Anthocyanins, which belong to the flavonoid family, are reddish natural pigments occurring ubiquitously in fruits and berries(Reference Clifford1). Their average daily intake was estimated at about 12·5 mg/d in the United States and at 47 mg/d in Finland(Reference Wu, Beecher and Holden2, Reference Ovaskainen, Torronen and Koponen3). However, high intakes (>100 mg/d) could be easily achieved with regular consumption of red fruits or berries. Natural food colourants rich in anthocyanins may also significantly contribute to the total anthocyanin dietary intake(Reference Erdman, Balentine and Arab4).
Anthocyanins have been reported to promote health and prevent various chronic diseases(Reference Galvano, La Fauci and Vitaglione5, Reference Prior and Wu6). They may reduce the risk of CVD, exert anticarcinogenic and neuroprotective activities, reduce inflammatory insult and modulate the immune response(Reference Cooke, Steward and Gescher7–Reference Misikangas, Pajari and Paivarinta11). They are also reported to exert antidiabetic and antiobesity effects(Reference Tsuda, Horio and Uchida12, Reference Prior, Wu and Gu13).
Health-enhancing properties of anthocyanins depend on their absorption, metabolism, tissue distribution and excretion. In the last few years, a number of studies have been conducted on the absorption of anthocyanins in human subjects or experimental animals (for review, see reference(Reference McGhie and Walton14)). Most of them reported that the proportion of total anthocyanins absorbed and excreted in the urine (in the native form and as metabolites) was below 1 % of the ingested amount, which is lower than that found for most flavonoids(Reference Galvano, La Fauci and Vitaglione5, Reference McGhie and Walton14). Anthocyanins are rapidly absorbed from both stomach and small intestine, and they appear in blood circulation and urine as intact, methylated, glucurono- and/or sulphoconjugated forms(Reference Felgines, Talavéra and Gonthier15–Reference Talavéra, Felgines and Texier19). Knowledge on tissue distribution of various anthocyanin metabolites is important in understanding the mechanisms by which health effects could occur. However, to date, little is known about their distribution outside the digestive tract. In rats, a few studies have shown the presence of anthocyanins in some organs such as liver, kidney, brain and eyes(Reference Talavéra, Felgines and Texier20–Reference Hassimotto, Genovese and Lajolo24). We have recently shown that anthocyanin feeding in rats resulted in the distribution of anthocyanin metabolites (native as well as glucurono and/or methylated anthocyanins) to several organs (prostate, bladder, testes, adipose tissue and heart) albeit at low concentrations(Reference Felgines, Texier and Garcin25). Recent studies in mice have also detected anthocyanins in various tissues such as liver, kidney, prostate, testes and lungs(Reference Marczylo, Cooke and Brown26, Reference Sakakibara, Ogawa and Koyanagi27). Most authors have suggested that other metabolites may also be present in organs, but to date, they are unidentified and their amounts are unknown. Indeed, anthocyanin chemistry is complex, and anthocyanins could be converted into colourless forms that are not detected owing to their chemical instability at physiological pH(Reference Mazza and Miniati28) or metabolised into various compounds such as phenolic acids(Reference Aura, Martin-Lopez and O'Leary29–Reference Fleschhut, Kratzer and Rechkemmer31).
The aim of the present work was thus to follow anthocyanin distribution in mice after oral administration of a radiolabelled substrate, 14C-cyanidin 3-O-glucoside (Cy3G). Cy3G was chosen as it was the most representative dietary anthocyanin. We first followed the radioactivity derived from the labelled anthocyanin in tissues over a 24 h period. Next, we tentatively established a complete evaluation of the recovery of radioactivity 3 h after the administration of a higher amount of 14C-labelled Cy3G.
Materials and methods
Chemicals
l-[U-ring-14C]-Phenylalanine was purchased from Isobio (Fleurus, Belgium) and isopropanol from Atlantic Labo (Bruges, France) and methyl jasmonate and H2O2 were purchased from Sigma-Aldrich (St-Quentin Fallavier, France). Hionic-Fluor liquid scintillation cocktail and Soluene-350 were obtained from Perkin Elmer (Courtaboeuf, France). Cy3G and malvidin 3-O-glucoside were purchased from Extrasynthèse (Genay, France). Blackberry anthocyanin extract was supplied by Ferlux-Mediolanum (Cournon d'Auvergne, France).
Radiotracer production
14C-Cy3G was obtained as described previously(Reference Vitrac, Krisa and Decendit32). Briefly, the l-[U-ring-14C]-phenylalanine (92·5 kBq/ml of cell suspension) and unlabelled phenylalanine (final concentration of 0·5 mM) were added to grape cell suspension culture (50 ml) on days 6 and 8. Methyl jasmonate (10 μm) was applied on day 6. Then, anthocyanins were extracted from cells harvested on day 14, and Cy3G was purified by a combination of chromatographic techniques(Reference Vitrac, Krisa and Decendit32). The purity of 14C-labelled Cy3G was estimated by HPLC analysis, and it was over 97 %. The specific radioactivity of 14C-Cy3G used for the two experiments described below (study over time and study of tissue distribution) was 23·9 and 29·5 MBq/g, respectively. Given the position of the 14C on phenylalanine and the anthocyanin biosynthetic pathway(Reference Springob, Nakajima and Yamazaki33), Cy3G was labelled on its B-ring (Fig. 1). 14C-Cy3G was dissolved in water immediately before administration.
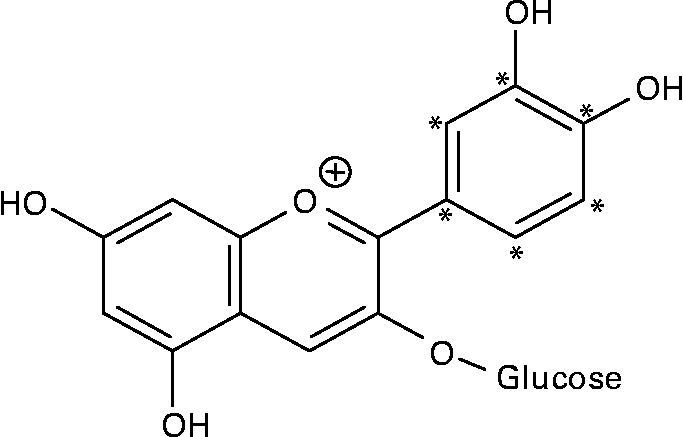
Fig. 1 Structure of 14C-labelled cyanidin 3-O-glucoside. * 14C-labelled positions.
Animals
Male Balb/c mice of 10 weeks of age weighing approximately 23 g (Charles River, L'Arbresle, France) were maintained in a temperature-controlled room with a dark period from 08.00 to 20.00 hours. They were fed ad libitum with a standard chow (113, SAFE, Augy, France) and water for at least 1 week before the experiments. For the first experiment (study over a 24 h period), mice (n 12) were kept in wire-bottomed cages. For the second experiment, mice (n 3) were individually housed in metabolic cages fitted with urine/faeces separators. Mice were deprived of food for 16 h before the administration of the radiolabelled anthocyanin, and they were maintained and handled according to the recommendations of the Institutional Ethics Committee in accordance with the French decree no. 87-848.
Radiolabelled anthocyanin administration and sampling procedure
In the first experiment (study over a 24 h period), mice (n 12) received 0·7 ml of a 14C-Cy3G solution by direct stomach intubation. Each mouse received 22·2 kBq 14C-Cy3G (0·93 mg). Mice were then sacrificed at various times post administration (1, 3, 6 or 24 h, n 3 per time). Mice sacrificed 24 h after anthocyanin administration had access to food from 4 h after the gavage.
In the second experiment (tissue distribution), mice (n 3) received 0·7 ml of a 14C-Cy3G solution containing 141 kBq 14C-Cy3G (4·76 mg) by direct stomach intubation, and they were then sacrificed 3 h after anthocyanin administration.
Anthocyanin intake corresponds to about a 110–600 mg/d intake in human consumers, which is an amount corresponding to less than two servings of blackberries(Reference Wu, Beecher and Holden2).
At the end of the experimental period, mice were sacrificed after being anaesthetised with sodium pentobarbital (40 mg/kg body weight). All the blood was withdrawn from the abdominal aorta into heparinised tubes. Plasma and erythrocytes were separated by centrifugation at 12 000 g for 5 min. Intracardiac perfusion of a NaCl solution (5 ml; 0·15 m) allowed to remove residual blood from the tissues. Inner tissues (liver, kidneys, heart, gallbladder, lungs, epididymal adipose tissue, brain, cerebellum, thymus, prostate gland, testes, gastrocnemius muscle, spleen, bladder, eyes and pancreas) were collected from bloodless mice and weighed.
In the first experiment, stomach, a fraction of small intestine (duodenum) and caecum were collected and washed with deionised water, and radioactivity was analysed in these tissues as described below. Bladder was removed with its urine content, and faeces were collected.
In the second experiment, stomach, whole small intestine, caecum and colon were removed. The contents of the stomach, caecum and colon were collected from their respective organ by washing twice with 0·5 ml of 0·15 m-NaCl. These contents and organs were analysed separately for radioactivity counting. On account of the difficulty in washing small intestine, whole small intestine (i.e. wall and content) was crushed using a homogeniser (Ultra-Turrax) in 1 ml of 0·15 m-NaCl, and three aliquots were analysed for radioactivity content. In this experiment, urine was collected from the bladder by puncture and from the metabolic cage after it was rinsed with 500 μl of distilled water, and analysed separately. Faeces were also collected.
Non-labelled anthocyanin administration and sampling procedure
Mice (n 12) received 0·7 ml of a blackberry anthocyanin solution by direct stomach intubation, and were sacrificed 3 h later. The blackberry anthocyanin solution was obtained by dissolution of the blackberry extract at a concentration of 11·2 mg/ml in water. Hence, the mice received 3·03 mg (6·26 μmol) of Cy3G equivalents. The blackberry extract contained 38·7 % anthocyanins. They are only derivatives of cyanidin, with Cy3G being the main anthocyanin (90 %)(Reference Talavéra, Felgines and Texier20).
At the end of the experimental period, mice were sacrificed after being anaesthetised with sodium pentobarbital (40 mg/kg body weight). All the blood was withdrawn from the abdominal aorta into heparinised tubes. Blood samples were centrifuged at 12 000 g for 5 min, and the plasma was removed. Urine was collected from the metabolic cage after it was rinsed with 500 μl of distilled water. When present, urine was collected from the bladder and pooled with the urine collected from the cage. Urine and plasma samples were acidified with 240 mm-HCl and stored at − 20°C until HPLC analysis.
Radioactivity quantification
Whole organs (weight < 150 mg) or aliquots were digested immediately after collection. Plasma, erythrocytes, faeces and urine samples were treated in the same manner. Digestion of organs was carried out with 2 ml of Soluene-350 for 4 h at 50°C. Then, 0·2–0·5 ml of H2O2 was added until a clear solution was obtained. Absolute quantification of radioactivity in selected organs and biological liquids was performed by scintillation counting. Radioactivity was determined after colour quench correction by adding 10 ml of Hionic-Fluor liquid scintillation cocktail to each sample. Counts were measured three times over a 10 min period each in a Tricarb 2200 CA liquid scintillation counter (Perkin Elmer), and were corrected for background (estimated at 20 dpm). The counting efficiency was on average 96 %. Radioactivity was detected in all the studied organs at levels clearly higher than the background one.
HPLC analysis
Urine samples were centrifuged at 12 000 g for 5 min, and the supernatant (20 μl) was injected and analysed by HPLC as described below.
Plasma anthocyanins were extracted with a Sep-Pak C18 Plus solid-phase extraction cartridge (Waters, Milford, MA, USA) as described previously(Reference Talavéra, Felgines and Texier19) using malvidin 3-O-glucoside as the internal standard, and were then analysed by HPLC (60 μl).
Analysis of anthocyanins was performed by HPLC using a UV–vis detector (785A; Perkin-Elmer) at 524 nm. Samples were loaded onto an Uptisphere 3 ODB C18 3-μm column (150 × 4·6 mm internal diameter) protected by an Uptisphere 3 ODB C18 3-μm guard column (10 × 4 mm internal diameter; Interchim, Montluçon, France). Elution was done using water–H3PO4 (99:1) as solvent A and acetonitrile as solvent B at a flow rate of 1·0 ml/min. Analyses were carried out with linear gradient conditions from 100 to 90 % A for 10 min and then to 75 % A for 30 min. Anthocyanin concentrations are expressed as Cy3G equivalents.
Statistical analysis
Values are given as means with their standard errors. Significant differences between means were determined by one-way ANOVA followed by the Student–Newman–Keuls test (GraphPad, Instat, San Diego, CA, USA).
Results
Distribution of radioactivity in various tissues was determined after the administration of 22·2 kBq 14C-Cy3G in mice and sacrifice at various times post administration (Fig. 2). The amount of radioactivity in stomach and duodenum decreased from 1 h 30 min to 24 h, whereas in most of the other organs, the highest radioactivity was detected 3 h after 14C-Cy3G administration, and it decreased thereafter to become very low 24 h after the ingestion. In some organs (heart, thymus, spleen, brain, cerebellum, eyes and muscle), the amount of radioactivity was very low throughout the experiment (data not shown). The amount of radioactivity detected in the tissues outside the gastrointestinal (GI) tract accounted for 0·74, 1·44, 0·55 and 0·17 % of the administered dose at 1 h 30 min, 3 h, 6 h and 24 h post administration, respectively. No radioactivity was detected in faeces collected 1 h 30 min and 3 h after 14C-Cy3G administration. However, 9·5 and 44·5 % of the ingested amount was associated with faecal materials collected 6 and 24 h after the ingestion, respectively.

Fig. 2 Time course distribution of radioactivity in tissues after the ingestion of 22·2 kBq 14C-cyanidin 3-O-glucoside by mice. Mean values with their standard errors (n 3). a,b,c Mean values for a tissue with unlike superscript letters were significantly different (P < 0·05). □, 1 h 30 min; ![]() , 3 h;
, 3 h; ![]() , 6 h; ■, 24 h.
, 6 h; ■, 24 h.
Tissue distribution of radioactivity was then evaluated more thoroughly in mice that received a higher amount of radioactivity (141 kBq 14C-Cy3G per mouse). Animals were sacrificed 3 h later. The entire GI tract and urine were collected and analysed in order to establish a complete evaluation of radioactivity recovery. After 3 h, most of the radioactivity (87·9 % of intake) was in the GI tract, especially in the small intestine (50·7 %) and caecum (23 %; Table 1). At this time, 3·3 % of the radioactivity was recovered in urine (Table 2). There was a minimal accumulation of radioactivity in tissues outside the GI tract, with the total amount of radioactivity in these tissues being 0·76 % of the ingested amount (Table 2). Distribution of radioactivity varied among organs, with liver, gallbladder and kidneys showing the highest counts. No radioactivity was detected in faeces collected 3 h after administration. A total amount of 128 (sem 3) kBq (92·0 (sem 1·1) % of the administered radioactivity) was recovered in these mice.
Table 1 Distribution of radioactivity in the gastrointestinal tract 3 h after oral administration of 141 kBq of 14C cyanidin 3-O-glucoside in mice
(Mean values with their standard errors for three mice)
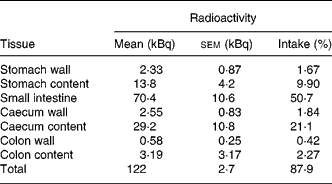
Table 2 Distribution of radioactivity in urine, plasma and tissues outside the gastrointestinal tract 3 h after oral administration of 141 kBq of 14C cyanidin 3-O-glucoside in mice
(Mean values with their standard errors for three mice)

To evaluate the proportion of anthocyanin metabolites with an intact flavylium skeleton (absorbing at 524 nm) present in urine and plasma, a blackberry anthocyanin extract, as a source of Cy3G, was administered to mice. Plasma and urine were collected 3 h after the administration of blackberry anthocyanins and were analysed by HPLC (Fig. 3). The total urinary excretion of anthocyanins accounted for 1·1 (sem 0·1) % (n 3) of anthocyanin intake. Total amount of anthocyanins detected in plasma was 0·45 nmol/mouse, which corresponded to 0·007 % of ingested anthocyanins.

Fig. 3 HPLC chromatograms of blackberry anthocyanins (a) and urine (b) and plasma (c) collected from mice 3 h after oral administration of a blackberry anthocyanin solution. Detection was performed at 524 nm. Peaks are as follows: 1, cyanidin 3-O-glucoside; 2, methylated cyanidin 3-O-glucoside; IS, internal standard (malvidin 3-O-glucoside).
Discussion
Numerous studies conducted in human subjects or animals have shown that total urinary excretion of anthocyanins is typically < 0·1 % of the administered dose (for review, see reference(Reference Galvano, La Fauci and Vitaglione5)). However, only a few authors have evaluated their distribution in body tissues and organs, and when this was the case, only parent compounds and their metabolites detectable at approximately 525 nm have been quantified(Reference Talavéra, Felgines and Texier20, Reference Kalt, Blumberg and McDonald22, Reference Ichiyanagi, Shida and Rahman23, Reference Felgines, Texier and Garcin25, Reference El Mohsen, Marks and Kuhnle34). Oral administration of a labelled substrate such as 14C-Cy3G used in the present study allows to follow the distribution of radioactivity in the body. Cy3G was labelled on its B-ring. 14C will thus be present in all the anthocyanins and metabolites retaining the core flavonoid structure as well as in metabolites derived from the B-ring such as protocatechuic acid. Although quantification of radioactivity provided no information regarding the nature of the labelled metabolites, it allowed, however, to evaluate the amount of anthocyanin-derived metabolites distributed in the body more thoroughly. Excision of tissues was carried out in bloodless mice after perfusion of saline to remove residual blood in organs. Radioactivity detected in tissues was thus a reflection of the presence of anthocyanin derivatives in the tissues and not in the contaminating blood.
The distribution of 14C-Cy3G and labelled metabolites in tissues was first examined over a 24 h period. In plasma and in most organs outside the GI tract, the highest amount of radioactivity was detected 3 h after oral administration of labelled Cy3G. Maximum plasma anthocyanin concentration was usually found between 15 and 30 min after anthocyanin administration in rats(Reference Ichiyanagi, Shida and Rahman23, Reference Hassimotto, Genovese and Lajolo24, Reference Ichiyanagi, Shida and Rahman35, Reference Tsuda, Horio and Osawa36). Moreover, a recent study carried out in mice has shown that in organs such as liver, kidney, lungs, heart and prostate, anthocyanin peak levels occurred within 30 min after oral administration of 500 mg/kg body weight Cy3G and decreased thereafter(Reference Marczylo, Cooke and Brown26). So, the peak of radioactivity observed 3 h after feeding was probably associated mainly to anthocyanin metabolites not detected at 524 nm which could in part result from anthocyanin skeleton degradation.
One and a half hours after feeding the radiolabelled anthocyanin, the bulk of the radioactivity was present in the upper part of the GI tract (stomach and duodenum). Then, the radioactivity progressed down the GI tract, and it was found in faeces from 6 h after anthocyanin intake. After 24 h, faeces contained nearly half of the administered radioactivity (45 %). This radioactivity is more important than that detected in the faeces 24 h after the administration of [2-14C]-quercetin 4′-glucoside in rats (11 % of the administered amount)(Reference Mullen, Rouanet and Auger37). At the same time, the authors also found 64 % of the administered radioactivity in urine. This radioactivity was associated with phenolic acids and other unknown metabolites, but not with quercetin conjugates(Reference Mullen, Rouanet and Auger37). The relatively large excretion of radioactivity in the faeces and low radioactivity in urine observed here for Cy3G, when compared to quercetin 4′-glucoside, reflects its relatively poor systemic bioavailability. The radioactivity found in the faeces corresponds mainly to anthocyanin degradation products since it has been reported in rats fed with various berries (raspberry, bilberry and chokeberry) that < 0·8 % of the administered anthocyanins were recovered in faeces 12–24 h after their administration(Reference Borges, Roowi and Rouanet38, Reference He, Magnuson and Giusti39). Various in vitro studies have shown that anthocyanins incubated with faecal microflora are degraded into phenolic acids originating from the anthocyanin B-ring, and into aldehydes derived from the A-ring(Reference Aura, Martin-Lopez and O'Leary29–Reference Fleschhut, Kratzer and Rechkemmer31, Reference Forester and Waterhouse40). As an example, Cy3G is deglycosylated and degraded into protocatechuic acid and phloroglucinol aldehyde(Reference Keppler and Humpf30). In human subjects, 28 % of the ingested cyanidin glycosides are recovered as protocatechuic acid in the faeces(Reference Vitaglione, Donnarumma and Napolitano41). A limitation of this investigation is the non-identification of phenolic acids. However, analytical determination of phenolic acids could not be carried out due to the limited sample size of mice.
After the administration of 22 kBq/mouse, the amount of radioactivity present in some organs was very low, and thus it was difficult to determine it with accuracy. Thus, in order to evaluate the distribution of radioactivity more thoroughly, a higher amount of 14C-labelled Cy3G (141 kBq per animal) was administered to mice, and the animals were killed at the time of highest radioactivity in the inner tissues, i.e. 3 h after gavage. Most of the radioactivity (88 %) was still present in the GI tract (small intestine: 50·7 % and caecum: 23 %), with the major part being in the digestive content. Nearly half of the administered radioactivity was found in the small intestine and is likely largely derived from Cy3G. Indeed, Borges et al. (Reference Borges, Roowi and Rouanet38) have shown that 3 h after raspberry anthocyanin ingestion by rats, 40 % of the anthocyanins were in the ileum as their native forms, but only 2 % were recovered in the caecum and colon. It could thus be suggested that the labelled compounds in the small intestine were principally anthocyanins, whereas the later parts of the GI tract mainly contained anthocyanin degradation products. Anthocyanins in the lumen of the upper GI tract may be stabilised by binding to mucus, secretions and food residues(Reference He, Wallace and Keatley42). Their limited absorption in the gut and high concentrations in the GI tract could explain the chemoprevention against colorectal cancer as reported in rodents for various anthocyanin-rich extracts or berries(Reference Misikangas, Pajari and Paivarinta11, Reference Cooke, Schwarz and Boocock43–Reference Lala, Malik and Zhao45). Furthermore, in vitro studies on various colon cancer cell lines have shown antiproliferative actions of anthocyanins and anthocyanidins(Reference Zhang, Vareed and Nair46–Reference Renis, Calandra and Scifo49). Microbial Cy3G metabolites such as protocatechuic acid could also participate in the chemopreventive action of anthocyanins since this phenolic acid has been shown to inhibit the growth of human colon cancer cells(Reference Hudson, Dinh and Kokubun50).
Three hours after anthocyanin feeding in mice, 1·1 % of the administered blackberry anthocyanins (mainly Cy3G) were found in urine as metabolites with an intact flavylium skeleton (absorbing at 524 nm). Urinary excretion of radiolabelled metabolites was higher (3·3 %) indicating that about two-third of the urinary metabolites did not possess a flavylium skeleton. Urinary excretion of the labelled metabolites was in the same order of magnitude as that reported after [2-14C]-quercetin 4′-glucoside administration in rats (0·7 and 6·8 % 1 and 6 h after feeding, respectively)(Reference Mullen, Rouanet and Auger37).
Minimal accumulation of radioactivity (approximately 3 %) was observed in tissues outside the GI tract 1–6 h after [2-14C]-quercetin 4′-glucoside feeding to rats(Reference Mullen, Rouanet and Auger37). Similarly, we observed that the amount of radioactivity distributed to tissues beside the GI tract was very low, and that it accounted for only 0·76 % of the administered dose 3 h after administration. Plasma contained about sixfold more labelled metabolites (0·044 % of the administered 14C) than anthocyanin metabolites with intact flavylium skeleton absorbing at 524 nm (0·007 % of the non-labelled blackberry anthocyanins). Protocatechuic acid was described as a major anthocyanin metabolite in various in vitro studies(Reference Aura, Martin-Lopez and O'Leary29–Reference Fleschhut, Kratzer and Rechkemmer31) and in human plasma(Reference Vitaglione, Donnarumma and Napolitano41). However, its presence in rat plasma after Cy3G oral administration remains controversial. Whereas Tsuda et al. (Reference Tsuda, Horio and Osawa36) found protocatechuic acid in plasma at a concentration almost eight times higher than that of Cy3G, Ichiyanagi et al. (Reference Ichiyanagi, Rahman and Hatano51) failed to detect this phenolic acid in plasma. Labelled metabolites without intact flavylium skeleton could correspond to protocatechuic acid as well as to other colourless unidentified metabolites.
Large differences in radioactivity concentration were observed between tissues. The high amount of radioactivity in liver, gallbladder, kidney and bladder is probably caused by the excretion of some anthocyanin metabolites into the bile(Reference Talavéra, Felgines and Texier18) and urine. We have previously reported a relatively high anthocyanin concentration in rat kidney and bladder(Reference Talavéra, Felgines and Texier20, Reference Felgines, Texier and Garcin25). A high concentration of labelled metabolites was also observed in the prostate. Anthocyanins were shown to be cytotoxic and to induce apoptosis in prostate cancer cells(Reference Zhang, Seeram and Lee47, Reference Munoz-Espada and Watkins52–Reference Hafeez, Siddiqui and Asim54). By accumulation of their various metabolites in this gland, anthocyanins could thus play a role in the prevention of prostate cancer.
Low radioactivity was also found in pancreas, lungs and thymus, and very low radioactivity was found in other organs such as heart, adipose tissue, brain and eyes. We have detected previously small amounts of Cy3G and its metabolites in brain, adipose tissue, testes and heart from rats fed with blackberry anthocyanins(Reference Talavéra, Felgines and Texier20, Reference Felgines, Texier and Garcin25). When fed to mice, bilberry anthocyanins were also found in liver, kidney, testes and lungs, whereas no detectable levels were found in heart, thymus, brain, white fat and eyes(Reference Sakakibara, Ogawa and Koyanagi27).
Altogether, 92 % of the administered radioactivity could be recovered from the various tissues and fluids 3 h after 14C-Cy3G feeding. The remaining radioactivity may be distributed in tissues that were not collected such as skeletal muscles, skin, bone and most of the adipose tissues. Indeed, all the analysed tissues accounted for < 25 % of the mouse whole body weight. Moreover, the high overall recovery of radioactivity suggests that losses through degradation into 14CO2 are, at best, minimal at this time. This is in accordance with the recent study of Mullen et al. (Reference Mullen, Rouanet and Auger37) carried out on rats receiving [2-14C]-quercetin 4′-glucoside, but it contrasts with the study of Walle et al. (Reference Walle, Walle and Halushka55) where 52 % of the radioactivity from [4-14C]-quercetin was recovered in the expired air as 14CO2 in human subjects.
In conclusion, the present study is the first one to describe the distribution of the metabolites derived from a radiolabelled anthocyanin in the body. Most of the radiolabelled metabolites (88 %) were present in the digestive tract, and only small amounts were found in inner tissues, confirming the limited systemic bioavailability of anthocyanins. There was thus minimal accumulation of radioactivity in tissues outside the GI tract. The radioactivity found in various tissues may correspond not only to labelled anthocyanins and their metabolites with intact flavylium skeleton, but also to other metabolites that remain to be identified.
Acknowledgements
C. F., O. T. and A. S. designed the research and wrote the paper. C. F., O. T., S. K., A. M. and C. B. conducted the research. S. K. and J.-M. M. provided the essential materials, and A. S., J.-L. L. and J.-M. M. managed the implicated laboratories. The authors have no conflicts of interest to disclose. The present work has no funding.







