Development of the rumen is an important physiological challenge for young ruminants. It entails growth and cellular differentiation of the rumen, and results in a major shift in the pattern of nutrients being delivered to the intestines and liver, and thus to the peripheral tissues of the animal( Reference Baldwin, McLeod and Klotz 1 ). Numerous studies have shown that the rumen development process consists of anatomic development (increase in rumen mass and growth of the rumen papillae)( Reference Wang, Xu and Wang 2 , Reference Reynolds, Dürst and Lupoli 3 ), functional achievement (fermentation capacity and enzyme activity)( Reference Faubladier, Julliand and Danel 4 , Reference Rey, Enjalbert and Monteils 5 ), as well as microbial colonisation (bacteria, fungi, methanogenic archaea and protozoa)( Reference Fouts, Szpakowski and Purushe 6 , Reference Fonty, Gouet and Jouany 7 ). Insufficient rumen development will negatively affect nutrient digestion and absorption, or even lead to diseases such as respiratory disease and diarrhoea( Reference Baldwin, McLeod and Klotz 1 ). In contrast, complete rumen development facilitates digestion of feed components, thereby providing nutrients for the physiological requirements of the animal.
Previous studies( Reference Lane, Baldwin and Jesse 8 , Reference Wardrop and Coombe 9 ) have demonstrated that the rumen development process occurs in three phases: non-rumination phase (0–3 weeks); transition phase (3–8 weeks); rumination phase (from 8 weeks onwards). In young calves, ruminal enzyme activities have been observed as early as 2 d of age( Reference Rey, Enjalbert and Monteils 5 ). It has also been found that highest xylanase and amylase activities in the rumen occurred at 7 and 10 d of age, respectively, suggesting degradation capacity, even before the availability of substrate( Reference Rey, Enjalbert and Monteils 5 ). In contrast, Siddons( Reference Siddons 10 ) claimed that ruminal amylase activities increased with age, which was confirmed by observations that the ruminal amylolytic bacterial community increased with age( Reference Anderson, Nagaraja and Morrill 11 ). Furthermore, it has been noted that colonisation of bacteria in the rumen is sequential, and begins in the first week of life( Reference Li, Connor and Li 12 , Reference Jami, Israel and Kotser 13 ). Compared with studies focusing on ruminal bacteria, less attention has relatively been given to colonisation mechanisms of archaea, fungi and protozoa, especially by using non-culture-based molecular methods. Collectively, despite the importance of rumen development, there is still little information available on changes in rumen anatomic development and functional achievement, especially the microbial colonisation process, during these three different phases of rumen development.
In intensive farming, supplemental feeding is a preferred method of providing nutrients with emphasis on offering young ruminants solid starter concentrates at a relative early age( Reference Rey, Enjalbert and Monteils 5 , Reference Jami, Israel and Kotser 13 , Reference Belanche, Balcells and De La Fuente 14 ). During the past few decades, research on rumen development has been mainly focused on this type of feeding system. Concomitantly, factors affecting rumen development processes in ruminants receiving supplemental feeding have been extensively illustrated( Reference Owens, Dubeski and Hanson 15 ), with primary attention on diet composition( Reference Coverdale, Tyler and Quigley 16 – Reference Suárez, Van Reenen and Stockhofe 19 ). For instance, total volatile fatty acid (TVFA) concentrations were higher for calves fed ground- v. coarse-grain starter( Reference Coverdale, Tyler and Quigley 16 ). Moreover, addition of starter concentrates to forage diets positively influenced the anatomic structure of the rumen wall( Reference Suárez, Van Reenen and Stockhofe 19 ).
Feed supplementation for young ruminants is not prevalent in many areas of the world where ruminant production is based primarily on grazing pastures with limited supplemental feed( Reference Liu, Cai and Zhu 20 , Reference Haenlein 21 ). It has been reported that an artificial feeding system with a milk replacer led to lower rumen weight, lower volatile fatty acid (VFA) concentrations and greater pH, when compared with a natural system wherein the offspring remained with its mother( Reference Abecia, Ramos-Morales and Martinez-Fernandez 22 ). However, it is not clear whether the information available on rumen development in supplement-fed ruminants is relevant for grazing ruminants. There is a paucity of knowledge on rumen development in grazing animals, and no studies have been reported in the peer-reviewed literature on differences between these two feeding systems.
Therefore, the present study aimed at (1) describing the age-related sequential dynamic changes in anatomic, functional and microbial variables during time intervals that span the three phases of rumen development, and (2) determining the effect of the feeding system on the rumen development process. In the present study, solid feed (20 d) and weaning (40 d) trials were conducted during the transition phase. The effect of age was tested from birth to 70 d, while the effect of the feeding system was tested after the solid feed was provided (from 20 to 70 d).
Materials and methods
The experiment was approved by the Animal Care Committee, Institute of Subtropical Agriculture, the Chinese Academy of Sciences, Changsha, China.
Animals, diets and management
A total of forty-four 1-d-old male and female goats were separated from their dams. The experiment start for each goat was staggered to accommodate the differing birth dates. To ensure that the environmental conditions were similar throughout the experiment, all the goats were housed in a well-ventilated room with controlled temperature and humidity. The goats weighed an average of 1·35 (sem 0·12) kg at birth. Once each birth was noticed, the kid was immediately placed in an individual pen to avoid direct contact with adult animals. The goats were then maintained within an individual pen for the duration of the study. The animals allocated to the grazing procedure (see below) were returned to their individual pens overnight. From birth to 20 d, all the goats consumed only goat milk ad libitum. Each goat was offered twice daily a bucket of 1 litre of goat milk (per meal) at 08.00 and 17.00 hours. A total of four goats were slaughtered at each of the following ages: 0, 7 and 14 d. The remaining thirty-two goats were randomly divided into two treatment groups, based on different feeding systems: supplemental feeding and grazing. For the supplemented group, between 20 and 40 d, the goats were gradually weaned off goat milk and fed a diet of forage supplemented with starter concentrate. Feeds were provided individually twice daily at 08.00 and 17.00 hours; at each meal, the animals received a bucket of goat milk (0·5 litres), and a separate bucket containing a mixture of forage (fresh grass; 0·04 kg DM/meal) and starter concentrate (0·12 kg DM/meal). After 40 d, the goats consumed only forage (0·06 kg DM for each of the two meals provided daily at 08.00 and 17.00 hours) supplemented with starter concentrate (0·17 kg DM at each meal) until 70 d.
The starter concentrate (per kg DM) was composed of 74·1 g whey powder, 211 g maize flour, 320 g bean meal, 65 g fishmeal, 220 g fat powder, 51 g milk powder, 8·6 g CaCO3, 25·3 g CaHPO4, 5 g NaCl and 20 g premix. The forage fed to the supplemented group was harvested from the same pasture as grazed by the grazing group. Between 20 and 40 d, goats in the grazing group were weaned off goat milk and fed a diet of only forage, which was achieved by grazing on pasture. The goats were offered goat milk (0·5 litres/meal) for 10 min at 08.00 and 17.00 hours, and grazed 8 h daily on pasture. After 40 d, goats in the grazing group received no milk, and just grazed on pasture for 8 h daily until 70 d. In both the groups, four goats were slaughtered at each of the following ages: 28, 42, 56 and 70 d. All goats had free access to water.
Collection procedures and sampling
After slaughtering the goats, live weight and eviscerated hot carcass weight (hide removed) were measured. The rumen was removed, and the content was divided into three parts. The first one-third of the rumen content was filtered through four layers of cheesecloth, and the rumen fluid pH was immediately determined (pH meter model 2000; Beckman Instruments, Inc.). A 4 ml sample of the rumen fluid was collected and stored at − 20°C for the analysis of NH3-N and VFA.
Another one-third of the rumen content was used to isolate solid-associated microbes (SAM) and liquid-associated microbes (LAM), according to the procedures of Yang et al. ( Reference Yang, Beauchemin and Rode 23 ). To avoid sample contamination between samples collected on the same day, the rumen tissue samples were taken using individual sterilised scalpels, and put into individual DNase- and RNase-free centrifuge tubes. Similarly, each rumen content sample was immediately put into an individual container prefilled with CO2, and the isolation procedure of SAM and LAM was conducted under anaerobic conditions. The isolated SAM and LAM were stored in individual DNase- and RNase-free centrifuge tubes at − 80°C until extraction of DNA. Microbial isolation was performed because LAM and SAM differed in composition and function, and could be affected by dietary factors. Many previous studies have demonstrated that SAM account for 70 % of all rumen contents, and thus they are considered to be pivotal to feed digestion in the rumen( Reference Yang, Beauchemin and Rode 23 , Reference Chen, Wang and Wu 24 ).
The final one-third of the rumen content was used to isolate extracellular and intracellular enzymes, as described by Ha et al. ( Reference Ha, Lee and Ahn 25 ). Extracellular enzymes play an essential role in feed digestion. Intracellular enzymes also play a similar role, and finding ways to release these enzymes may also increase ruminal forage fermentation( Reference Ha, Lee and Ahn 25 ). The reason for measuring both extracellular and intracellular enzymes was to establish how age and the feeding system affect the relative proportions, as a means of understanding the development of digestive capacity within the rumen. After the rumen was emptied of its contents, it was rinsed repeatedly with PBS (pH 7·4) until clean, and was then drained of excess PBS and reweighed. Overall, four sections of the rumen wall (approximately 1 cm2) were taken according to the procedures suggested by Lesmeister et al. ( Reference Lesmeister, Tozer and Heinrichs 26 ), and fixed in 10 % formalin solution for anatomic analysis.
Assessment of anatomic structure
Rumen tissue samples were removed from buffered formalin, trimmed and dehydrated using graded ethanol (50, 70, 80, 90 and 100 %) and xylene. Samples were then embedded in paraffin, sectioned and stained with haematoxylin and buffered eosin( Reference Wang, Xu and Wang 2 ). Rumen papillae length and area were measured on the stained sections at 4 × magnification by digital planar morphometry, using a fluorescence microscope (Olympus). A minimum of twenty intact and well-oriented papillae were measured for each sample.
Chemical analysis
The NH3-N concentrations of the rumen fluid were determined by the phenol-hypochlorite method using a UV–visible spectrophotometer (UV-2450; Shimadzu) at 550 nm. VFA (acetate, propionate, butyrate, valerate, isobutyrate and isovalerate) concentrations were analysed by a gas chromatograph (7890A; Agilent). Procedures have been described in detail in our previous work( Reference Jiao, Wang and He 27 ).
Determination of enzyme activity potentials
The preparation of samples for enzyme activity potential measurements was done as suggested by Ha et al. ( Reference Ha, Lee and Ahn 25 ). Extracellular and intracellular enzyme activity potentials of carboxymethylcellulase (CMCase), xylanase and amylase in rumen contents were determined by measuring the release of reducing sugars from substrates (carboxymethylcellulose, xylan and starch, respectively)( Reference Rey, Enjalbert and Monteils 5 ). The reaction times were 30, 15 and 15 min, respectively. One enzyme activity unit (U) was defined as the amount of enzyme required to release 1 μmol of reducing sugars (xylose or glucose equivalents)/min per g of wet rumen content. Extracellular and intracellular protease activity potentials were assayed using azocasein as a substrate, according to the method of Eun & Beauchemin( Reference Eun and Beauchemin 28 ). The hydrolysis of azocasein released an azo group, which induced a coloration measured by spectrophotometry at 420 nm. For each enzyme, the potential of total enzyme activity is the sum of the extracellular and intracellular enzyme activities.
DNA extraction and real-time quantitative PCR
Genomic DNA of LAM and SAM was extracted from 1 ml of fluid using the QIAamp DNA Stool Mini Kit (Qiagen GmbH), according to the manufacturer's instructions with a slight modification. The fluid was incubated at 95°C for 10 min instead of the original 70°C for 5 min after the addition of ASL buffer, in order to lyse both Gram-positive and Gram-negative microbial cells. The quality and quantity of DNA were measured on the basis of absorbance at 260 and 280 nm using a NanoDrop ND1000 (NanoDrop Technologies, Inc.). Primers used for quantification of bacteria and fungi( Reference Denman and McSweeney 29 ), methanogenic archaea( Reference Hook, Northwood and Wright 30 ), and protozoa( Reference Sylvester, Karnati and Yu 31 ) were from previous studies. The quantitative PCR was performed using procedures detailed in our earlier work( Reference Jiao, Wang and He 27 ). Briefly, a standard curve was generated for each microbial group, using plasmid DNA containing the exact 16S or 18S ribosomal RNA gene inserts. The quantitative PCR assays were performed on an ABI 7900HT system (Applied Biosystems) with a total volume of 10 μl, using SYBR® Premix Ex Taq™ (Takara). By relating the C t value to the standard curves, the final copy numbers of targeted bacteria, fungi, methanogenic archaea and protozoa per g of rumen contents were calculated as described by Chen et al. ( Reference Chen, Penner and Li 32 ). The values were converted to log10 for further statistical analysis.
Statistical analysis
The effect of the feeding system (supplemental feeding and grazing) was examined from 28 to 70 d. Data were analysed as a completely randomised design using the MIXED procedure of SAS (SAS Institute, Inc.) with a model that included the fixed effects of feeding system, age and feeding system × age interaction, with individual animal as the experimental unit because each goat was individually fed and measurements were taken from individual goats. The slice option was used when the feeding system × age interaction was significant, to partition and test the effect of the feeding system on age. To test the effects of age on rumen development of goats from 0 to 70 d, the MIXED procedure of SAS (SAS Institute, Inc.) was used, with animal nested within age as the random effect and individual animal as the experimental unit. Orthogonal contrasts were used to test for linear, quadratic and cubic effects of age. Quartic, quintic and sextic effects were not examined because they could not be interpreted biologically. If there was no feeding system × age interaction from 28 to 70 d, the linear and quadratic effects of age from 0 to 70 d were averaged over the two feeding systems (supplemental feeding and grazing). If the interaction was significant, the effects of age from 0 to 70 d for the supplemented and grazing groups were presented separately. Statistical significance was accepted at P< 0·05, and a trend was considered at P< 0·10. All presented data are expressed as least square means.
Results
In the present study, goats were offered fixed amounts of goat milk until 40 d (1 litre/meal from 0 to 20 d and 0·5 litres/meal from 20 to 40 d), and typically all of this milk was consumed. Intakes of the fixed amounts of solid feed provided as forage and starter concentrate to the supplemented group were not recorded in the present study. In addition, it was not possible to monitor the intake for the grazing group under the grazing conditions at the farm. Nonetheless, average weight gain was nearly identical between the supplemented and grazing groups (Table 1); therefore, energy and N intakes were probably similar. Furthermore, for the supplemented goats, a parallel trial (data not shown) indicated that during the period when only goat milk was offered, the goats consumed almost all of the goat milk provided. This separate trial also showed that both during and after weaning, forage and concentrate consumption increased with age.
Table 1 Live weight and carcass weight of the goats at different ages as affected by the different feeding systems (Least square means and standard errors)
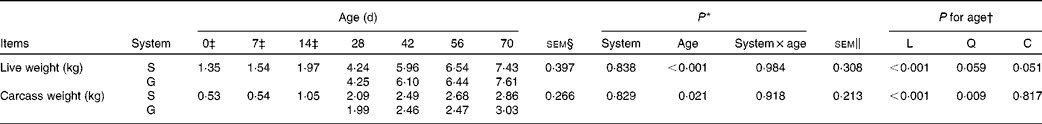
L, linear effect of age; Q, quadratic effect of age; C, cubic effect of age; S, supplemental feeding; G, grazing.
* P value for both feeding systems from 28 to 70 d.
† P for age from 0 to 70 d.
‡ During 0, 7 and 14 d, all goats consumed only milk. After 20 d, goats were randomised into two groups and received solid feed either in S or as G. Four goats were analysed at 0, 7 and 14 d, while eight goats (four goats each for diets S and G) were analysed at 28, 42, 56 and 70 d. The data for 0, 7 and 14 d were merged for S and G because these two groups were fed similarly during this period.
§ For system × age interaction: from 28 to 70 d.
∥ For age: from 0 to 70 d.
Live weight and carcass weight of goats at different ages as affected by the different feeding systems
There were no interaction effects between feeding system and age on live weight (P= 0·984) and carcass weight (P= 0·918), and the feeding system did not affect these parameters (P>0·10) (Table 1). In both groups of goats, an increasing cubic trend of age was observed from 0 to 70 d on live weight (P= 0·051), while a quadratic increasing effect of age was observed on carcass weight (P= 0·009), with the greatest rate of increase occurring between 14 and 28 d.
Anatomic development of rumen parameters of goats at different ages as affected by the different feeding systems
As presented in Table 2, the feeding system did not affect rumen weight (P= 0·655), rumen wall thickness (P= 0·330) or rumen papilla number (P= 0·160), and there were no feeding system × age interactions (P>0·10) for these variables. Irrespective of the feeding system, rumen weight and rumen wall thickness increased cubically with age (P< 0·05), rumen papilla number decreased quadratically with age (P< 0·001), and decreased rapidly between 14 and 28 d. Rumen papilla length in both the groups increased linearly with age (P< 0·001), and its value tended to be greater (P= 0·097) for supplemental feeding v. grazing.
Table 2 Rumen anatomic parameters of goats at different ages as affected by the different feeding systems (Least square means and standard errors)
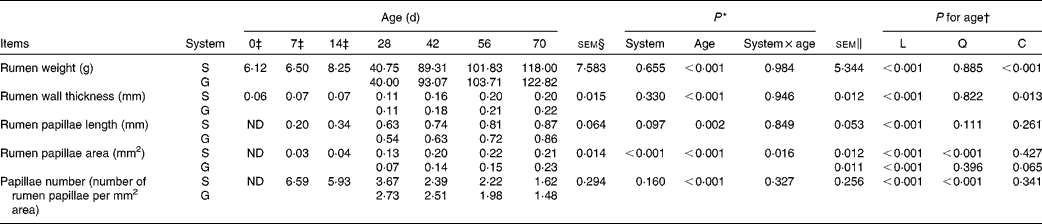
L, linear effect of age; Q, quadratic effect of age; C, cubic effect of age; S, supplemental feeding; G, grazing; ND, not detected due to insufficient sample size.
* P value for both feeding systems from 28 to 70 d.
† P for age from 0 to 70 d.
‡ During 0, 7 and 14 d, all goats consumed only milk. After 20 d, goats were randomised into two groups and received solid feed either in S or as G. Four goats were analysed at 0, 7 and 14 d, while eight goats (four goats each for diets S and G) were analysed at 28, 42, 56 and 70 d. The data for 0, 7 and 14 d were merged for S and G because these two groups were fed similarly during this period.
§ For system × age interaction: from 28 to 70 d.
∥ For age: from 0 to 70 d.
In contrast, an interaction between feeding system and age (P= 0·016) for rumen papillae area was observed, with the papillae area being greater for the supplemented group than for the grazing group at 28, 42 and 56 d, but less than that for the grazing group at 70 d. Furthermore, from 0 to 70 d, in the supplemented goats, rumen papillae area increased quadratically with age (P< 0·001), while in the grazing goats, it tended to increase cubically with age (P= 0·065).
Rumen fermentation capacity of goats at different ages as affected by the different feeding systems
There was no feeding system × age interaction (P>0·10) on rumen pH or NH3-N concentration, and the grazing group had higher pH (P< 0·001) and lower NH3-N concentrations (P< 0·001) than those of the supplemented group (Table 3). From 0 to 70 d, in both groups, age had no effect on pH (P= 0·103), but had a quadratic increasing effect (P= 0·005) on NH3-N concentration.
Table 3 pH, NH3-N and volatile fatty acids (VFA) of the rumen fluid of goats at different ages as affected by the different feeding systems (Least square means and standard errors)
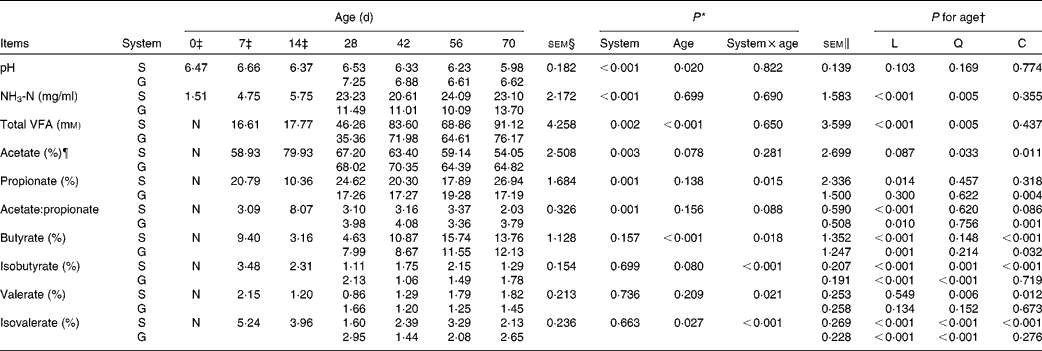
L, linear effect of age; Q, quadratic effect of age; C, cubic effect of age; S, supplemental feeding; G, grazing; N, not detectable because the values were too low.
* P value for both feeding systems from 28 to 70 d.
† P for age from 0 to 70 d.
‡ During 0, 7 and 14 d, all goats consumed only milk. After 20 d, goats were randomised into two groups and received solid feed either in S or as G. Four goats were analysed at 0, 7 and 14 d, while eight goats (four goats each for diets S and G) were analysed at 28, 42, 56 and 70 d. The data for 0, 7 and 14 d were merged for S and G because these two groups were fed similarly during this period.
§ For system × age interaction: from 28 to 70 d.
∥ For age: from 0 to 70 d.
¶ Molar proportions of VFA.
No VFA were detected in rumen samples collected on the day of birth. No feeding system × age interactions were observed for TVFA concentration (P= 0·650) and acetate molar proportion (P= 0·281). The molar proportion of acetate was higher (P= 0·003) while TVFA concentration was lower (P= 0·002) for grazing v. supplemental feeding. From 7 to 70 d, in both groups, age had a quadratic effect on TVFA concentration (P= 0·005), while a cubic effect was observed on acetate molar proportion (P= 0·011). The feeding system × age interaction tended to be significant (P= 0·088) for the acetate:propionate ratio. Age tended to have a cubic effect on the acetate:propionate ratio in the supplemented group (P= 0·086), and had a cubic effect on the acetate:propionate ratio in the grazing group (P= 0·001).
Interaction effects between feeding system and age (P< 0·05) were observed on other molar proportions of VFA except acetate because at different ages, there was no consensus on whether the supplemented group exceeded the grazing group. From 7 to 70 d, age had cubic effects on propionate molar proportion in the grazing goats (P= 0·004), butyrate molar proportion in both groups (P< 0·05), and isobutyrate (P< 0·001), valerate (P= 0·012) and isovalerate (P< 0·001) molar proportions in the supplemented goats. Furthermore, a quadratic effect of age (P< 0·001) was observed on the molar proportion of isobutyrate and isovalerate in the grazing goats, and their values fluctuated with age.
Potentials of ruminal enzyme activities of goats at different ages as affected by the different feeding systems
The enzyme activity potentials of rumen contents were too low to be detected on the day of birth, and at 7 d, amylase and protease could not be detected because the sample size was insufficient to conduct the analytical procedures. The feeding system × age interaction was observed for intracellular xylanase (P= 0·022), extracellular CMCase (P= 0·001) and total CMCase (P= 0·012) because most of these activities were greater at 28 and 42 d, while less at 56 and 70 d for grazing v. supplemental feeding (Table 4). In addition, interactions tended to be significant for extracellular xylanase (P= 0·058), total xylanase (P= 0·068) and intracellular CMCase (P= 0·075). From 14 to 70 d, in the supplemented goats, the activity potentials of xylanase and CMCase (extracellular, intracellular and total) increased linearly with age (P< 0·01), reaching maximum values at 70 d. In grazing goats, the activity potentials of extracellular xylanase (P< 0·001) and total xylanase (P= 0·017) increased linearly with age, while age had quadratic effects on the activity potentials of CMCase (P< 0·05) and intracellular xylanase (P= 0·008).
Table 4 Enzyme activity potentials of the rumen contents of goats at different ages as affected by the different feeding systems (Least square means and standard errors)
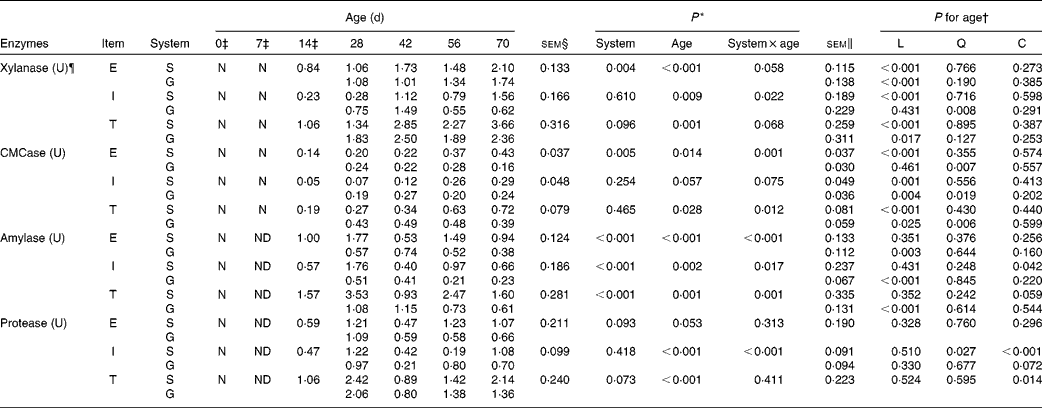
L, linear effect of age; Q, quadratic effect of age; C, cubic effect of age; E, extracellular; S, supplemental feeding; N, not detectable because the values were too low; G, grazing; I, intracellular; T, total; CMCase, carboxymethylcellulase; ND, not detected due to insufficient sample size.
* P value for both feeding systems from 28 to 70 d.
† P for age from 0 to 70 d.
‡ During 0, 7 and 14 d, all goats consumed only milk. After 20 d, goats were randomised into two groups and received solid feed either in S or as G. Four goats were analysed at 0, 7 and 14 d, while eight goats (four goats each for diets S and G) were analysed at 28, 42, 56 and 70 d. The data for 0, 7 and 14 d were merged for S and G because these two groups were fed similarly during this period.
§ For system × age interaction: from 28 to 70 d.
∥ For age: from 0 to 70 d.
¶ For xylanase, CMCase and amylase, 1 U enzyme activity is the amount of enzyme required to release 1 μmol of reducing sugars (xylose or glucose equivalents)/min per g of rumen content; 1 U protease activity is expressed as mg of azocasein hydrolysed/min per g.
For amylase, there were interactions between feeding system and age on the activity potentials of extracellular (P< 0·001), intracellular (P= 0·017) and total (P= 0·001) amylase: the supplemented group exceeded the grazing group for amylase activity potentials at 28, 56 and 70 d, but it was inferior to the grazing group at 42 d. From 14 to 70 d, in the supplemented goats, age had a cubic effect on intracellular amylase (P= 0·042) and a cubic effect tendency on total amylase (P= 0·059). In contrast, in the grazing goats, amylase activity potentials decreased linearly with age (P< 0·01). Similarly, the feeding system × age interaction (P< 0·001) was also detected on intracellular protease, as its value in the supplemented group was higher than that in the grazing group at 28, 42 and 70 d, while lower at 56 d. From 14 to 70 d, despite the fact that protease activity potentials were highest at 28 d in both groups, age had cubic effects on intracellular protease activity potentials in the supplemented goats (P< 0·001), and on total protease activity potentials in both groups (P= 0·014). Furthermore, extracellular protease (P= 0·093) and total protease (P= 0·073) activity potentials in the supplemented goats tended to be greater than those in grazing goats.
Ruminal microbial copy numbers of goats at different ages as affected by the different feeding systems
As illustrated by quantitative PCR results, no feeding system × age interactions (P>0·10) were observed on rumen bacterial and methanogenic archaeal copy numbers (Table 5). The copy numbers of liquid-associated bacteria (P= 0·001) and archaea (P= 0·007) were less, while copy numbers of solid-associated bacteria (P= 0·001) and archaea (P= 0·008) were greater for the grazing group v. the supplemented group. From 0 to 70 d, irrespective of the feeding system, there were increased cubic effects of age (P< 0·05) on liquid-associated and solid-associated bacterial copy numbers. Furthermore, both liquid-associated and solid-associated archaeal copy numbers increased quadratically with age (P< 0·001).
Table 5 Microbial copy numbers (log10 copies/DM rumen contents) of rumen contents of goats at different ages as affected by the different feeding systems (Least square means and standard errors)

L, linear effect of age; Q, quadratic effect of age; C, cubic effect of age; S, supplemental feeding; G, grazing; N, not detectable because the values were too low.
* P value for both feeding systems from 28 to 70 d.
† P for age from 0 to 70 d.
‡ During 0, 7 and 14 d, all goats consumed only milk. After 20 d, goats were randomised into two groups and received solid feed either in S or as G. Four goats were analysed at 0, 7 and 14 d, while eight goats (four goats each for diets S and G) were analysed at 28, 42, 56 and 70 d. The data for 0, 7 and 14 d were merged for S and G because these two groups were fed similarly during this period.
§ For system × age interaction: from 28 to 70 d.
∥ For age: from 0 to 70 d.
Fungi and protozoa were first detected in rumen samples collected at 7 and 14 d, respectively. Feeding system × age interactions were observed on copy numbers of solid-associated fungi (P= 0·024) and liquid-associated protozoa (P= 0·014), for the solid-associated fungi copy number was less while the liquid-associated protozoa copy number was greater for grazing v. supplemental feeding at only 28 d. Furthermore, there were no feeding system × age interactions (P>0·10) on liquid-associated fungi and solid-associated protozoa copy numbers. The solid-associated protozoa copy number (P= 0·019) was greater for grazing v. supplemental feeding. From 7 to 70 d, irrespective of the feeding system, a quadratic effect of age (P= 0·007) was observed on the liquid-associated fungi copy number, with values increasing from 7 to 28 d, and declining thereafter. The solid-associated fungi copy number in the supplemented group was highest at 28 d, while it increased linearly (P= 0·001) with age in the grazing group. Moreover, except for the liquid-associated protozoa from 14 to 70 d, copy number in the supplemented goats (cubic tendency, P= 0·057), age had quadratic effects on protozoa copy number in both groups (P< 0·01), with their values showing an increase with age during the first 56 d, and a slight decline at 70 d.
Discussion
The rumen development process of young ruminants plays a vital role in host health and nutrition. In ruminants, one of the primary roles of the ruminal microbial ecosystem is to secrete various enzymes that break down plant polymers, and to ferment the released sugars into VFA( Reference Fouts, Szpakowski and Purushe 6 , Reference Krause, Nagaraja and Wright 33 ). Furthermore, VFA (mainly butyrate and propionate) are chemical stimuli for the development of the rumen epithelium and papillae, promoting its structural development and absorption activity, thereby providing energy to the host( Reference Bergman 34 ). Moreover, the rumen epithelium provides a critical barrier between the host and the rumen milieu, and epithelial cells play a key role in recognising the rumen microbiome( Reference Malmuthuge, Li and Fries 35 ). Taken together, these observations suggest that anatomic, functional and microbial development in the rumen is an integrated system, and it is essential to study them together for a better understanding of the process of rumen development.
Effects of the feeding systems on anatomic development, functional achievement and microbial colonisation during the rumen development process
To our knowledge, the present study was the first to demonstrate that supplemental feeding is better than grazing in the promotion of rumen development in young goats. Our outcomes revealed that relative to supplemented goats, more of SAM and less of LAM colonised the rumen of grazing goats. This finding may indicate that the grazing goats consumed more forage than the supplemented goats due to the absence of starter concentrate, and to digest plant polymers, more SAM were needed to colonise the forage( Reference Yang, Beauchemin and Rode 23 ). This confirms the findings of previous studies on cattle( Reference Petri, Schwaiger and Penner 36 ) and dairy cows( Reference de Menezes, Lewis and O'Donovan 37 ), which indicate that diet has a clear effect on shaping microbial communities. It has also been reported that the solid-associated bacteria account for approximately 70 % of the total bacteria( Reference Yang, Beauchemin and Rode 23 ), thus playing a pivotal role in fibre digestion. The present study revealed that irrespective of the feeding system, liquid-associated bacteria exceeded solid-associated bacteria in numbers in young goats. A primary reason for this might be the fact that the feed intake of the goats in the present study was not as much as that of adult goats, given that live weight at 70 d was only one-third of mature weight. Nevertheless, in grazing goats, solid-associated fungi exceeded liquid-associated fungi in numbers, suggesting the significance of fungi in the digestion of fibre during early days of ruminant development( Reference Fouts, Szpakowski and Purushe 6 ).
Simultaneously, variation in the feeding system also led to the observation that the rumen fluid in the supplemented group had greater TVFA concentrations and propionate molar proportions than those in the grazing group. This implies that the consumption of some concentrate from 28 d resulted in an earlier initiation of rumen fermentation( Reference Wang, Xu and Wang 2 ). The greater TVFA accumulation and propionate molar proportions in the rumen of supplemented goats probably accounted for the greater rumen papillae area in the present study. Moreover, greater concentrate consumption can lead to higher amylase activity potentials( Reference Suárez, Van Reenen and Beldman 38 ). Therefore, it is not surprising to find that both extracellular and intracellular amylase activity potentials were higher in supplemental feeding v. grazing. Intriguingly, the greater activity potentials of extracellular xylanase and CMCase in supplemented goats suggest that the optimal fibre-degrading capacity occurred when supplemental concentrate was offered( Reference Faubladier, Julliand and Danel 4 ). In general, more effective fibre digestion is related to greater solid-associated bacteria number in mature ruminants because solid-associated bacteria exceeded liquid-associated bacteria in number (about 70 v. 30 %), thus playing a pivotal role in rumen feed digestion( Reference Yang, Beauchemin and Rode 23 , Reference Chen, Wang and Wu 24 ). Nonetheless, the present study demonstrated in young ruminants the significance of liquid-associated bacteria in digesting fibre, given that the numbers of liquid-associated bacteria exceeded those of solid-associated bacteria, which contrasts with the situation in mature animals. Despite this observation, greater xylanase activity potentials were observed at 28 d, and greater CMCase activity potentials at 28 and 42 d in the grazing group, reflecting enhanced ability to degrade fibre in grazing goats during the transition phase.
During weaning, forage provision was also a major determinant, affecting the rumen development process. In young calves, providing chopped hay (3–4 cm) improved fibre digestibility to a greater extent than finely ground hay (2 mm) during the week after weaning, suggesting that the physical form of forage could affect rumen development( Reference Montoro, Miller-Cushon and DeVries 39 ). Meanwhile, provision of chopped hay to calves can promote solid feed DM intake and rumen development (with higher pH)( Reference Khan, Weary and von Keyserlingk 17 , Reference Castells, Bach and Aris 40 ). In the present study, although the forage (fresh grass) offered to both supplemented and grazing goats were of the same chemical composition, different forage intake levels (not measured) might also account for variations in rumen development.
Anatomic development, functional achievement and microbial colonisation during the rumen development process
The present study confirmed that the anatomic, functional and microbial development of the rumen is a temporal and successional process, with age being an important factor, irrespective of the feeding system( Reference Jami, Israel and Kotser 13 ). The ruminal milieu of newborn goats was devoid of VFA and enzyme activities with very low NH3-N concentration, suggesting the absence of fermentation capacity and enzyme degradation, which concurred with previous data for calves( Reference Rey, Enjalbert and Monteils 5 ). Not surprisingly, bacteria colonised rapidly during the first week, similar to a previous observation on calves( Reference Rey, Enjalbert and Combes 41 ).
In the study of Rey et al. ( Reference Rey, Enjalbert and Combes 41 ), the animals were separated from their dams immediately after birth, and placed in individual pens to avoid contact between calves and their dams. Since attendance was not that complete during the birth of the goats in the present study, there was still a possibility of some suckling being unnoticed.
Evidence from previous research on lambs grazing on pasture with dams showed that archaeal colonisation was found at 1–3 d after birth and their population densities reached near 104 copy numbers per g at 1 week of age( Reference Skillman, Evans and Naylor 42 ), within the range of the present study, even though there was a possibility of contamination with adult animals and between lambs( Reference Fonty, Gouet and Jouany 7 , Reference Belanche, Balcells and De La Fuente 14 ). In lambs, whether raised with dams or separately in individual pens, fungi and protozoa appeared relatively later (1–3 weeks), a finding supported by the present results. The maternal vagina microflora directly transmitted to the kid goat( Reference Rey, Enjalbert and Combes 41 , Reference Mändar and Mikelsaar 43 ) and the mother's milk( Reference Hunt, Foster and Forney 44 , Reference Wise and Anderson 45 ) were two main sources of microbes on the first day. As reported in a study on calves( Reference Tamate, McGilliard and Jacobson 46 ), growth of the rumen papillae was also minimal and only slight VFA concentrations were detected in goats during the non-rumination phase. This slow evolution of fermentation capacity could be associated with the intake of only milk without solid feed( Reference Cozzi, Gottardo and Mattiello 18 ). The present results showed a slight increase in acetate molar proportion at 14 d, thereafter a surge in the acetate:propionate ratio. Such a modification in the VFA profile could be attributed to the fact that microbes could have degraded lactose and other oligosaccharides in the milk into acetate and lactate, as supported by previous studies in calf faeces( Reference Shimomura and Sato 47 ). Furthermore, amylase activity potentials were relatively high at 14 d, suggesting a vigorous activity of amylolytic microbes that needs further investigation. This might demonstrate the existence of starch degradation capacity even before the availability of solid feed substrate. Collectively, these results suggest that microbes colonise before functional achievements.
During the transition period (3–8 weeks), feed intake was not recorded in the present study. However, it probably increased with age, as the intake of solid feed usually increases significantly after the first month of life( Reference Anderson, Nagaraja and Morrill 11 , Reference Belanche, Balcells and De La Fuente 14 ). Solid feed provides substrate for microbial fermentation in the rumen, which would account for the surge in microbial copy numbers. Fungi and protozoa colonised fastest during this period (28 d), implying that solid feed plays an indispensable role in their colonisation. On the contrary, Fonty et al. ( Reference Fonty, Gouet and Jouany 7 ) claimed that fungi disappeared in lambs when a solid diet was given. Variations in animal species (lambs v. goats), diet composition, housing conditions, as well as measurement methods (culture method v. molecular method) might account for the discrepancy between the findings of Fonty et al. ( Reference Fonty, Gouet and Jouany 7 ) and the present study.
Along with colonisation of microbes, NH3-N concentration was found to be comparable with that in adult goats( Reference Zeng, Tan and Tang 48 ), and TVFA concentration evolved progressively, which agreed with previous studies on calves( Reference Rey, Enjalbert and Monteils 5 , Reference Sahoo, Kamra and Pathak 49 ). In accordance with the literature( Reference Anderson, Nagaraja and Morrill 11 ), we observed a decline in the acetate:propionate ratio from 8·1 to 3·1–4·0 from 14 to 28 d, which could be attributed to the shift in the goats' diet from milk to forage supplemented with starter concentrate. Lactate resulting from amylolytic microbes that degrade starch in the concentrate and forage can be further converted to propionate by lactate-utilising microbes( Reference Bernalier-Donadille 50 ). Possible enhanced lactate production could account for the increase in the molar proportion of propionate at 28 d.
Presumably increased propionate and butyrate concentrations stimulated the morphological development of the rumen( Reference Tamate, McGilliard and Jacobson 46 ). The rumen papillae length and area increased progressively from 14 to 42 d, which was similar to the findings of a study in calves( Reference Suárez, Van Reenen and Beldman 38 ). It has also been observed that the number of papillae per unit surface area of rumen declined as the goats matured and grew in size, resulting from the development of the rumen (almost ten times larger in size compared with that at 14 d), with higher age and intake, which stretched the basal epithelial tissue and extended the separation distance between the papillae( Reference Reynolds, Dürst and Lupoli 3 ). Furthermore, xylanase and CMCase activity potentials increased markedly during the transition phase, indicating a direct reflection of the surge in fibre-degrading capacity.
Presumably such changes in cellulolytic activities reflect changes in cellulolytic bacteria, which reached their highest values at 30 d in lambs( Reference Fonty, Gouet and Jouany 7 ). Another explanatory factor could be the progressive increase in fungi, which possess the ability to secrete cellulolytic activity and degrade fibre( Reference Fouts, Szpakowski and Purushe 6 ). In the present study, fungi numbers at 42 d were almost thirty times greater than that at 14 d. In contrast, amylase and protease activity potentials surged from 14 to 28 d, followed by a sharp decline at 42 d. In calves, a similar trend in amylase potential has been observed pre- and post-weaning( Reference Sahoo, Kamra and Pathak 49 ), which might be due to the stress of weaning.
From 8 weeks onwards, the goats appeared to be mature ruminants with respect to functional achievement of the rumen. At 56 and 70 d, most of the functional parameters did not evolve any further, irrespective of the feeding system, which was in accord with a previous report on calves( Reference Sahoo, Kamra and Pathak 49 ), demonstrating that functional achievement occurred at 2 months in goats. The quantitative PCR did not detect changes in microbial numbers after 2 months. Similarly, in foals( Reference Faubladier, Julliand and Danel 4 ) and lambs( Reference Skillman, Evans and Naylor 42 ), microbial numbers fluctuated very slightly. In contrast, goat weight gain and rumen enlargement continued beyond 2 months of age( Reference Reynolds, Dürst and Lupoli 3 ). Thus, rumen weight continued to increase, and rumen papillae length and area continued to enlarge, despite complete functional achievement and microbial colonisation of the rumen. Moreover, Tamate et al. ( Reference Tamate, McGilliard and Jacobson 46 ) also observed in calves an increase in rumen empty weight with a decline in the number of papillae per mm2 from 8 to 12 weeks. These results suggest that rumen anatomic development is achieved after 2 months.
It was not feasible to measure the forage intake of grazing goats in the present study, and this is an unfortunate constraint on understanding and interpreting the various responses observed. In addition, variation between individuals is an important determinant during the rumen development process. This is particularly noticeable when presenting microbial data. For example, even though most of the microbial copy numbers of the same group were similar, we still observed that bacterial copy number from one goat at 14 d was almost sixty times greater than that of another milk-fed goat of the same age. Chen et al. ( Reference Chen, Penner and Li 32 ) also found a significant variation in epimural bacterial diversity among individuals within the same group. This suggests that individual variation needs to be taken into account when exploring the interactions between host and commensal microbes.
Conclusion
From 20 d onwards, when solid feed was started to be offered to the goats, supplemental feeding was superior to grazing, in shaping rumen development, as it promoted greater rumen papillae area, fermentation capacity and enzyme activity potentials. Moreover, irrespective of the feeding system, most of the anatomic, functional and microbial parameters of the rumen development process showed an increase with age, with anatomic and functional parameters evolving progressively from 14 to 42 d of age, while microbial colonisation occurring from birth to 28 d. These results imply that microbial colonisation occurred earlier than functional achievement, with anatomic development occurring last. Offering solid feed and weaning played a vital role in shaping the rumen development process. Further investigations are required to determine the changes in microbial composition and diversity, as well as their interactions with anatomic and functional development.
Acknowledgements
The authors appreciate G. E. Lobley (Rowett Institute of Nutrition and Health, University of Aberdeen, Bucksburn, Aberdeen, UK) for assistance with the revision of the paper.
The authors acknowledge the financial support received from the National Natural Science Foundation of China (grant no. 31320103917), the ‘Strategic Priority Research Program – Climate Change: Carbon Budget and Relevant Issues’ (grant no. XDA05020700), the ‘CAS Visiting Professorship for Senior International Scientists’ (grant no. 2010T2S13, 2012T1S0009) and the Hunan Provincial Creation Development Project (2013TF3006).
The authors' contributions are as follows: J. J. and Z. T. designed the research; J. J. and X. L. conducted the research; C. Z. was involved in the animal experiments; J. J., K. A. B. and S. T. performed the statistical analysis and interpreted the results; J. J. wrote the initial draft of the manuscript; K. A. B. critically revised the article for important intellectual content. All authors read and approved the final manuscript.
There are no conflicts of interest to declare.







