Age is a major non-modifiable risk factor for CVD(Reference Genest, McPherson and Frohlich1, 2), with individuals aged 50 years and older having worse lipid profiles compared with younger age groups(Reference Reeder, Liu and Horlick3, Reference Reeder, Liu and Horlick4). Important to consider are interventions that can lower modifiable risk factors in older individuals because our population is projected to age substantially over the next 25 years due to the ageing of the baby-boomer generation(Reference Martini, Garrett and Lindquist5). This will result in a disproportionate increase in health care costs, especially in the area of CVD(Reference Martini, Garrett and Lindquist5).
Pulses (i.e. beans, peas, chickpeas and lentils) are high in many components considered beneficial for cardiovascular health, including soluble and insoluble fibre, vegetable protein, oligosaccharides, saponins, folic acid, phytosterols and other antioxidants(Reference Chibbar, Ambigaipalan and Hoover6). Pulses have a very low glycaemic index, which results in lower blood glucose and insulin response compared with other foods(Reference Jenkins, Wolever and Taylor7). Pulses are effective for improving lipid profile(Reference Crujeiras, Parra and Abete8–Reference Dumesnil, Turgeon and Tremblay10) and glycaemic control(Reference Sievenpiper, Kendall and Esfahani11). Pulse-based foods have also been reported to reduce systolic blood pressure and C-reactive protein (a marker of inflammation) in overweight and obese subjects(Reference Hermsdorff, Zulet and Abete12). Determining whether a pulse-based diet can be effective in older individuals, as it is in younger individuals(Reference Crujeiras, Parra and Abete8, Reference Jenkins, Wong and Patten9), is important because dietary intake may have less influence on CVD risk factors in older compared with younger subjects(Reference Porrini, Simonetti and Testolin13). A few studies of pulse diets have included individuals over the age of 50 years(Reference Anderson, Story and Sieling14–Reference Pittaway, Ahuja and Robertson20), the age category most at risk for CVD(Reference Genest, McPherson and Frohlich1–Reference Reeder, Liu and Horlick4); however, no study has focused exclusively on individuals aged 50 years and older. A pulse-based diet may be an optimal intervention for improving cardiovascular risk factors since pulse consumption in North America currently is very low(Reference Leterme21).
The purpose of the present study therefore was to evaluate the effect of a pulse-based diet on risk factors for CVD, including blood lipids (with LDL-cholesterol (LDL-C) as the primary outcome), fasting glucose and insulin, inflammation, blood pressure and body composition (waist circumference, fat mass) in individuals aged 50 years and older. Based on past studies of younger participants(Reference Crujeiras, Parra and Abete8, Reference Jenkins, Wong and Patten9, Reference Sievenpiper, Kendall and Esfahani11, Reference Hermsdorff, Zulet and Abete12), we hypothesised that a pulse-based diet would reduce these risk factors for CVD in older participants.
Methods
Experimental design and study protocol
This clinical trial employed a randomised single-blind cross-over design to compare a pulse-based and regular diet (Clinical Trials Government identifier no. NCT00800033). Researchers collecting and analysing the data were blinded to whether participants were on the pulse-based diet or their regular diet. Sample size was calculated as that required to show a 0·2 mmol/l decrease in LDL-C, which reduces CHD-related mortality and total events by 6 %(Reference Gould, Davies and Alemao22), a level considered clinically significant(Reference Pan, Yu and Demark-Wahnefried23). We assumed a baseline LDL-C of 3·63 mmol/l with a standard deviation of 0·82, based on our previous work with participants aged 50 years and older(Reference Cornish, Chilibeck and Paus-Jennsen24). Using an α level of 0·05, a power of 80 %, and assuming a correlation between repeated measures of 0·86(Reference Cornish, Chilibeck and Paus-Jennsen24), the required sample size was calculated as thirty-nine. We recruited additional participants to account for those who might drop out or be lost to follow-up and used an intent-to-treat analysis. Subjects were inactive men and women (defined as participating in moderate aerobic exercise two times or less per week), not allergic to any of the components of the pulse diet, not on medication that affects blood lipids, and aged 50 years or older. A total of 108 subjects (thirty-seven males) were randomised to receive the pulse-based diet or their regular diet for 2 months and then crossed over to receive the other diet for 2 months, separated by a 1-month ‘washout’. Randomisation was carried out using a computer-generated allocation schedule using a fixed block size of four. Stratification was by sex. The allocation sequence was concealed from the research assistant enrolling and assessing participants by using central allocation (i.e. telephone). All participants were given advice on following the Canadian Physical Activity Guidelines as part of standard of care(Reference Warburton, Charleworth and Ivey25). Ethical approval for the study was received from the Bio-medical Research Ethics Board at the University of Saskatchewan and all subjects gave their written informed consent.
The pulse-based diet included snacks, salads, soups and meals prepared with lentils, chickpeas, beans and peas (150 g dry weight or 250 g wet weight cooked daily). The specific pulses included green lentils, red split lentils, chickpeas, yellow split peas, and pinto, fava, broad, black and kidney beans. The type of pulses incorporated into meals was rotated so that participants received an equal percentage (i.e. 25 % each) of meals containing lentils, chickpeas, peas and beans. These pulses represented a mixture of varieties that are available in the food system (i.e. they came from a wide variety of sources and suppliers). A total of two servings (75 g dry weight or 125 g wet weight cooked pulses per serving) were supplied daily (i.e. lunch, dinner or snacks). This serving size is similar to the serving size recommended for legumes in the ‘meat and alternatives’ food group from Canada's Food Guide, which is two or three servings of legumes per d for individuals aged 51 years and older, where one serving is equal to 0·75 cups or about 123 g(26). Meals containing the different pulses were rotated. No foods were given during the control period; participants were told to follow their usual diet. The subjects were asked to keep a log of all pulse foods eaten and compliance to the diet was determined from these logs. They had to fill out a detailed validated FFQ at baseline, and at 2, 3 and 5 months so that we could compare intake of nutrients during the pulse-diet and regular-diet phases. This FFQ is a 110-item survey designed to estimate the customary dietary intake for a wide variety of food items and nutrients in adults (Block 98.2 FFQ, Block Dietary Data Systems). The FFQ was modified for our use by the addition of foods unique to Canada. The proportions of soluble and insoluble fibre from the pulse-based meals were estimated from the CRC Handbook of Dietary Fiber in Human Nutrition (Reference Spiller27).
Laboratory analyses
Blood sampling and serum separation
Sampling was performed in the morning (07.00 to 09.00 hours) by a phlebotomist after participants had fasted for 12 h. The blood was allowed to clot for at least 30 min and then spun in a centrifuge for 10 min at 3000 rpm. Then serum was stored at − 80°C.
Serum assays
An LX20 Beckman Coulter analyser (Beckman Coulter Canada Inc.) was used to analyse the following components by enzymic kits: total cholesterol, LDL-C and HDL-cholesterol (EnzyChrom™ HDL and LDL/VLDL Assay Kit, EHDL-100); TAG (EnzyChrom™ TAG Assay Kit, ETGA-200); glucose (QuantiChrom™ Glucose Assay Kit, DIGL-200) (BioAssay Systems). Commercially available ELISA kits were used to analyse serum insulin (ALPCO™ Insulin EIA) and C-reactive protein (ALPCO™ C-Reactive Protein [hsCRP] EIA) (ALPCO Diagnostics). Samples from all of the time points for each individual were analysed in the same assay to eliminate between-assay variability. Within-assay CV ranged from 1·7 to 7·6 %.
Physical data collection and measurements
BMI was obtained by dividing a subject's total body mass (kg) by the square of height (m). Waist circumference was measured at the superior border of the iliac crest. Blood pressure was measured after the participant was in a comfortable seated position for 5 min.
Dual-energy X-ray absorptiometry (Hologic Discovery) was used for the determination of whole body fat mass, percentage body fat and abdominal fat mass. Abdominal fat was determined following the protocol utilised by Svendsen et al. (Reference Svendsen, Hassager and Bergmann28). In our laboratory, the precision (CV) of the abdominal fat mass is 4·7 %(Reference Vatanparast, Chilibeck and Cornish29). The CV for whole body fat mass from our laboratory is 3·0 %(Reference Cornish, Chilibeck and Paus-Jennsen24).
Physical activity was assessed by questionnaire(Reference Godin and Shephard30) to determine whether physical activity levels differed across diet phases, since this could have an effect on cardiovascular risk factors(Reference Cornish, Chilibeck and Paus-Jennsen24).
Participants were contacted three times per week to probe for adverse events and these were recorded according to the guidelines for good clinical practice(31).
Statistical analyses
Dependent variables were measured at four time points: baseline, after the first 2-month diet, before the second 2-month diet (i.e. after the washout) and at the end of the second 2-month diet. These variables included: body weight, BMI, total body fat, percentage fat, abdominal fat, waist circumference, blood pressure, serum TAG, total cholesterol, HDL-cholesterol, LDL-C, C-reactive protein (as an inflammatory marker), glucose and insulin.
Dependent variables were analysed by a sex (male v. female) × diet (pulse v. regular diet) × time (baseline v. 2 months) ANOVA with repeated measures on the last two factors. Here, and in the results section, ‘baseline’ refers to the beginning of each study arm. When interactions were significant, Bonferroni post hoc tests were performed to determine differences between pairs of means. Statistical tests were run including all participants and then including only participants who were classified as having elevated total cholesterol, LDL-C, glucose, TAG, C-reactive protein, blood pressure or waist circumference, or low HDL-cholesterol as defined by the National Cholesterol Education Program(2). For blood pressure we also ran separate analyses for those who were taking blood pressure medication and those who were not taking blood pressure medication. For body composition (i.e. percentage fat), statistics were run for all participants and separately for those who had percentage fat greater than the normative values (as determined by dual-energy X-ray absorptiometry) for their age group(Reference Kyle, Genton and Hans32). The α level was set at 0·05. All values are presented as means and standard deviations, except on graphs where error bars represent standard errors, for clarity. All analyses were carried out using Statistica 7.0 (StatSoft).
Results
A total of eighty-seven participants (thirty males and fifty-seven females; aged 59·7 (sd 6·3) years; body mass 76 (sd 16) kg; height 167 (sd 9) cm; BMI 27·5 (sd 4·5) kg/m2) completed the study (see study flow diagram; Fig. 1). Numbers reported for each measure throughout the present results vary slightly because some participants missed testing sessions or insufficient blood was available for assays on all blood measurements. Of the participants, nineteen were taking blood pressure medication during the study and three were on oral agents for control of blood glucose. Other medications and the number of participants on each included thyroid medication (n 6), medication for acid reflux (n 4), pain medication (n 3), antibiotics (n 2), cancer medications (n 3), medications for attention deficit disorder (n 2) and medication for atrial fibrillation (n 1). No differences between males and females in response to the different diets over time were found (i.e. there were no sex × diet × time interactions); therefore, for clarity all means are presented with males and females combined.
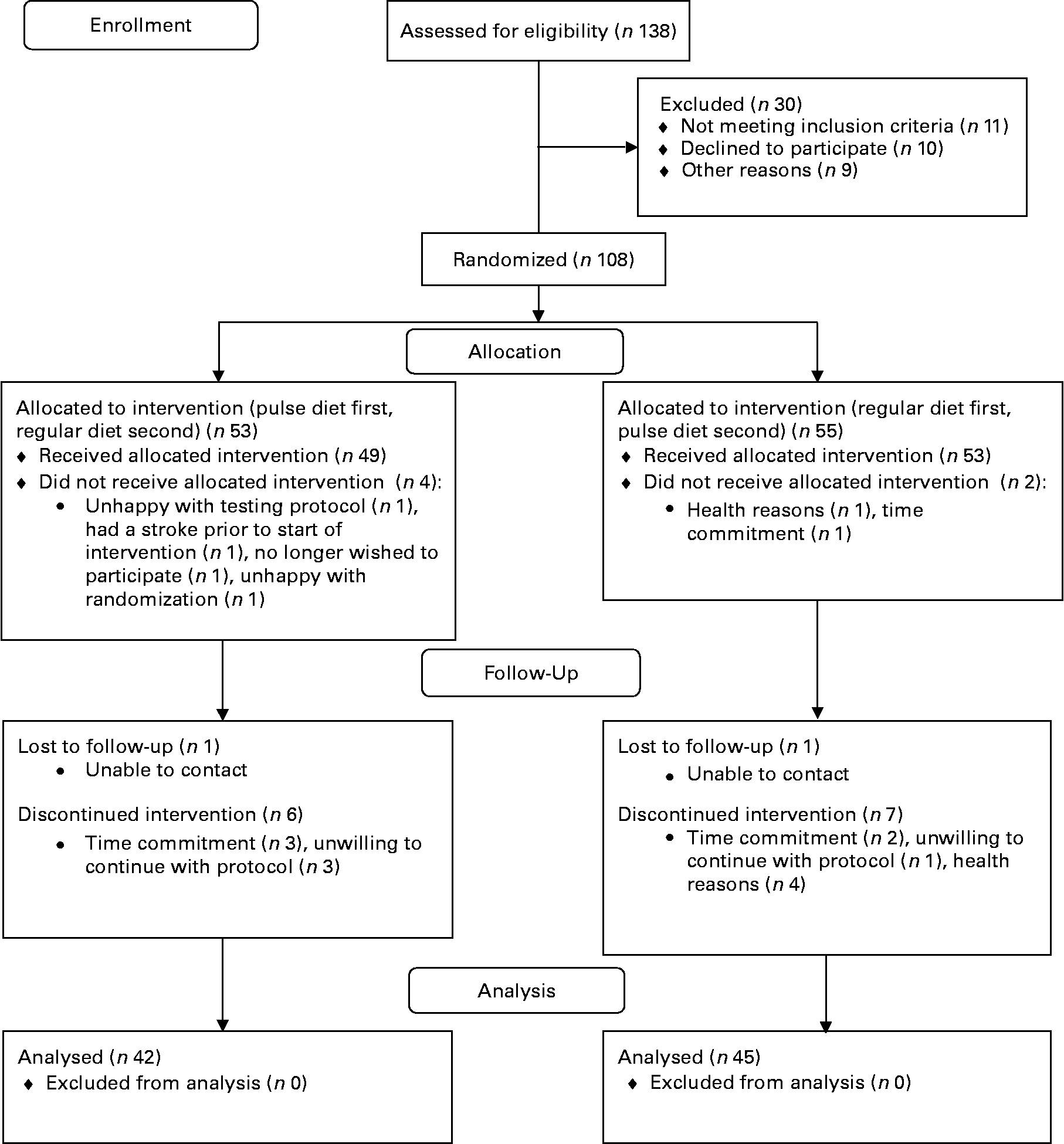
Fig. 1 Participant flow through the study.
Dietary analyses
Mean energy and nutrient intakes at baseline and the end of each diet phase are presented in Table 1. There were no differences between the pulse-based and regular diet at baseline. At the end of the diet phases, the only variable that differed was dietary fibre, which was higher during the pulse-based diet, as expected (P < 0·01). Of the 30 (sd 15) g dietary fibre consumed daily during the pulse intervention, 21 (sd 10) g were provided by the pulse-based meals. The relative amount of insoluble fibre in the total fibre content for the pulse-based meals was 69 (sd 11) %, with the remainder (31 (sd 11) %) from soluble fibre. From the dietary logs, participants consumed 74 (sd 24) % (range 2–100 %) of the pulse-based meals supplied.
Table 1 Nutrient intake during the pulse and regular diets (n 70)†
(Mean values and standard deviations)
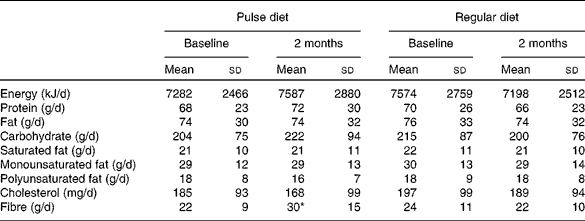
* Fibre content was greater at the end of the pulse diet than at all other time points (P < 0·01).
† Micronutrients (vitamins and minerals) were not analysed.
Serum chemistry
The pulse diet significantly decreased total cholesterol and LDL-C compared with the regular diet (diet × time P < 0·001 and P = 0·01, respectively; Table 2). When analyses were performed on participants with levels of total cholesterol or LDL-C that were ‘borderline high’ or greater (i.e. 5·17 mmol/l or greater for total cholesterol and 3·36 mmol/l or greater for LDL-C)(2), the pulse-based diet decreased total cholesterol significantly more than the regular diet (diet × time P = 0·05; Fig. 2), whereas there was no significant difference between diets for LDL-C (Fig. 3). There were no significant differences between diets for changes in any of the other dependent variables measured from blood sampling (Table 2). Analyses with participants that had values outside of the optimal ranges for these other variables showed no differences between diets (data not shown).
Table 2 Serum measures during the pulse and regular diets
(Mean values and standard deviations)
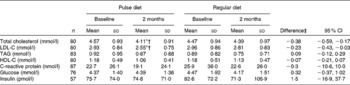
LDL-C, LDL-cholesterol; HDL-C, HDL-cholesterol.
* Mean value was significantly different from that at baseline for the pulse diet (P < 0·001).
† Mean value was significantly different from the 2-month value for the regular diet (P < 0·01).
‡ Difference between change in pulse diet and change in regular diet.
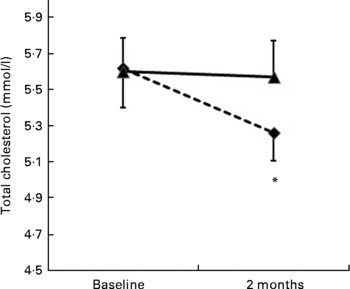
Fig. 2 Change in cholesterol levels for participants with elevated total cholesterol ( ≥ 5·17 mmol/l) at baseline (n 20). (- -♦- -), Pulse diet; (–▲–), regular diet. Values are means, with standard errors represented by vertical bars. * Mean value was significantly different from that at baseline (P = 0·05).

Fig. 3 Change in LDL-cholesterol levels for participants with elevated LDL-cholesterol ( ≥ 3·36 mmol/l) at baseline (n 27). (- -♦- -), Pulse diet; (–▲–), regular diet. Values are means, with standard errors represented by vertical bars.
Physical measures
No differences over time were found between diets for blood pressure or any of the anthropometric measures (Table 3) except with participants that had percentage fat above the normative value for their age(Reference Kyle, Genton and Hans32) (n 73; group × time P = 0·045). For these participants, there was a small, but statistically significant, decrease in percentage body fat during the pulse diet (34·2 (sd 6·4) % at baseline and 33·5 (sd 6·4) % at 2 months; P = 0·008) with no change during the regular diet (33·5 (sd 6·3) % at baseline and 33·4 (sd 6·5) % at 2 months). There were no differences between diets in our sub-analyses of individuals with elevated blood pressure or those taking or not taking blood pressure medication (data not shown). Also, no differences in physical activity scores during the different diet phases were apparent. Physical activity scores (arbitrary units) were: 27 (sd 22), 24 (sd 21), 27 (sd 18) and 30 (sd 31) before the pulse diet, at the end of the pulse diet, before the regular diet, and at the end of the regular diet, respectively.
Table 3 Physical measures during the pulse and regular diets
(Mean values and standard deviations)
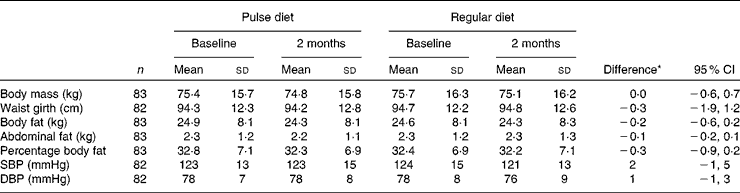
SBP, systolic blood pressure; DBP, diastolic blood pressure.
* Difference between change in pulse diet and change in regular diet.
Adverse events
Of the participants, eight reported adverse events that were considered related to the pulse-based diet. Of these, seven participants reported one adverse event each, and one participant reported two adverse events. The adverse events were: upset stomach (n 4), flatulence (n 2), bloating (n 2) and diarrhoea (n 1). Adverse events were rated as ‘mild’ (n 6) or ‘moderate’ (n 3) in severity. No serious adverse events were related to the pulse-based diet. None of the participants withdrew from the study because of these adverse events.
Discussion
The most important results from the present study were that a pulse-based diet reduced total cholesterol and LDL-C in individuals aged 50 years and older, an age at which risk of CVD is elevated(Reference Genest, McPherson and Frohlich1, 2). Total cholesterol was reduced by about 8·3 % and LDL-C was reduced by about 7·9 % on the pulse-based diet compared with the regular diet (Table 2). Total cholesterol was also reduced by 6 % on the pulse-based diet in a sub-analysis of participants who had cholesterol levels that were higher than desirable(2) at baseline (Fig. 2). These results are considered clinically significant(Reference Pan, Yu and Demark-Wahnefried23). A reduction in total cholesterol by 8·3 % is estimated to reduce risk of CHD by 17 to 25 %(Reference Manson, Tosteson and Ridker33). For participants who had cholesterol levels classified as ‘higher than desirable’ (i.e. 5·17 mmol/l or higher)(2) at baseline, the mean cholesterol level after the pulse-based diet (5·26 mmol/l; Fig. 2) was close to the level described as ‘desirable’ ( < 5·17 mmol/l) by cholesterol guidelines(2). The reduction of LDL-C by 0·23 mmol/l (for all participants) on the pulse-based diet compared with the regular diet (Table 2) is estimated to reduce CHD-related mortality and total events by 5 to 9 %(Reference Genest, McPherson and Frohlich1, Reference Gould, Davies and Alemao22, Reference Grundy, Cleeman and Merz34). Although the reduction in LDL-C for the sub-analysis of individuals with high LDL-C levels at baseline ( ≥ 3·36 mmol/l)(2) was not statistically significant (Fig. 3), the reduction may still be of clinical significance: The reduction of 0·33 mmol/l on the pulse-based diet compared with the regular diet is estimated to reduce CHD-related mortality and total events by 7 to 13 %(Reference Genest, McPherson and Frohlich1, Reference Gould, Davies and Alemao22, Reference Grundy, Cleeman and Merz34). The absolute difference of 0·33 mmol/l between diets is equal to the absolute difference between individuals from the Quebec Cardiovascular Study who developed IHD over a 5-year follow-up v. those who did not develop heart disease(Reference Lemieux, Lamarche and Couillard35). For our participants with elevated LDL-C at baseline the change in LDL-C from 3·76 to 3·24 mmol/l during the pulse diet (Fig. 3) moves them from the classification of ‘borderline high’ (i.e. 3·36 mmol/l or greater) to ‘near optimal’ (i.e. less than 3·36 mmol/l) according to cholesterol guidelines(2). This change would move many of our participants into a category of patients (i.e. ≤ 3·5 mmol/l) where initiation of treatment with statins would no longer be recommended according to the Canadian Cardiovascular Society guidelines(Reference Genest, McPherson and Frohlich1).
The reductions in total cholesterol and LDL-C in our older participants during a pulse-based diet are similar to those observed in younger participants(Reference Crujeiras, Parra and Abete8, Reference Jenkins, Wong and Patten9). Younger subjects (i.e. < 50 years of age) who were given mixed pulse diets (lentils, chickpeas, peas and beans) for 2–4 months reduced total cholesterol by 6 to 13 %, and LDL-cholesterol by 8 %(Reference Crujeiras, Parra and Abete8, Reference Jenkins, Wong and Patten9). The similar response to pulse-based diets in individuals aged over 50 years found in the present study is important because it has been suggested that diet may have less of an influence on blood lipid levels in older compared with younger individuals(Reference Porrini, Simonetti and Testolin13).
The present study is unique in that it is the first study to investigate the effect of a pulse-based diet on blood lipids exclusively in participants aged 50 years and older. A number of previous studies have included participants of age 50 years or older amongst their subject participants. Our findings of reduced total cholesterol and LDL-C are in agreement with most of these studies. Participants who ranged in age from 22 to 78 years given pulse diets including chickpeas, beans and/or lentils at doses ranging from 120 to 140 g/d (dry weight) or from 120 to 300 g/d (wet weight) for 3–16 weeks reduced total cholesterol by 4–19 % and LDL-C by 5–24 %(Reference Jenkins, Wong and Patten9, Reference Anderson, Story and Sieling14–Reference Winham, Hutchins and Johnston18), comparable with our findings of reductions in total cholesterol and LDL-C of approximately 8 %. In contrast, two additional studies that included participants aged 28–66 years given 80–440 g (wet weight) beans daily for 28–42 d over 3–7 weeks resulted in no improvement in total cholesterol or LDL levels(Reference Cobiac, McArthur and Nestel36, Reference MacKay and Ball37).
A number of components in pulses may account for their cholesterol-lowering effects(Reference Chibbar, Ambigaipalan and Hoover6). In the present study participants consumed approximately 36 % higher fibre during the pulse-based compared with the regular diet (Table 1). Approximately 69 % of the fibre supplied in the pulse diets was insoluble fibre and 31 % soluble. Soluble and insoluble fibre can bind to bile acids in the intestinal lumen and prevent their reabsorption. The consequent increased hepatic production of bile acids decreases the hepatic cholesterol pool, necessitating increased cholesterol uptake from the blood, and thereby decreasing serum cholesterol levels(Reference Chibbar, Ambigaipalan and Hoover6, Reference Duane15, Reference Van Bennekum, Nguyen and Schulthess38, Reference Kishimoto, Wakabayashi and Takeda39). Also, fermentation of soluble fibre in the colon produces SCFA which contribute to decreased cholesterol synthesis in the liver(Reference Chibbar, Ambigaipalan and Hoover6, Reference Kishimoto, Wakabayashi and Takeda39, Reference Wright, Anderson and Bridges40).
In addition to improving total cholesterol and LDL-C levels, we hypothesised that a pulse-based diet would improve other markers of CVD risk (i.e. fasting TAG, C-reactive protein, fasting insulin and glucose, and blood pressure). We observed no benefit of the pulse-based diet on these risk factors (Tables 2 and 3). Most other studies that have included participants over the age of 50 years have also reported no significant changes in TAG after 3–8 weeks of diets containing pulses(Reference Anderson, Story and Sieling14–Reference Winham, Hutchins and Johnston18). The only exception was the study by Jenkins et al. (Reference Jenkins, Wong and Patten9) who found that 140 g (dry weight) daily of chickpeas, beans or lentils (similar to the present study) given over 4 months to seven hypercholesterolaemic men ranging in age from 31 to 65 years decreased TAG by 25 %. Their intervention was twice as long as our intervention and this may account for the differences between studies. One previous study showed that four servings per week of 160 to 235 g (wet weight) of lentils, beans, chickpeas or peas significantly reduced C-reactive protein and blood pressure in younger (aged 36 years) obese and overweight participants over an 8-week period(Reference Hermsdorff, Zulet and Abete12), identical to the length of our diet. Their pulse diet significantly reduced body weight, whereas we observed no changes in body weight nor was weight loss expected based on the present study design (Table 3). We observed only minor changes in body composition (i.e. a small decrease in percentage fat in our subgroup of participants with above-average percentage fat). It is possible that a decrease in body weight is required to improve C-reactive protein and blood pressure. The older age of our participants may also account for lack of improvement in C-reactive protein. Levels of inflammation are chronically elevated with ageing and may be difficult to improve with dietary interventions(Reference Cornish, Chilibeck and Paus-Jennsen24, Reference Cornish and Chilibeck41). Multiple interventions may be required to lower the chronic inflammation observed with ageing(Reference Cornish and Chilibeck42). A few studies that have included older participants have evaluated changes in C-reactive protein with half a cup to 250 g (wet weight) of beans per d over 4–8 weeks and none found significant reductions in C-reactive protein or changes in body weight(Reference Winham and Hutchins17, Reference Winham, Hutchins and Johnston18, Reference Hartman, Albert and Zhang43), similar to the results of the present study.
Sievenpiper et al. (Reference Sievenpiper, Kendall and Esfahani11) performed a meta-analysis of eleven trials of pulses and their effect on fasting glucose and insulin(Reference Sievenpiper, Kendall and Esfahani11). Expressed as a standardised mean difference, fasting blood glucose was reduced by 0·82 (a large effect size), and fasting blood insulin by 0·49 (a moderate effect size). Pulses included chickpeas, black-eyed peas and various beans at a dose of 152 g (wet weight) per d given over a mean of 6·7 weeks. The total number of participants was 253. The present study may have lacked power to detect differences in these variables. The present study included few participants with very high fasting glucose or insulin levels and, therefore, our participants may have been less responsive to the pulse-based diet. Other studies that have included participants over the age of 50 years are in agreement with the present results: 3 to 8 weeks of pulse-based diets failed to reduce fasting glucose or insulin(Reference Anderson, Story and Sieling14, Reference Winham and Hutchins17–Reference Pittaway, Ahuja and Robertson20, Reference Hartman, Albert and Zhang43).
The present study had a number of limitations. Aside from providing the two servings of pulses per d, the pulse-based and control (regular) diets were not controlled. This approach would decrease the internal validity of the present study, but would increase the external validity, as it would be more applicable to everyday living. Despite the lack of dietary control, minimal differences in macronutrient intake, aside from dietary fibre, were found between the diet phases (Table 1). Another limitation was that although the present study was adequately powered to detect changes in cholesterol and LDL-C for our complete set of study participants, some of the sub-analyses of participants who had elevated risk factors involved smaller numbers of participants and may have lacked statistical power. Although the overall compliance to our diet was good, with 74 % of the pulse-based foods consumed, there was much variability in compliance, with a range of 2 to 100 %. Not all participants therefore could tolerate the pulse-based diet. A final limitation is that the analysis of our FFQ could not partition total fibre intake into soluble and insoluble fractions (Table 1). We were only able to determine the soluble and insoluble fractions from the meals given during the pulse diet phase by hand searching the CRC Handbook of Dietary Fiber in Human Nutrition (Reference Spiller27).
In conclusion, a 2-month diet containing two servings (150 g/d dry weight) of mixed pulses (lentils, chickpeas, beans and peas) daily lowers total cholesterol and LDL-C by clinically significant amounts in participants 50 years and older, an age group who are at increased risk of CVD. The pulse-based diet had no effect on other risk factors for CVD including TAG, C-reactive protein, blood pressure, abdominal fat mass, and fasting glucose and insulin. Multiple interventions may be needed to improve these latter risk factors in older adults(Reference Genest, McPherson and Frohlich1). Future studies should focus on the long-term effectiveness and long-term compliance to a pulse-based diet.
Acknowledgements
This research was funded as part of the Pulse Innovation Project, through Canada's Agricultural Policy Framework (APF), a Federal-Provincial-Territorial initiative, and by the Saskatchewan Pulse Growers. There were no restrictions on publishing the study. H. V. was supported by a post-doctoral fellowship from the Saskatchewan Health Research Foundation.
S. A. was involved in data collection, sample analyses, food preparation and writing the manuscript, P. D. C. was involved with study planning, data analyses and writing the manuscript, H. V. was involved in data collection, body composition analyses and writing the manuscript, and G. A. Z. was involved in study and diet planning and writing the manuscript.
The authors declare no conflict of interest.







