or tea is 226·8 g and one serving of sweets and desserts is 30 g.
† Scoring is based on scoring standards used in the Healthy Eating Index (HEI)-2015(Reference Krebs-Smith, Pannucci and Subar23).
‡ Scoring is based on scoring standards using in the Alternative Healthy Eating Index (AHEI) adjusted to per 1000 kcal(Reference Chiuve, Fung and Rimm24).
§ Scoring is based on the scoring standards used in the plant-based diet index (PDI) by Satija et al.(Reference Satija, Bhupathiraju and Spiegelman19). The quintiles were Q1 = 13·0, Q2 = 15·5, Q3 = 17·6, Q4 = 19·8 and Q5 = 22·6 for vegetable oils (g/2000 kcal (to convert energy values from kcal to kJ, multiply it by 4·184)); Q1 = 0·38, Q2 = 0·93, Q3 = 1·68, Q4 = 2·38 and Q5 = 3·43 for tea/coffee (cup equiv. per 2000 kcal); Q1 = 6·8, Q2 = 2·5, Q3 = 2·0, Q4 = 1·65 and Q5 = 1·34 for sweets/desserts (serving per 2000 kcal); Q1 = 1·05, Q2 = 1·22, Q3 = 1·40, Q4 = 1·61 and Q5 = 1·90 for poultry (oz. equiv. per 2000 kcal); and Q1 = 2·63, Q2 = 0·85, Q3 = 0·65, Q4 = 0·53 and Q5 = 0·43 for eggs (oz. equiv. per 2000 kcal).
|| Scoring is based on the scoring standards used in the American Heart Association (AHA) diet score based on the AHA 2020 Strategic Goals for Diet(Reference Rehm, Penalvo and Afshin25), corresponding to 80th percentile of intake among US adults in NHANES 1999–2014.
Mortality
The primary outcome was mortality from all causes and the secondary outcomes were mortality from heart disease and cancer. Mortality outcomes were identified from linkage to the National Death Index up to 31 December 2015(Reference Menke, Muntner and Batuman26). Death from heart disease was defined as I00–I09, I11, I13 and I20–I51 being the underlying cause of death using the International Statistical Classification of Disease, 10th revision, and death from cancer was defined as C00–C97 being the underlying cause of deaths. Other cause-specific mortality was not assessed due to the small number of deaths due to other specific causes. Follow-up length was defined as the interval of time from the 24-h recall interview to the date of death for those individuals who had died or to the 31 December 2015 for those participants who were censored.
Demographic, lifestyle and co-morbidity conditions
Demographic and lifestyle factors including age, sex, race/ethnicity, education and income were collected during household interview. Alcohol intake, smoking, physical activity, body weight and height were obtained among participants who received physical examinations in a Mobile Examination Center. Race and ethnicity were categorised as non-Hispanic whites, non-Hispanic blacks, Hispanic and other racial/ethnic groups. Family income was classified as poverty-to-family income ratio and was categorised as low income (poverty-to-family income ratio < 1·85) and higher income (poverty-to-family income ratio ≥ 1·85). Smokers were defined as individuals who reported smoking at least 100 cigarettes during their lifetime, with former smokers defined as not currently smoking and current smokers defined as currently smoking. Participants who drank a minimum of twelve drinks in any given year were classed as drinkers with moderate drinkers defined as those who consumed <1 drink/d for women and <2 drinks/d for men and heavy drinkers being defined as those who consumed ≥1 drink/d for women and ≥2 drinks/d for men. Metabolic equivalent (MET)-hours of moderate-to-vigorous leisure-time physical activity was calculated by summarising minutes of reported activity per week with the metabolic equivalent of physical activities with different intensities. BMI was calculated using the formula: weight (kg)/height (m)2. Co-morbidity conditions (cancer, congestive heart failure, CHD, myocardial infarction, stroke, high cholesterol, hypertension and diabetes) were defined if participants reported that they have ever been told by a healthcare professional that they had such conditions and/or to take prescribed medications because of these conditions, or they are currently taking medication for such a condition.
Statistical analysis
We first categorised the quality of plant-based, animal and both foods based on the sex-specific quartiles of the total score of cDQI, pDQI and aDQI and compared the distribution of demographic, lifestyle factors and co-morbidity conditions across quartiles of pDQI, aDQI and cDQI, using ANOVA for continuous variables and χ 2 test for categorical variables.
To examine the association between the quality of plant-based, animal and both foods and mortality, we first evaluated pDQI, aDQI and cDQI individually in association with mortality using Cox proportional hazard models with multivariable adjustments. The proportional hazard assumption was evaluated by comparing the log–log survival curves by quartiles of cDQI, pDQI and aDQI. The parallel survival curves suggested that the proportional hazard assumption was met. To evaluate the relative importance of the quality of plant-based v. animal foods, we included pDQI and aDQI simultaneously in the same model. We also evaluated each component of cDQI in association with mortality outcomes. All analyses were adjusted for age, sex, race/ethnicity, total energy intake, education, physical activity, cigarette smoking, alcohol drinking, BMI and co-morbidity conditions. We further investigated whether the associations between diet quality and mortality differed by age, sex, income, weight status, levels of physical activity and presence of co-morbidity conditions at baseline. Sensitivity analyses were conducted by restricting the analyses to participants who participated in the first (1999–2006) v. the last four cycles (2007–2014) and by scoring eggs positively.
Sampling weights were incorporated in all analyses to account for unequal probabilities of sample selection due to complex sample design and oversampling of certain subgroups. All analyses were conducted using SAS version 9.4 (SAS Institute). P < 0·05 was considered statistically significant.
Results
The mean total score of the cDQI among US adults was 43·8 (out of maximum score 85), among which the mean score for plant-based components was 27·6 (out of maximum score 55) and the mean score for animal-based components was 16·2 (out of maximum score 30) (Table 1). Among the seventeen food components, three plant-based food components had mean score below 50 % of the maximum score: whole grains (25·4 %), sugar-sweetened beverages (26·8 %) and vegetables excluding white potatoes (46·0 %), suggesting that US adults had particularly poor adherence to the recommended intake of these foods.
Compared with individuals in the lowest quartile of cDQI, those in the highest quartile were older and more likely to be non-Hispanic white, college graduates with a higher income, physically active and moderate drinkers; report co-morbidity conditions at baseline and were less likely to be heavy smokers or obese (Table 2). Individuals with a higher pDQI score were older and more likely to be non-Hispanic whites, overweight and report co-morbidity conditions compared to those with a lower pDQI score, whereas individuals with a higher score of aDQI were younger and more likely to be non-Hispanic blacks and have a healthy weight and less likely to report co-morbidity conditions compared to those with a lower aDQI score.
Table 2. Characteristics of US adults aged ≥20 years by plant- and animal-based diet quality scores, National Health and Nutrition Examination Survey (NHANES) 1999–2014
(Mean values and standard deviations; numbers and percentages)
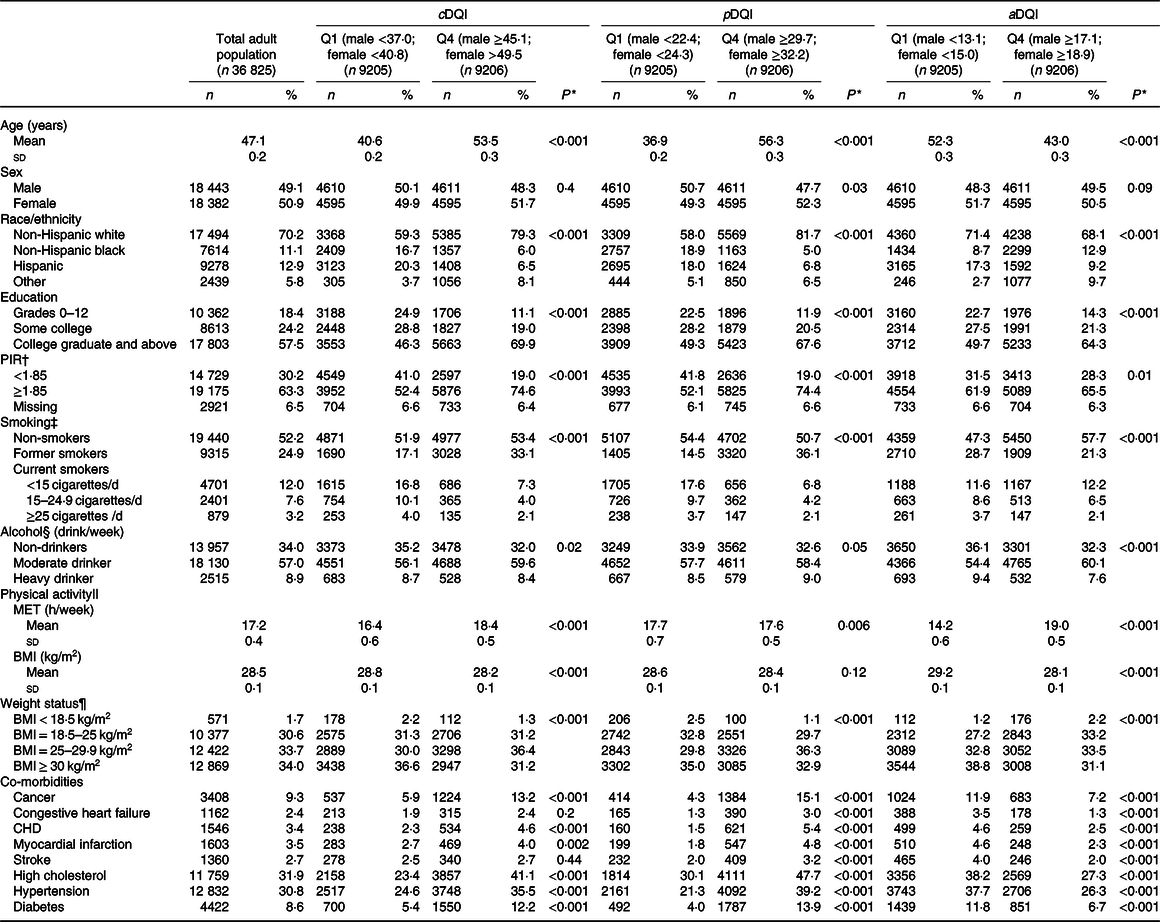
cDQI, Comprehensive Diet Quality Index; pDQI, plant-based Diet Quality Index; aDQI, animal-based Diet Quality Index; Q, quartile; PIR, poverty-to-income ratio; MET, metabolic equivalents.
* ANOVA was used to assess the difference in the distribution of continuous characteristic variables and χ 2 test was used to assess the difference in the distribution of categorical variables by quartiles of cDQI, pDQI and aDQI scores.
† Poverty-to-income ratio (PIR) represents ratio of family income to the federal poverty threshold, adjusting for household size. For reference, the federal poverty threshold in 2014 for a family of four was $23 850 per year. A family of four earning $44 123 per year would have a ratio of 1·85. A lower ratio indicates a lower level of income.
‡ Smokers were defined as individuals who reported smoking at least 100 cigarettes during their lifetime, with former smokers defined as not currently smoking and current smokers defined as currently smoking.
§ Participants who drank a minimum of twelve drinks in any given year were classed as drinkers with moderate drinkers defined as those who consumed <1 drink/d for women and <2 drinks/d for men and heavy drinkers being defined as those who consumed ≥1 drink/d for women and ≥2 drinks/d for men.
|| MET-hours of moderate-to-vigorous physical activity per week was by summarising minutes of reported activity with the metabolic equivalent of physical activities with different intensities.
¶ BMI was calculated by dividing weight in kilograms (kg) by height in metres squared (m2).
During a median 8·3 years of follow-up, 4669 total deaths occurred, including 798 deaths due to heart disease and 1021 deaths due to cancer. Compared with individuals in the lowest quartile, those in the highest quartile of cDQI had 25 % lower all-cause mortality (Q4 v. Q1: hazard ratio (HR) 0·75, 95 % CI 0·65, 0·86; P trend < 0·001) (Table 3 and Fig. 1). After controlling for aDQI, individuals in the highest quartile of pDQI had 34 % lower all-cause mortality (Q4 v. Q1: HR 0·66, 95 % CI 0·58, 0·74; P trend < 0·001) compared with those in the lowest quartile. The aDQI was not associated with any of the mortality outcomes after controlling for pDQI. When each component of cDQI was evaluated individually, lower all-cause mortality was associated with higher intake of vegetables (excluding white potatoes) (HR 0·75, 95 % CI 0·64, 0·88), whole fruits (HR 0·72, 95 % CI 0·57, 0·91), nuts/seeds/legumes (HR 0·77, 95 % CI 0·67, 0·89), vegetable oils (HR 0·82, 95 % CI 0·71, 0·94) and coffee/tea (HR 0·81, 95 % CI 0·70, 0·94). No associations were found for other plant-based food components or any animal food components (online Supplementary Table S2). Scoring eggs positively did not change the results. No associations were found between cDQI, pDQI and aDQI and heart disease- or cancer-specific mortality. Similar associations were found among individuals who participated in the earlier (1999–2006) v. later NHANES (2007–2014) cycles, although the associations were slightly stronger among those who participated in the later cycles (online Supplementary Table S3).
Table 3. Plant- and animal-based diet quality and all-cause, heart disease, cancer and other mortality among US adults, National Health and Nutrition Examination Survey (NHANES) 1999–2014
(Hazard ratios (HR) and 95 % confidence intervals)
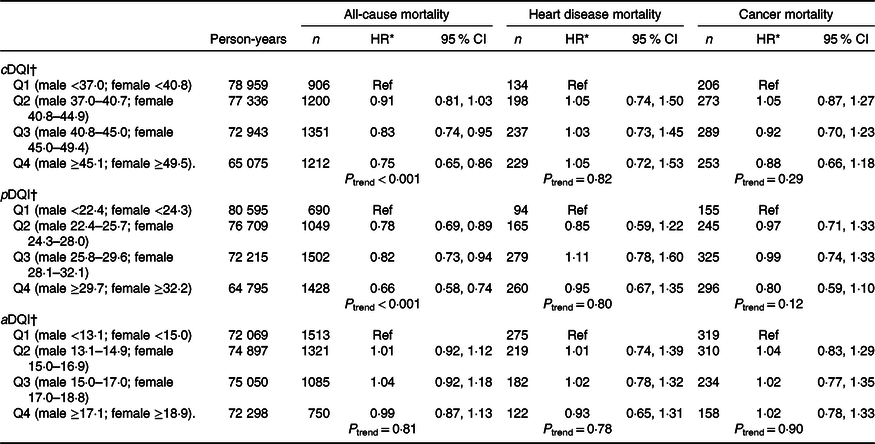
cDQI, Comprehensive Diet Quality Index; Ref, reference; pDQI, plant-based Diet Quality Index; aDQI, animal-based Diet Quality Index.
* Cox proportional hazard models were used to evaluate the associations between diet quality indices and mortality. HR and 95 % CI were adjusted for age, sex, race/ethnicity, education, total energy intake, physical activity, cigarette smoking, alcohol consumption, BMI, co-morbidities and accounted for NHANES survey weights. pDQI and aDQI were simultaneously adjusted in the same model.
† The median scores in each quartile of cDQI were 33·8, 38·8, 43·0 and 48·9 among males and 37·4, 43·0, 47·6 and 53·5 among females; the median scores in each quartile of pDQI were 20·3, 24·1, 27·6 and 32·7 among males and 21·8, 26·2, 30·0 and 35·2 among females and the median scores in each quartile of aDQI were 11·4, 14·0, 16·0 and 18·4 among males and 13·6, 16·1, 18·0 and 20·5 among females.
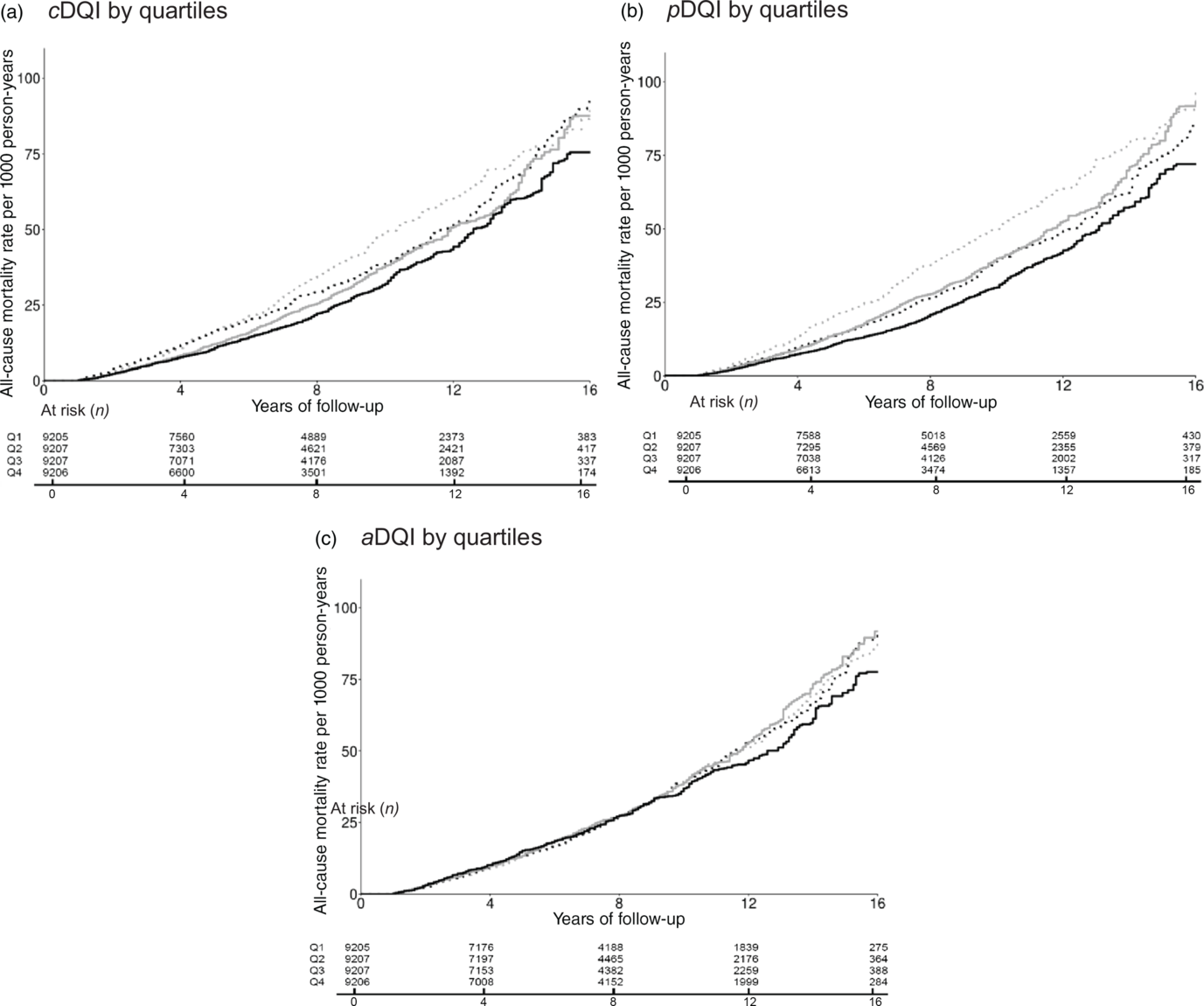
Fig. 1. Cumulative incidence of all-cause mortality by quartiles (Q) of Comprehensive Diet Quality Index (cDQI), plant-based Diet Quality Index (pDQI) and animal-based Diet Quality Index (aDQI) among US adults, National Health and Nutrition Examination Survey 1999–2014. (a) ![]() , Q1 (male <37·0; female <40·8);
, Q1 (male <37·0; female <40·8); ![]() , Q2 (male 37·0–40·7; female 40·8–44·9);
, Q2 (male 37·0–40·7; female 40·8–44·9); ![]() , Q3 (male 40·8–45·0; female 45·0–49·4);
, Q3 (male 40·8–45·0; female 45·0–49·4); ![]() , Q4 (male 45·1+; female 49·5+). (b)
, Q4 (male 45·1+; female 49·5+). (b) ![]() , Q1 (male <22·4; female <24·3);
, Q1 (male <22·4; female <24·3); ![]() , Q2 (male 22·4–25·7; female 24·3–28·0);
, Q2 (male 22·4–25·7; female 24·3–28·0); ![]() , Q3 (male 25·8–29·6; female 28·1–32·1);
, Q3 (male 25·8–29·6; female 28·1–32·1); ![]() , Q4 (male 29·7+; female 32·2+). (c)
, Q4 (male 29·7+; female 32·2+). (c) ![]() , Q1 (male <13·1; female <15·0);
, Q1 (male <13·1; female <15·0); ![]() , Q2 (male 13·1–14·9; female 15·0–16·9);
, Q2 (male 13·1–14·9; female 15·0–16·9); ![]() , Q3 (male 15·0–17·0; female 17·0–18·8);
, Q3 (male 15·0–17·0; female 17·0–18·8); ![]() , Q4 (male 17·1+; female 18·9+).
, Q4 (male 17·1+; female 18·9+).
Subgroup analyses revealed that among individuals with co-morbidity conditions at baseline, those in the highest quartile of cDQI (HR 0·72, 95 % CI 0·64, 0·82; P trend < 0·001) or pDQI (HR 0·66, 95 % CI 0·58, 0·75; P trend < 0·001) had a lower risk of all-cause mortality compared with those in the lowest quartile. In contrast, no associations were found among individuals without co-morbidity conditions at baseline. The inverse association between pDQI and all-cause mortality was slightly stronger among individuals who were overweight (Q4 v. Q1: HR 0·59, 95 % CI 0·48, 0·73; P trend < 0·001) compared to those with a healthy weight (HR 0·69, 95 % CI 0·52, 0·91; P trend = 0·007) or obese individuals (HR 0·68, 95 % CI 0·51, 0·89; P trend = 0·02). The inverse association between cDQI and all-cause mortality was slightly stronger among individuals who were physically active (HR 0·62, 95 % CI 0·42, 0·93; P trend = 0·008) compared with those physically inactive (HR 0·80, 95 % CI 0·68, 0·94; P trend = 0·003). Although no association was found for aDQI and all-cause mortality among older individuals, younger individuals (20–44 years old) in the highest quartile of aDQI had a lower risk of all-cause mortality compared with those in the lowest quartile (Q4 v. Q1: HR 0·65, 95 % CI 0·43, 0·97; P trend = 0·09). In contrary, the association between pDQI and all-cause mortality was only found among older individuals (45–59 years old: HR 0·67, 95 % CI 0·47, 0·96; P trend = 0·05; 60+ years old: HR 0·73, 95 % CI 0·61, 0·88; P trend < 0·001) but not younger ones. Similar associations between cDQI, pDQI, aDQI and mortality were observed between men and women and by income.
Discussion
In a nationally representative sample of US adults, we found that eating a diet with both high-quality plant-based and animal foods (i.e. scoring high in both the pDQI and the aDQI) was associated with a lower risk of all-cause mortality. This association largely reflects the inverse relationship between quality of plant-based foods and all-cause mortality. No independent associations were found for the quality of animal foods with mortality.
The public health and environmental impact of a plant-based diet has gained increased attention in recent years(Reference Willett, Rockstrom and Loken28). It remains debatable whether Americans will eat more plant-based foods and less animal foods, and if so, whether eating a plant-based diet is affordable(Reference Forouhi, Krauss and Taubes29,Reference Hirvonen, Bai and Headey30) . Earlier studies reporting potential health benefits of eating plant-based foods were largely conducted among specific populations such as the Seventh Day Adventists(Reference Orlich, Singh and Sabate6,Reference Appleby, Crowe and Bradbury7,Reference Crowe, Appleby and Travis31,Reference Chang-Claude, Frentzel-Beyme and Eilber32) who have demonstrated a wide range of healthy behaviours such as higher levels of physical activity and lower BMI than the general population regardless of dietary choices(Reference Key, Appleby and Spencer33,Reference Waldmann, Koschizke and Leitzmann34) . More recently, studies among the healthcare professionals (e.g. nurses from the Nurses’ Health Study and physicians from the Health Professionals Follow-up Study) reported that high intakes of healthy plant-based foods (e.g. whole grains, fruits/vegetables, nuts/legumes, oils and tea/coffee) were associated with a lower risk of CHD, whereas consumption of unhealthy plant-based foods (e.g. juices/sweetened beverages, refined grains, potatoes/fries and sweets) was associated with a higher CHD risk. Similar findings were observed for all-cause and CVD mortality(Reference Baden, Liu and Satija35,Reference Kim, Caulfield and Garcia-Larsen36) .
Among the general US population, we found that consuming high-quality plant-based foods (pDQI) was associated with a lower risk of all-cause mortality. Although the associations with cancer and heart diseases-specific mortality were not statistically significant, the HR were below one, suggesting a non-significant inverse association. The bioactive components in healthy plant-based foods such as fibre and phytochemicals have been shown to decrease oxidative stress, reduce inflammation and inhibit cell proliferation(Reference Wang, Khor and Shu37), which can confer a protective effect on chronic diseases(Reference Liu38,Reference Liu39) . Our findings support the dietary recommendations(4) that promote the consumption of high-quality plant-based foods for improving health. In particular, the inverse association between high-quality plant-based foods and all-cause mortality was observed among individuals with co-morbidity conditions at baseline but not among those without co-morbidity conditions. It is reasonable to suspect that individuals with co-morbidity conditions might change their diet due to diet therapy and consequently their prognosis could be improved, contributing to reduced mortality.
We extended the diet quality index used in the previous studies by scoring the quality of animal foods based on the healthfulness of animal foods. However, we did not find that high-quality animal foods were associated with all-cause mortality after controlling for the quality of plant-based foods. Americans are experiencing improving trends in animal-based components of their diet. For example, there was a decreasing trend in red meat consumption among US adults in the past 10–15 years(40). Meanwhile, Americans fall significantly short for several healthy plant-based foods such as whole grains, fruits and vegetables and have excess intake of unhealthy plant-based foods such as those high in added sugars(Reference Rehm, Penalvo and Afshin25). These trends may have made it more difficult to detect associations with animal-based components compared with plant-based ones.
These results may suggest that the relationship between the quality of animal foods and all-cause mortality is not as strong as that for plant-based components. Indeed, when each plant-based and animal food component was evaluated individually, several plant components (non-starchy vegetables, whole fruits and nuts/seeds/legumes) were associated with lower all-cause mortality, but none of the animal components had a significant association with all-cause mortality. Thus, the public health efforts to improve population health may be more effective to focus on increasing the consumption of healthful plant-based foods such as fruits, vegetables, whole grains, nuts/seeds and legumes. Our findings also support the recommendations made by the EAT-Lancet initiative to eat a diet rich in healthful plant-based foods with fewer animal foods for achieving both health and environment benefits(Reference Willett, Rockstrom and Loken28). Importantly, future dietary recommendations shall address not only the health aspects of a diet but also the sustainability of the diet through its environmental, economic and social influences.
Interestingly, eating high-quality animal foods was associated with lower all-cause mortality among younger individuals and yet such an association was not found among older individuals. In contrast, eating high-quality plant-based foods was associated with lower all-cause mortality among older individuals but not younger individuals. This may reflect the different trends of eating high-quality animal v. plant-based foods by age: younger individuals consumed a higher quality of animal foods than older individuals, whereas older individuals had a better quality of plant-based foods than younger individuals. The heterogeneous results among young v. old individuals may also reflect the difference in mortality risk and relative contribution of causes of death by age. Further investigations are needed to understand the potentially different roles of animal v. plant-based foods in chronic disease prevention by age and other lifestyle factors.
Our study has several strengths. First, assessing diet quality as a whole is a more powerful approach to evaluate the impact of humans’ diet on health than studying individual foods/nutrients because humans do not consume foods/nutrients in isolation(Reference Hu41). Dietary guidelines are also moving away from a single food/nutrient approach to focusing on the overall diet quality and eating patterns(4). We assessed the quality of several key plant- and animal-based components in the context of dietary recommendations, which allows for more relevant and translatable findings. Second, we constructed a cDQI that scores the quality of animal foods along with plant-based foods. Different from previous diet quality indices that are also constructed to take healthy and unhealthy types of foods into account, the cDQI facilitates the evaluation of the relative importance of plant v. animal foods in association with health outcomes. Indeed, our results suggested that while eating a diet with both high-quality plant-based and animal foods contributes to a lower risk of all-cause mortality, this association is largely driven by consuming high-quality plant-based foods not animal foods. Animal foods constitute a large proportion of our daily food intake. It is important to understand the different roles that animal v. plant-based foods may play in health. Third, our study included the use of a large-scale nationally representative sample of US adults and the results can be more readily applied to the general population in the USA. The longitudinal design minimises selection bias and recall bias. We also used dietary data collected using validated measures (i.e. 24-h diet recalls)(Reference Ahluwalia, Dwyer and Terry42,Reference Moshfegh, Rhodes and Baer43) .
There are some limitations that need to be considered. First, distribution of diet was estimated based on self-reported dietary intake subject to measurement error. The NHANES used 1 or 2 d of 24-h diet recalls as the primary source to measure dietary intake, which does not well capture usual intake due to large day-to-day variations in food intake. To improve the estimation of usual intake, we applied the National Cancer Institute method to reduce the measurement errors associated with usual intake estimation. Adjusting for energy intake also reduces measurement error(Reference Ahluwalia, Dwyer and Terry42). However, measurement error cannot be ruled out and is likely to be non-differential by mortality, which attenuates the associations. Second, a few food components included in cDQI were scored based on quintiles of consumption. Thus, the cut-offs may differ between studies where study participants have different consumption levels, which affects the comparability of study findings across studies. Third, diet quality is correlated with participants’ socio-economic status and lifestyle factors such as education, cigarette smoking, BMI, alcohol drinking and physical activity. Having chronic health conditions such as cancer, CVD or diabetes may also change one’s dietary intake patterns. We excluded individuals who died within 12 months of dietary assessment to minimise the chance of reverse causation. To reduce the chance of residual confounding, we carefully adjusted for all these factors in the multivariable models. In addition, we stratified the association by presence or absence of co-morbidity conditions. However, dietary intake patterns may be associated with factors that we have not identified or adjusted for, and residual confounding may still be present. Fourth, mortality was determined through probabilistic matching with the National Death Index. Although probabilistic matching is subject to misclassification, a prior validation study has shown that the accuracy of the method was high, with 96·1 % of the decedents and 99·4 % of the living participants classified correctly(Reference Menke, Muntner and Batuman26). Fifth, our sample size is limited to evaluate cause-specific mortality such as deaths due to cancer or heart disease. Thus, we treated cause-specific mortality analyses as secondary and the results should be interpreted with caution. Sixth, repeated assessment on dietary intake for the same individual was not available in NHANES. We were unable to evaluate how potential changes in dietary intake are associated with mortality outcomes.
Despite these limitations, our study is among the first to evaluate the relative importance of the quality of plant-based v. animal foods in association with mortality outcomes among a nationally representative sample of US adults. Our results suggest that eating better-quality plant-based foods is associated with a lower risk of all-cause mortality among US adults. Conversely, the quality of animal foods does not independently contribute to mortality. Findings support the current dietary recommendations that promote high-quality plant-based diet for chronic disease prevention.
Acknowledgements
The present study was supported by National Institutes of Health/National Institute on Minority Health and Health Disparities (NIH/NIMHD) 1R01MD011501. F. F. Z. and C. D. developed the proposal, M. R., F. C., J. W., S. Z., C. D., J. L. and M. D. completed the analysis, L. K. interpreted the results and drafted the manuscript, F. F. Z. had primary responsibility for final content and all authors approved the final version of the manuscript.
None of the authors has any conflicts of interest to declare.
Supplementary material
For supplementary material referred to in this article, please visit https://doi.org/10.1017/S0007114520003670







