Despite the rather high average daily fat intake in France, compared with other European countries, epidemiological surveys document a relatively low rate of cardiovascular mortality. This phenomenon, which is often called the French paradox, is thought to be explained by a fairly high red wine consumption by the French(Reference Renaud and de Lorgeril1, Reference Goldfinger2). Red wine is known to be rich in various polyphenolic compounds that might have a variety of health benefits. Among these polyphenols, the stilbene derivative resveratrol seems to be the most vigorously studied, which is probably due to the fact that it apparently affects a wide array of physiological and biochemical processes as shown in animal and cell culture studies(Reference Cucciolla, Borriello and Oliva3). On the other hand, human studies with conclusive results on resveratrol are regrettably lacking.
Resveratrol is considered to have beneficial effects on the cardiovascular system, as it has been found to improve vasodilatation(Reference Silan4), ischaemic preconditioning(Reference Ray, Maulik and Cordis5, Reference Palfi, Bartha and Copf6), both of which seem to be the result of the activation of the endothelial NO synthase enzyme(Reference Wallerath, Deckert and Ternes7), and to inhibit both platelet aggregation(Reference Cucciolla, Borriello and Oliva3) and vascular smooth muscle cell proliferation(Reference Poussier, Cordova and Becquemin8). In addition, resveratrol has also been demonstrated to show anti-inflammatory(Reference Radnai, Tucsek and Bognar9) and anti-tumour activities(Reference Cucciolla, Borriello and Oliva3), and it might even have considerable anti-ageing properties as it provokes changes in cell signalling that mimics those found upon energy restriction(Reference Cucciolla, Borriello and Oliva3).
Oxidative stress, which is the result of either the overproduction of free radicals or some impairment in the cellular antioxidant systems, can lead to serious deterioration of health. For instance, oxidative stress is likely to be involved in both the development and the advancement of diabetes mellitus, as several studies have demonstrated the involvement of oxygen free radicals (reactive oxygen species) in the appearance of insulin resistance, a distinctive characteristic of type 2 diabetes(Reference Delbosc, Paizanis and Magous10–Reference Wei, Whaley-Connell and Chen12). In addition, oxidative stress might even play some direct role in the subsequent surfacing of other diabetes-related complications(Reference Evans, Maddux and Goldfine13, Reference Baynes14).
Resveratrol itself is an efficient antioxidant, as evidenced by both in vitro (Reference Rüweler, Gülden and Maser15) and in vivo studies(Reference Bhavnani, Cecutti and Gerulath16), and, in parallel, it has also been shown to improve diabetes-related impairments in animals(Reference Sharma, Anjaneyulu and Kulkarni17, Reference Kumar, Kaundal and Iyer18). Thus, in the present study, we address the question whether resveratrol has beneficial effects in controlling and/or improving insulin resistance also in humans, and at the same time, whether it alters oxidative status. In addition, we also aim to elucidate the underlying biochemical mechanisms that might – at least in part – explain the effects of resveratrol, especially in relation to type 2 diabetes.
Patients and methods
Patients
The present study was conducted according to the guidelines laid down in the Declaration of Helsinki, and all procedures involving human patients were approved by the Research Ethics Committee, University of Pécs (Pécs, Hungary; certificate no. 2168). Written informed consent was obtained from all participating patients.
A total of nineteen Caucasian male patients previously diagnosed with type 2 diabetes (according to the WHO diagnostic guidelines) were included in the study. They underwent a blinded randomisation into two groups: ten patients to receive oral resveratrol twice daily (in gelatin capsules containing 5 mg resveratrol) and nine patients to placebo (two capsules daily, see also below). All nineteen patients were over 18 years of age, had normal creatinine clearance ≥ 90 ml/min and were on either angiotensin-converting enzyme inhibitor or angiotensin II receptor blocker medication. Exclusion criteria were receiving insulin treatment, receiving corticosteroids, alcohol or drug abuse, severe liver or cardiac (New York Heart Association III-IV) disease, existing autoimmune disease, acute infection and any type of malignancy. The patients were instructed to abstain from any alcoholic beverages and foods containing substantial amounts of resveratrol (e.g. wine, red grapes, peanuts and berries). Compliance with these instructions was confirmed by verbal declaration of patients.
Study protocol
Before the initiation of the trial, all participants went through a general examination including past medical history, general physical survey, electrocardiogram, blood pressure, pulse rate and blood chemistry analysis (serum Na, K, urea, creatinine, total protein, albumin, HbA1c, fructosamine, alanine transaminase, aspartate aminotransferase, alkaline phosphatase, lactate dehydrogenase, γ-glutamyl transferase, bilirubin and prothrombin). The general examination was followed by a 4-week washout period before the trial began (during which all lipid-lowering medication was ceased).
The day before the initiation of the trial (designated as baseline), 24 h urine samples from all patients were collected, from which creatinine, albumin and ortho-tyrosine (o-Tyr) concentrations were measured. Estimated glomerular filtration rate values were computed according to the Cockroft–Gault equation(Reference Cockcroft and Gault19). During the same day, 24 h blood pressure (ambulatory blood pressure monitor) and a 48 h continuous tissue glucose monitoring (CGM; Medtronic MINIMED, SOF-SENSOR, MMT-7002) were initiated. This was followed by drawing blood to measure glucose, insulin, C-peptide, TAG, LDL, HDL, total cholesterol, fructosamine, high-sensitivity C-reactive protein, fibrinogen, homocysteine, general blood chemistry (see also above), erythropoietin from the plasma and phosphorylated protein kinase B (pAkt):protein kinase B (Akt) ratio in platelets (prepared from the peripheral blood).
During the study period, the aforementioned measurements were repeated twice: at the end of the second (week 2) and the fourth weeks (week 4). After initiation of the CGM, the patients received a test meal (one portion, see later), which was also followed by the collection of blood samples to determine plasma glucose, serum insulin, C-peptide and TAG at 30, 60, 90 and 120 min. In addition, urine was collected to measure 4 h creatinine clearance and albumin secretion.
Analytical procedures
Routine blood and urine tests were carried out by the Department of Laboratory Medicine at the University of Pécs Medical School according to standard clinical laboratory procedures. The values of homeostasis model of assessment for insulin resistance and related to β-cell function (HOMAIR and HOMAβ, respectively) were calculated as in Nagaretani et al. (Reference Nagaretani, Nakamura and Funahashi20) and Matthews et al. (Reference Matthews, Hosker and Rudenski21), respectively. Insulin sensitivity index (ISIStumvoll) and glucose metabolic clearance rate (MCRStumvoll) were calculated as described previously(Reference Stumvoll, Mitrakou and Pimenta22).
Oxidative stress was quantified by determining urinary o-Tyr levels using a reverse-phase HPLC (Shimadzu LC-10 ADVP HPLC system; Shimadzu USA, Canby, OR, USA) equipped with a fluorescence detector (Shimadzu RF-10 AXL) as described previously(Reference Molnár, Wagner and Markó23). Tyr and its isomers were excited at 275 nm, and their emission was measured at 305 nm. Urinary amino acid level was normalised to urinary creatinine concentration.
Platelets were isolated from peripheral blood samples as follows: to obtain washed human platelets, whole blood was collected from fasted (10–12 h) patients and was mixed with trisodium citrate (9:1; 130 mm). Then, the citrated blood was centrifuged at 250 g for 10 min to obtain the platelet-rich plasma by saving the supernatant into a separated polypropylene tube. The resultant platelet-rich plasma was mixed with an equal volume of washing buffer (pH 7·4) containing 20 mm-Tris–HCl and 150 mm-NaCl. The platelet-rich plasma was subsequently centrifuged at 500 g for 10 min, and the platelet pellet was suspended in HEPES–Tyrode's buffer (pH 7·4) containing 140 mm-NaCl, 4·5 mm-KCl, 2·5 mm-CaCl2, 1·0 mm-MgCl2, 11 mm-glucose and 20 mm-HEPES, and was adjusted to a final concentration of 5·0 × 108 platelets/ml. Isolated platelets were lysed on ice for 30 min in NP40 buffer containing 20 mm-Tris–HCl, 137 mm-NaCl, NP40 (1 %), glycerol (5 %) and 1·0 mm-EDTA, supplemented with phosphatase and protein kinase inhibitors containing 1·0 mm-phenylmethylsulfonyl fluoride, 2·0 mm-sodium fluoride, 10 mm-tetrasodium pyrophosphate, 2·0 mm-sodium orthovanadate, 25 mm-aprotinin and 25 mm-leupeptin. Protein concentration of the platelet lysates was determined by the Bradford assay (Bio-Rad, Hercules, CA, USA) using bovine serum albumin as standard. All chemicals were purchased from Sigma (St Louis, MO, USA).
The ratio of the phosphorylated form of Akt and total Akt (pAkt (Ser473):Akt) was determined by Western blot as described previously(Reference Wagner, Laczy and Tamaskó24).
Glucagon-like peptide 1 (GLP-1), glucose-dependent insulinotropic peptide (GIP) and amylin were quantified using ELISA kits commercially available from Millipore (Billerica, MA, USA): EGLP-35K for GLP-1; EZHGIP-54K for human GIP; EZHAT-51K for human amylin.
Materials
Resveratrol of herbal origin (with >98 % t-resveratrol content) and the placebo (both in gelatin capsules) were obtained from Argina Nutraceuticals (previously Admarc Nutraceuticals, Fót, Hungary) and dosed 5 mg/capsule. The identical placebo capsules contained only the carrier microcrystalline cellulose. A known number of (either test or placebo) capsules were boxed to check patient compliance at the end of the trial. The protein, carbohydrate and fat contents of the capsules were negligible. During the study, adverse effects of resveratrol or any signs of drug interaction have not been observed.
The test meal (Diben) was purchased from Fresenius Kabi (Bad Homburg, Germany). It was given to patients in 225 ml portions; one portion had an energy content of 945 kJ from 10·13 g protein, 20·81 g carbohydrate and 11·25 g fat.
Statistical analysis
The measured parameters (CGM data, time until maximal interstitial glucose level, HOMAIR values, o-Tyr:urinary creatinine ratio and pAkt:Akt ratio) showed normal distribution. The data from patients of the placebo and resveratrol groups were arranged respectively and evaluated by employing ANOVA using the program Origin (Microcal, Northampton, MA, USA), and are presented as means and standard deviations. Correlation between clinical parameters was analysed by Pearson's parametric correlation test using the program SPSS 13.0 for Windows (SPSS, Chicago, IL, USA).
As after the first 2 weeks into the trial no significant differences (for any of the measured parameters) between the two groups (i.e. resveratrol v. placebo) were observed, the data of week 2 are not shown. Because of the very large individual variations even at baseline in the HOMAIR and o-Tyr values within the same group, both measures were analysed on the individual level, i.e. for each participant, the value measured at baseline was subtracted from that measured at week 4 (i.e. Δ HOMAIR and Δ o-Tyr:creatinine ratio), and then the resulting values were averaged within the respective groups.
Results
The study was completed with the full compliance of all patients enrolled. The patients who were randomly assigned to the resveratrol and placebo groups initially formed an apparently homogeneous population regarding all the assessed parameters (Table 1), even considering their previous lipid medication usage. Significant differences between the two groups in the assessed clinical and biochemical parameters surfaced at the end of the fourth week of the trial (week 4).
Table 1 Clinical parameters at baseline of patients in the resveratrol and placebo groups* (Mean values and standard deviations)

CRP, C-reactive protein; eGFR, estimated glomerular filtration rate.
* Mean values were not significantly different between the two groups (resveratrol v. placebo) in the listed parameters.
† eGFR was calculated by the Cockroft–Gault formula.
Fig. 1(a) shows a typical example of resveratrol-treated patients' CGM records after consuming the test meal at baseline and at week 4. Some noticeable differences between the two groups (i.e. placebo group v. resveratrol group) emerged at week 4. While the records of placebo group at both baseline and week 4, and those of the resveratrol group at baseline were virtually indistinguishable (not shown), significant differences were found, when these records were statistically analysed for either the two groups (i.e. placebo group v. resveratrol group) at week 4 or the resveratrol group at baseline v. week 4. For instance, the CGM records of the resveratrol group at week 4 displayed a distinct initial drop followed by a recovery phase (see Fig. 1(a)), which was reflected in two statistically analysable parameters. First, the time to the maximum glucose level significantly differed within the resveratrol group at baseline v. week 4 (49·50 (sd 13·83) v. 81·25 (sd 20·49) min, P = 0·006), as well as between the two groups at week 4 (81·25 (sd 20·49) v. 58·1 (sd 18·42) min, P = 0·03). Meanwhile, the time to the maximum glucose appearance in the placebo group did not significantly differ at baseline v. week 4 (42·00 (sd 20·19) v. 56·43 (sd 18·42) min, P = NS; Fig. 1(b)). Second, as shown in Fig. 1(c), the extent of the initial drop (between 23 and 35 min after the test meal) was again significantly different between the two groups at week 4 (6·79 (sd 2·95) v. 8·64 (sd 4·42) mmol/l, P = 0·023).
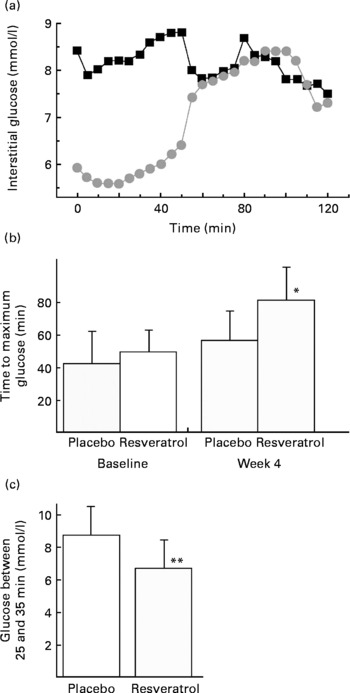
Fig. 1 Effect of resveratrol treatment on continuous glucose monitoring records. (a) An example before and after resveratrol treatment. (■, Baseline; ![]() , week 4). (b) Statistical analysis of time until maximum interstitial glucose is reached after the test meal in the placebo and resveratrol groups. (c) Glucose levels at 25–35 min after the test meal (at week 4) in the placebo and resveratrol groups. Values are means, with standard deviations represented by vertical bars. * Mean values were significantly different for baseline v. week 4 within the resveratrol group (P = 0·006). ** Mean values were significantly different for the resveratrol group v. placebo group (P = 0·023).
, week 4). (b) Statistical analysis of time until maximum interstitial glucose is reached after the test meal in the placebo and resveratrol groups. (c) Glucose levels at 25–35 min after the test meal (at week 4) in the placebo and resveratrol groups. Values are means, with standard deviations represented by vertical bars. * Mean values were significantly different for baseline v. week 4 within the resveratrol group (P = 0·006). ** Mean values were significantly different for the resveratrol group v. placebo group (P = 0·023).
No difference in serum insulin levels between the resveratrol and the placebo groups was observed at any time during the trial (i.e. at baseline v. week 2 or 4).
Because of the large individual variations in the measured HOMAIR values within the groups, a statistical comparison of the groups showed only a tendency of decrease upon resveratrol treatment at week 4 but with no statistical significance (i.e. P = 0·112; not shown). However, as shown in Fig. 2, if the changes in HOMAIR values at week 4 v. baseline on the level of individuals were compared, then the differences were averaged within the respective group, a significant dissimilarity between the two groups emerged (resveratrol group: − 1·52 (sd 1·18) v. placebo group: 0·04 (sd 1·4), P = 0·044). No differences were observed for HOMAβ values (data not shown).

Fig. 2 Decrease in insulin resistance homeostasis model of assessment for insulin resistance (HOMAIR) upon resveratrol treatment. Values are means between baseline and week 4 for the placebo and resveratrol groups, with standard deviations represented by vertical bars. * Mean values were significantly different for the resveratrol group v. the placebo group (P = 0·044).
The test values of the urinary o-Tyr:creatinine ratio, which were averaged within the respective group, showed a noticeable decrease for the resveratrol group at week 4, which, however, was not significant probably due to the extremely large individual variations among the participants (not shown). On the other hand, a clearly significant difference between the two groups again surfaced (0·02 (sd 0·046) v. − 0·015 (sd 0·014) μmol/mmol, P = 0·043; Fig. 3), if the values of difference (i.e. the week 4 − baseline) on the individual level were considered.

Fig. 3 Changes in urinary ortho-tyrosine:creatinine excretion after resveratrol treatment. The test values measured at week 4 were subtracted from those at baseline for each participant separately. Values are means, with standard deviations represented by vertical bars. * Mean values were significantly different (P = 0·043).
Fig. 4 illustrates that a 4-week-long treatment with resveratrol significantly increased the ratio of phosphorylated v. total Akt (pAkt:Akt) ratio in platelets (0·78 (sd 0·25) v. 1·41 (sd 0·36), P = 0·032), while the pAkt:Akt ratio in the placebo group did not change during the trial (0·81 (sd 0·54) v. 0·72 (sd 0·37)).

Fig. 4 Increase in protein kinase B phosphorylation (pAkt) in platelets upon resveratrol treatment. Values are means, with standard deviations represented by vertical bars. * Mean values were significantly different for baseline v. week 4 within the resveratrol group (P = 0·032).
No significant changes were found for amylin, GIP and GLP-1 throughout the trial. At week 4, the amylin level was 2·98 (sd 0·69) pm in the resveratrol group and 3·11 (sd 2·93) pm in the placebo group (NS); the GIP level was 38·22 (sd 9·44) pg/ml in the resveratrol group and 36·23 (sd 8·17) pg/ml in the placebo group (NS); and the GLP-1 level was 7·73 (sd 1·93) pm in the resveratrol group and 7·21 (sd 0·72) pm in the placebo group (NS).
However, we noticed that the initial negative correlation between systolic hyperbaric impact and ISIStumvoll disappeared as a result of resveratrol treatment (R visit 1 − 0·826, P = 0·003 v. R visit 3 − 0·028, P = 0·943 for the resveratrol group) and (R visit 1 − 0·757, P = 0·029 v. R visit 3 − 0·716, P = 0·046 for the placebo group). Similar results were obtained, when the systolic hyperbaric impact was correlated with MCRStumvoll: (R visit 1 − 0·834, P = 0·003 v. R visit 3 − 0·023, P = 0·953 for the resveratrol group) and (R visit 1 − 0·757, P = 0·029 v. R visit 3 − 0·717, P = 0·045 for the placebo group), respectively.
Discussion
In the present double-blind, placebo-controlled study, we tested the effects of trans-resveratrol on male patients with type 2 diabetes, and found that oral resveratrol in reasonably low dosages (2 × 5 mg daily) improved insulin resistance and, probably as a consequence, decreased blood glucose levels and delayed the appearance of glucose peaks after a test meal. On the other hand, no resveratrol-induced changes were found in GLP-1, GIP and amylin levels. In addition, we also found that resveratrol seemed to decrease oxidative stress, as assessed by measuring urinary o-Tyr excretion, and increase Akt phosphorylation. At the same time, we found no evidence that resveratrol would affect β-cell function (HOMAβ).
The present findings appear to be in accordance with the results of previous studies, which have suggested an antioxidant role for resveratrol. For instance, resveratrol has previously been found to lessen oxidative stress in isolated K562 human leukaemia cells(Reference Chan25) and in rats with streptozocin-induced diabetes(Reference Sharma, Anjaneyulu and Kulkarni17, Reference Kumar, Kaundal and Iyer18), which might relate to resveratrol's potential to scavenge oxygen free radicals in vitro (Reference Rüweler, Gülden and Maser15). On the other hand, it also seems to be capable of both decreasing the production of oxygen free radicals and increasing certain antioxidant enzyme levels/activity(Reference Yen, Duh and Lin26). In this regard, it is interesting to note that a simple calculation that considers the amounts of the excreted o-Tyr and the effective resveratrol dosage applied would suggest that the ‘antioxidant’ effect of resveratrol observed in the present study might not be due to a direct free radical-scavenging mechanism.
It has previously been shown that a negative correlation exists between hypertension and insulin sensitivity (i.e. ISIStumvoll(Reference Kanauchi, Kimura and Akai27)). Thus, it is worth noting that the negative correlation between systolic hyperbaric impact, ISIStumvoll and MCRStumvoll seemed to disappear upon resveratrol treatment, suggesting that resveratrol might affect the nature of the association between insulin resistance and hypertension, which itself seems to warrant further studies on its vascular effects in relation to its influence on glucose regulation.
The blood glucose-lowering effect of resveratrol in diabetic rats has been documented and found to be associated with a resveratrol-induced activation of Akt and endothelial NO synthase(Reference Thirunavukkarasu, Penumathsa and Koneru28, Reference Rivera, Morón and Zarzuelo29). The phosphorylation of Akt is known to be an essential step of insulin signalling(Reference Avogaro, de Kreutzenberg and Fadini30). The present findings with human subjects support the notion that the insulin resistance-lowering effect of resveratrol also occurs via the activation of the Akt signalling pathway. On the other hand, oxidative stress is now widely accepted to be a part of the aetiology of insulin resistance(Reference Evans, Maddux and Goldfine13, Reference Baynes14). The present results seem to suggest that, in parallel with reducing insulin resistance, resveratrol might decrease oxidative stress. Although at this time it is not possible to pinpoint a clear cause-and-effect relationship between oxidative stress and some impairment in Akt-activating mechanisms, it is tempting to speculate that such causative coupling might exist.
Whether resveratrol (or some of its future derivatives) becomes a useful tool in combating type 2 diabetes is difficult to tell, although the fact that recent studies(Reference Baur and Sinclair31, Reference Perdew, Hollingshead and Dinatale32) (including the present study) have demonstrated that the efficacy of resveratrol at low doses might increase the possibility for its medicinal application. On the other hand, the present study definitely suggests that resveratrol could become a useful tool in gaining a deeper understanding of the mechanisms underlying the development of insulin resistance and oxidative stress.
Acknowledgements
The present study received no specific grant from any funding agency in the public, commercial or not-for-profit sectors. P. B. organised the clinical visits, sample collection, statistical analysis and manuscript preparation. G. A. M. made the CGM system measurements, and helped in the statistical analysis and manuscript preparation. M. M. helped in the CGM system measurements and manuscript preparation. L. M. measured the oxidative stress markers (HPLC measurements) and helped in the manuscript preparation. B. L. performed the Western blotting experiments and helped in the manuscript preparation. J. C. helped in the Western blotting experiments and manuscript preparation. E. M. isolated platelets from blood samples for Western blotting and helped in the manuscript preparation. I. A. S. helped in the measurement of oxidative stress markers and manuscript preparation. Á. M. helped in the HPLC measurements and manuscript preparation. R. H. randomised the cases and controls and helped in the preparation of figures. L. G. M. helped in the study design, statistical analysis and participated in manuscript preparation. B. S. made the ELISA measurements, and was involved in the study design and application for funding. I. W. contributed to the idea generation and coordination of the study. There is no conflict of interest associated with the present study.







