Colibacillosis caused by the strains of enterotoxigenic Escherichia coli (ETEC) is a common diarrhoeic disease in neonatal and early weaned pigs(Reference Fairbrother, Nadeau and Gyles1) and is the most important disease worldwide in the swine industry(Reference Zhang, Zhao and Ruesch2). Specifically, ETEC K88 serotype, which expresses fimbrial adhesin F4, is the most prevalent serotype responsible for this disease. The susceptibility of piglets to ETEC K88 is inherited as an autosomal dominant trait and is determined by the expression of receptors on the enterocyte brush borders(Reference Jensen, Frydendahl and Svendsen3). So, the recognition of the intestinal receptors by the bacterial fimbriae and the attachment of E. coli to the brush border of epithelial cells are considered as the primary requisites for the pathogenesis of colibacillosis. After attachment, ETEC K88 can also produce enterotoxins that induce water and electrolyte secretion into the intestinal lumen, resulting in dehydration and metabolic acidosis(Reference Erume, Berberov and Kachman4).
Receptors for ETEC K88 fimbriae appear to be glycoconjugates, which are sparsely located on the mucosal surface of the small intestine of piglets, preferentially in the ileum(Reference Hermes, Molist and Francisco Perez5). Different antigenic variants, referred to as K88ab, K88ac and K88ad fimbriae, bind to their own set of receptors. Whereas the K88ab and K88ac adhesins preferentially bind to glycoproteins, the K88ad adhesin appears to bind to glycolipids(Reference Jin and Zhao6). In both cases, carbohydrates seem to play a key role in the binding of the receptors to ETEC K88 fimbrial adhesins(Reference Erickson, Baker and Bosworth7). In this sense, the importance of N-acetylgalactosamine, fucosylated tetra- and pentasaccharides, GalNAc(β1-4)Gal-containing sequences, β-galactose, N-acetyllactosamine (LacNAc) and phosphatidylethanolamine has been pointed out(Reference Shoaf-Sweeney and Hutkins8). Taking this into account, on the basis of their glycoside composition, the incorporation of some receptor analogues in the diet would be a practical strategy to reduce the number of some intestinal pathogens(Reference Lane, Mehra and Carrington9), including that of ETEC K88(Reference Ofek, Hasty and Sharon10). Until now, numerous reports on the ability of natural feed ingredients (FI) to bind to or block the attachment of ETEC K88 to the intestinal mucosa based on their complex carbohydrate composition have been published(Reference Roubos-van den Hil, Nout and Beumer11–Reference Hermes, Manzanilla and Martin-Orue14). Some vegetables have been proposed as attractive alternatives to antibiotics for swine production(Reference Windisch, Schedle and Plitzner15). Other natural products, such as casein glycomacropeptide (CGMP)(Reference Hermes, Molist and Francisco Perez5, Reference Lane, Mehra and Carrington9) and microbial by-products, such as exopolysaccharides (EPS) and mannan-oligosaccharides (MOS), have also been suggested to act as enteropathogen antiadhesives(Reference Ruas-Madiedo, Gueimonde and de los Reyes-Gavilan16–Reference Baurhoo, Letellier and Zhao19). However, no evidence about the specific interaction between these ingredients and ETEC K88 regarding their ability to block the adhesion of ETEC K88 to the intestinal mucus under in vitro conditions has been reported by solely evaluating their interactions.
The objective of the present study was to make an in vitro screening comparison of the ability of different compounds to bind to ETEC K88 and to block or reduce its attachment to the intestinal mucus using microtitration-based adhesion tests.
Materials and methods
Animals and mucus isolation
The present experiment received previous approval from the Animal Protocol Review Committee of the Universitat Autònoma de Barcelona (no. 689). The treatment, management, housing, husbandry and slaughtering conditions conformed to the European Union Guidelines (The Council of the European Communities, registered under no. 11GCE007-R).
For the present experiment, five weaned piglets (28 d of age) from a commercial farm were selected on the basis of their genotype for susceptibility to ETEC K88 using a DNA marker-based test(Reference Jensen, Frydendahl and Svendsen3), allowing for genomic characterisation for the presence of F4 receptors in the intestinal epithelium. The piglets were maintained at the experimental facilities of the Universitat Autònoma de Barcelona. The piglets were fed a commercial feed treated with colistin for five consecutive days (Coliplus® Solution, Divasa Farmavic SA; 102 500 UI/kg (4·22 mg/kg) of body weight) and complementarily treated by intramuscular administration (Trimixin, S.P. Veterinaria SA) to reduce the microbial load in their gastrointestinal tract(Reference Hermes, Molist and Francisco Perez5). The piglets were killed by intravenous sodium pentobarbital overdosing (200 mg/kg body weight), and the abdomen was immediately cut opened. The ileum was extracted and immersed in the binding buffer (3·84 mm-NaH2PO4, 6·16 mm-Na2HPO4 and 0·15 m NaCl, pH 7·2). After that, sections were split along the mesenteric border and washed with sterile PBS. The intestinal mucus was recovered following the Fang et al. (Reference Fang, Gan and Marquardt20) procedures. Briefly, the mucus was collected by gentle scraping with a glass slide and was then transferred into 20 ml of the binding buffer. All processes were carried out in an ice-cold bath. The scrapings of all the piglets were pooled, mixed and centrifuged at 10 000 g at 4°C for 15 min to remove particulate material. The aliquots of supernatants containing the ileal mucus were stored at − 80°C until use. Before its utilisation in the in vitro tests, the ileal mucus was analysed for protein concentration and for the absence of any indigenous microbial contamination by monitoring the optical density (OD) of its culture in Luria broth (650 nm, every 10 min for 12 h at 37°C). Before each blocking test (BT), mucus was thawed and diluted 1:2 in sterile PBS to prepare the coating suspension well.
Feed ingredient extraction
The FI evaluated in the present study are listed out in Table 1, including ingredients of vegetable origin, a dairy protein and three microbial by-products.
Table 1 Feed ingredients used in the adhesion and blocking tests, along with the feed ingredient abbreviation, the provider company and the country of origin
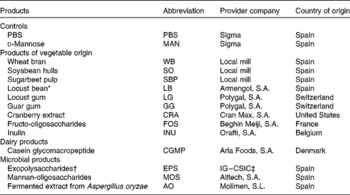
* Product obtained from the carob tree (Ceratonia siliqua) including a meal mixture of carob pods and carob seeds.
† Exopolysaccharides obtained from olive fermentation brines.
‡ Instituto de la Grasa – Consejo Superior de Investigaciones Científicas (Sevilla, Spain).
All the ingredients were prepared following the protocol described by Becker et al. (Reference Becker, Galletti and Roubos-van den Hil21). Briefly, coarse ingredients, such as wheat bran (WB), soyabean hulls and sugarbeet pulp (SBP), were finely ground in an analytical grinder. All the products were suspended in PBS at a solid:liquid ratio of 1:100 (w/v). Subsequently, the suspensions were sonicated three times for 30 s each (Unheated Ultrasonic Bath, JP Selecta) and then centrifuged at 460 g for 5 min (Mikro 220R, Hettich Zentrifugen). The supernatants were stored at − 20°C until used in the in vitro tests.
Wheat bran and locust bean fractionation
WB and locust bean (LB), which are ingredients rich in fibre and NSP, were also fractionated according to the Maes & Delcour(Reference Maes and Delcour22) procedures, which characterise different water-extractable and water-unextractable fibrous fractions from WB. Of each FI, three different fractions were obtained. The water-extractable material (WEM) was the first fraction obtained after enzymatic digestion (α-amylase (90°C – 30 min), protease (55°C – 4 h) and amyloglucosidase (60°C – overnight)), which in the case of WB, according to the authors, mainly comprises more or less 50 % of the total of non-cellulosic sugars (glucose, xylose and arabinose) and high protein content (33 %), but little arabinoxylan content (10 %)(Reference Maes and Delcour22). The first alkali treatment of the cellulosic residue led to a fraction, designated as AED1, and the second alkali treatment to the fraction AED2. During the dialysis step, more than 90 % of the ash content and 30 % of the proteins were removed. The authors reported that these two fractions (AED1 and AED2) have similar monosaccharide composition, and in both extracts, above 90 % are arabinoxylans when referring to WB samples(Reference Maes and Delcour22). Although Maes & Delcour(Reference Maes and Delcour22) applied this fractionation only to WB, in the present study, we also applied this procedure to LB looking for a similar physico-chemical fractionation of NSP. The three fractions obtained from WB and LB were included in the in vitro adhesion and BT.
Escherichia coli strains
In the present experiment, two different E. coli strains were used. Of these, one was an ETEC K88 strain isolated during a colibacillosis outbreak in Spain(Reference Blanco, Blanco and Gonzalez23), serotype (O149:K91:H10 (K-88)/LT-I/STb), which was generously provided by the E. coli Reference Laboratory, Veterinary Faculty of Santiago de Compostela. The other was a non-fimbriated E. coli (F4-, F6-, F18-, LT1-, ST1-, ST2+, Stx2e-) isolated from the faeces of a post-weaning piglet and kindly donated by the Departament de Sanitat i d'Anatomia Animals of the Universitat Autònoma de Barcelona. ETEC K88 was cultured in unshaken Luria broth at 37°C(Reference Snellings, Tall and Venkatesan24), while the non-fimbriated E. coli was cultured in shaking media. Bacteria were serially cultured every 48 h, at least three times.
Bacterial cells were collected by centrifugation of 15 ml of an overnight culture (1700 g, 5 min; Hettich Zentrifugen Mikro 220R). The supernatants were removed, and PBS buffer was added to the cell pellet to achieve an OD of the bacterial suspension of 1 (650 nm), which was used in the adhesion test (approximately log 9–8·5 colony-forming units (cfu)/ml). For the BT, bacterial suspensions were serially diluted to 6·5–7 log cfu/ml.
Adhesion test
The ability of the different FI to adhere to ETEC K88 was determined using an adaptation of the in vitro AT described by Becker et al. (Reference Becker, Galletti and Roubos-van den Hil21). The procedure involves the use of ninety-six-well high-binding polystyrene microtitration plates (Microlon F plate 655 092; Greiner Bio-One BV). Briefly, after overnight incubation at 4°C with 300 μl of the FI extracts, the plates were washed with sterile PBS to remove non-binding material. Afterwards, the non-specific adhesion sites were blocked by incubating the plates with 1 % bovine serum albumin and 0·5 % sodium azide in PBS (w/v) at 4°C for 1 h. Thereafter, the plates were washed twice with sterile PBS, and then 300 μl of the bacterial suspensions (ETEC K88 or non-fimbriated E. coli) were added. The plates were incubated for 30 min at room temperature and washed three times with sterile PBS to remove the non-attached bacteria. Finally, 300 μl of sterile Luria broth were added, and the sigmoidal growth of bacteria was measured using a microplate reader (Spectramax 384 Plus, Molecular Devices Corporation) at 37°C, following the protocol described by Becker et al. (Reference Becker, Galletti and Roubos-van den Hil21). Bacterial growth was monitored as OD at a wavelength of 650 nm at intervals of 10 min for 12 h. All the readings were taken in two independent assays and in triplicate per trial.
Blocking test
The BT was carried out by adapting the methodology described above. Briefly, 300 μl per well of the mucus suspensions were pipetted into the flat-bottomed ninety-six wells of high-binding polystyrene microtitration plates and incubated overnight at 4°C. The plates were washed with 300 μl of PBS to remove non-binding material. To avoid non-specific adhesion, the wells were treated with 350 μl of a mixture containing 1 % bovine serum albumin in PBS and 0·5 % sodium azide at 4°C for 1 h. Subsequently, two washing steps were carried out using 300 μl of PBS. To 500 μl of each tested FI extract (1 %), 500 μl of each bacterial suspension were added separately and incubated at 37°C for 30 min. After that, 300 μl of the co-incubated mixtures were added, in triplicate, to the wells of the microtitration plates and were allowed to adhere to the mucus at room temperature for 30 min. Afterwards, an equal number of washing steps and medium addition and bacterial growth monitoring procedures were carried out as in the in vitro AT protocol described above.
Additionally, the absence of any microbial growth in the FI extracts probably interfering with the test was evaluated by including controls of each extract co-incubated with PBS instead of with bacteria. To exclude the presence of antimicrobial compounds or nutrients in the FI extracts that could modify the number of viable bacteria after co-incubation, microbial counts in Luria agar plates were also conducted after the co-incubation step (30 min at 37°C and 30 min at room temperature). No differences related to PBS were detected. The supernatants of FI that exhibited the highest specific blocking properties (WB, CGMP and LB) were also tested at 0·2, 0·4, 0·6 and 0·8 % (w/v) in different dosage–response BT.
Bacterial counts and tOD=0·05 correlations
In trying to translate the OD values (t OD = 0·05) to cfu per well initially attached to the FI extracts (in the AT) or mucus (in the BT), an assay was carried out with ETEC K88 and the non-fimbriated E. coli strains. Both bacteria were serially diluted in Luria broth medium. The cfu were determined after serial dilutions in PBS, plating on Luria agar and incubation at 37°C for 48 h. At the same time, three replicates of each bacteria and dilution were incubated, adding 300 μl of each dilution per well to the microtitration plates. The growth characteristics were determined at 37°C for 18 h using a microplate reader (SPECTRAmax 384 Plus, Molecular Devices Corporation) as described above in the AT and BT protocols. The t OD = 0·05 (h) and cfu values obtained for each dilution were used to fit the following linear models: y= − 1·6371x+13·543 (R 2 0·994) for ETEC K88 and y= − 1·2875x+11·999 (R 2 0·996) for the non-fimbriated E. coli, where ‘y’ corresponds to t OD = 0·05 and ‘x’ to the log of cfu per well.
Statistical analyses
All the statistical analyses were carried out using SAS 9.2 (SAS, Inc.). The OD data from the AT and the BT were processed by a non-linear regression analysis using the non-linear P-NLIN (Gauss–Newton method) procedure (SAS 9.2, SAS, Inc.) following the equations described previously(Reference Becker, Galletti and Roubos-van den Hil21). From the time when the bacterial growth reached an OD of 0·05 (t OD = 0·05, h), the log cfu were calculated for each FI using the previously described linear models. Significant differences in the log cfu among the FI and between bacterial strains were determined using ANOVA. Linear, quadratic and cubic contrasts were conducted to analyse the dose response of each FI. Differences between the means were tested using the Tukey–Kramer adjustment for multiple comparisons.
Results
In vitro adhesion test
Table 2 summarises the ability of different FI to adhere to ETEC K88 and non-fimbriated E. coli as the number of bacteria attached to each well (log cfu per well). For most of the FI extracts, higher attachment was observed for ETEC K88 than for the non-fimbriated E. coli, indicating that fimbriae play an important role in bacterial adherence. The results indicated three different levels of adhesion to ETEC K88. A group including WB, CGMP and EPS exhibited the highest adhesion, with more than 7 log ETEC K88 cfu per well. Soyabean hull, locust gum (LG), guar gum and MOS had values between 7 and 6 log ETEC K88 cfu per well. Finally, the remaining FI, LB, fructo-oligosaccharides, d-mannose, inulin, fermented extract from Aspergillus oryzae, SBP and cranberry, yielded results similar to those of the negative control (PBS).
Table 2 Number of bacteria (log colony-forming units (cfu) per well) attached to wells coated with different feed ingredient extracts in the adhesion test*
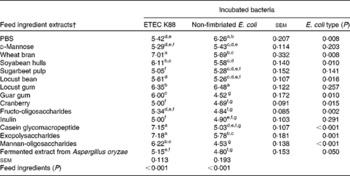
ETEC, enterotoxigenic Escherichia coli.
a,b,c,d,e,f,gValues with unlike superscript letters within a column were significantly different among the feed ingredients (P< 0·001).
* Each value (log cfu per well) was obtained from the average of three replicates in, at least, two independent in vitro assays according to the fitted equations.
† After extraction, all the feed ingredient extracts were tested at 1 % (w/v).
In vitro blocking test
Table 3 summarises the number of bacteria attached to the intestinal mucus after co-incubation with the different ingredient extracts (log cfu per well). In this case, a lower number of bacterial cells is associated with a higher blocking activity of the compounds. The results indicated that five ingredients, WB, LB, LG, guar gum and CGMP, significantly (P< 0·05) decreased the attachment of ETEC K88 to the mucus, when compared with PBS. By contrast, none of these ingredients was able to significantly decrease the number of the non-fimbriated E. coli attached to the mucus, thus demonstrating their specificity to ETEC K88. Among the FI, WB, LB and CGMP exhibited the highest effects with reductions of bacterial numbers by more than one log unit.
Table 3 Number of bacteria (log colony-forming unit (cfu) per well) that attached to the natural mucus after being co-incubated with different feed ingredient extracts tested in the blocking test*
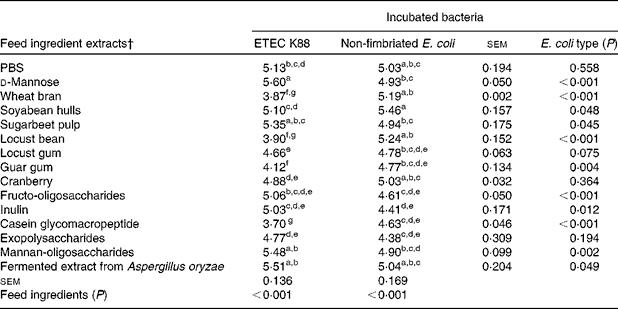
ETEC, enterotoxigenic Escherichia coli.
a,b,c,d,e,f,gMean values with unlike superscript letters within a column were significantly different among the feed ingredients (P< 0·001).
* Each value (log cfu per well) was obtained from the average of three replicates in, at least, two independent in vitro assays according to the fitted equations.
† After extraction, all the feed ingredient extracts were tested at 1 % (w/v).
The dose–response assay with the WB, CGMP and LB extracts revealed a significant linear response (P< 0·001) among the dosages evaluated for the three ingredients (Fig. 1). Nonetheless, the quadratic and cubic contrasts were also significant for LB (P< 0·001) and CGMP (P< 0·05), respectively.
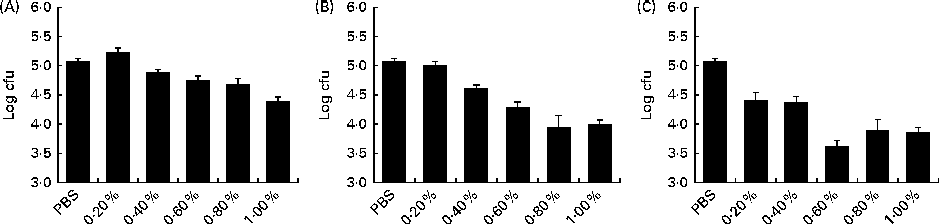
Fig. 1 Dose–response relationships of the ability of the (A) wheat bran, (B) casein glycomacropeptide and (C) locust bean extracts to block the attachment of enterotoxigenic Escherichia coli K88 to the natural ileal mucus. Log colony-forming units (cfu): number of bacteria attached to the natural ileal mucus that were not blocked by the feed ingredients in the blocking test. The lower the log cfu counts, the higher the adhesion-blocking ability. Linear ((A) P< 0·001; (B) P< 0·001; (C) P< 0·001), quadratic ((A) P= 0·095; (B) P= 0·399; (C) P< 0·001) and cubic ((A) P= 0·354; (B) P= 0·020; (C) P= 0·767) contrasts were conducted to analyse the dose response of each feed ingredient. Data were obtained from the experiments carried out in triplicate in two independent assays. Values are means, with their standard errors represented by vertical bars.
Fractionation of the carbohydrate components of wheat bran and locust bean
Carbohydrate fractions obtained from the two vegetable ingredients that exhibited the highest blocking activity (WB and LB) were evaluated in relation to their adhesive (Fig. 2) and anti-adhesive (Fig. 3) properties. All the WB fractions (WEM and AED1 and AED2) exhibited lower adhesive properties than did the sonicated WB extract, while the adhesive property of the AED2 fraction was not significantly different from that of PBS (Fig. 2). With regard to LB, neither the sonicated LB extract nor any of the LB fractions exhibited any relevant ability to adhere to ETEC K88.
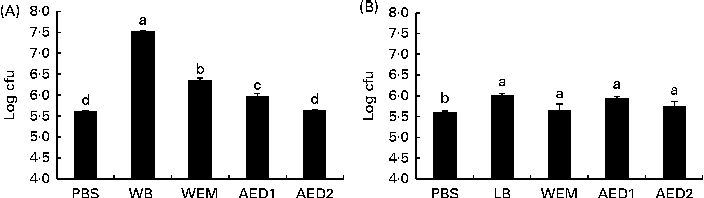
Fig. 2 Number of enterotoxigenic Escherichia coli K88 attached to wells (log colony-forming units (cfu) per well) coated with the different fractions obtained after the digestion process of (A) wheat bran (WB) and (B) locust bean (LB) in the adhesion test. WEM, water-extractable material after enzymatic digestion (α-amylase, protease and amyloglucosidase) and dialysis; AED1, fraction obtained after the first alkali treatment of the cellulosic residue; AED2, fraction obtained after the second alkali treatment. The higher the log cfu counts, the higher the adhesion ability. Values are means, with their standard errors represented by vertical bars. a,b,c,dMean values with unlike letters were significantly different between the fractions (P< 0·05). Data were obtained from the experiments carried out in triplicate in two independent assays.
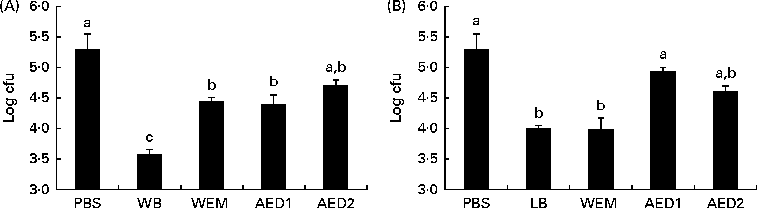
Fig. 3 Number of enterotoxigenic Escherichia coli K88 attached to wells (log colony-forming units (cfu) per well) coated with the natural ileal mucus after pre-incubation with the different fractions obtained after the digestion process of (A) wheat bran (WB) and (B) locust bean (LB) in the blocking test. WEM, water-extractable material after enzymatic digestion (α-amylase, protease and amyloglucosidase) and dialysis; AED1, fraction obtained after the first alkali treatment of the cellulosic residue; AED2, fraction obtained after the second alkali treatment. The lower the log cfu counts, the higher the adhesion-blocking ability. Values are means, with their standard errors represented by vertical bars. a,b,cMean values with unlike letters were significantly different between the fractions (P< 0·05). Data were obtained from the experiments carried out in triplicate in two independent assays.
The ability of these WB and LB fractions to block the attachment of ETEC K88 to the intestinal mucus is presented in Fig. 3. The WB fractions (WEM, AED1 and AED2) did not have the same ability to block the attachment of ETEC K88 as did the sonicated WB extract at 1 %. With regard to LB, the sonicated extract, together with the LB WEM fraction, demonstrated a similar ability to block the attachment of ETEC K88. However, the AED1 and AED2 fractions did not exhibit anti-adhesive properties.
Discussion
Using two simple in vitro methods, the present study shows differences among FI regarding their ability to bind to ETEC K88 and to interfere with its attachment to the intestinal mucus of piglets. These adhesive and blocking activities, when they occurred, were specific for the ETEC K88 strain but not for the non-fimbriated E. coli, thus confirming the role of fimbria F4 in the adhesion of the bacteria to different substrates. Among the FI, WB, CGMP and EPS exhibited the highest binding capacity for ETEC K88. WB and CGMP, together with LB, also exhibited the highest blocking activity in the in vitro blocking assay.
Casein glycomacropeptide
The ability of CGMP to block the adhesion of ETEC K88 to the ileal mucus showed a positive linear and cubic dose response in the range of 0·2–1 %. Brody(Reference Brody25) reviewed the evidence that CGMP obtained from bovine milk binds to E. coli toxins, inhibits bacterial and viral adhesion, promotes bifidobacterial growth and modulates the immune system response of the animals. Moreover, Nakajima et al. (Reference Nakajima, Tamura and Kobayashi-Hattori26) and Malkoski et al. (Reference Malkoski, Dashper and O'Brien-Simpson27) reported the anti-adhesive properties of CGMP against E. coli, arguing for the presence of glycoprotein structures. CGMP contains three glycosylation sites with a heterogeneous array of glycans, based on a core of Galβ(1 → 3)GalNAc- and NeuAc(2 → 5)Gal-, which may act as potential receptor analogues(Reference Grange, Mouricout and Levery28, Reference Rhoades, Gibson and Formentin29). In this sense, searching for new theranostic systems, the specific recognition of CGMP to ETEC K88, instead of to the non-fimbriated E. coli, was determined using chronoamperometric measurements(Reference Espinoza-Castañeda, de la Escosura-Muniz and Gonzalez-Ortiz30). In piglets, CGMP was able to reduce in vitro the attachment of ETEC K88 to porcine intestinal epithelial cells (IPEC-J2)(Reference Hermes, Manzanilla and Martin-Orue14) and also to reduce in vivo the incidence of diarrhoea and the attachment of E. coli to intestinal villi(Reference Hermes, Molist and Francisco Perez5).
Wheat bran
WB is the by-product of the wheat milling industry. It is the outermost covering of wheat grain, which is rich in carbohydrates (40 % of NSP and 34 % of starch) and protein (12 %)(Reference Palmarola-Adrados, Choteborska and Galbe31). In human medicine, WB has been proposed as a cholesterol reducer and for the prevention of certain gastrointestinal cancers(Reference Mohsin-Javed, Zahoor and Shafaat32). To our knowledge, the pathogenic anti-adhesive properties of WB have only been suggested in swine nutrition(Reference Hermes, Manzanilla and Martin-Orue14, Reference Hermes, Molist and Ywazaki33–Reference Molist, Hermes and de Segura35), which can be confirmed by the results obtained by our research group.
The WB extract yielded positive results in both the adhesion and blocking assays. In addition, a significant linear dose response was demonstrated. Other in vivo experiments carried out in piglets orally challenged with ETEC K88 also showed that the dietary inclusion of WB reduced the incidence of diarrhoea and the attachment of E. coli to the ileal mucosa(Reference Molist, Gómez de Segura and Pérez34). These results are also in agreement with other findings(Reference Hermes, Molist and Ywazaki33, Reference Molist, Hermes and de Segura35), reporting that WB in the diet reduces the number of ubiquitous E. coli in the intestinal tract. Moreover, the ability of the WB soluble extract to block the adhesion of ETEC K88 to IPEC-J2 has also been demonstrated(Reference Hermes, Manzanilla and Martin-Orue14).
The WEM did not exhibit the same ability as did the sonicated WB extract to adhere to ETEC K88. The alkaline extracts, AED1 and AED2, did not exhibit a higher adhesion capacity compared with the whole WB extract either. A similar response was observed in the BT, in which none of the fractions exhibited the same blocking ability as did the sonicated WB extract. Both the AED1 and AED2 fractions have been reported to contain xylose (40 %) and arabinose (33 %) as the main monosaccharides(Reference Maes and Delcour22). The failure of these fractions to block the adhesion of ETEC K88 to the intestinal mucus could be due to the digestion process carried out with α-amylase, protease and amyloglucosidase enzymes, which probably may have broken the functional structure of a putative glycoprotein or protein responsible for the recognition of ETEC K88 fimbriae. In this sense, the involvement of a glycoprotein or a complex of proteins deriving from WB could be the reason for the specific binding phenomenon. This possibility is no wonder, because some reports have suggested the ability of protein fractions from plant-derived compounds to act as anti-adhesive substrates(Reference Lengsfeld, Titgemeyer and Faller36, Reference Wittschier, Lengsfeld and Vorthems37). It could also suggest that the ability of WB to adhere to ETEC K88 can be reduced along the gastrointestinal tract through digesta. However, this hypothesis merits further in vivo studies.
Locust bean
The LB used in the present study comprised the milled mixture of carob pods and part of their seeds. This FI is highly rich in galactomannans, a neutral polysaccharide consisting of a β-(1 → 4)-mannan backbone to which single d-galactopyranosyl residues are attached via α-(1 → 6) linkages. Apart from the wide range of technological applications as feed additives(Reference Rinaudo38), galactomannans are also interesting because it has been reported that mannan residues are able to inhibit fimbrial adhesins of enteropathogens such as E. coli and Salmonella (Reference Swanson, Grieshop and Flickinger39).
The LB extract reduced the adhesion of ETEC K88 to the mucus in a similar way to WB; however, LB did not exhibit any ability to adhere to the pathogen. The linear and quadratic contrasts were observed significantly in the dose–response assay. Previous studies using plant products rich in galactomannans have demonstrated anti-adhesive properties against many pathogens. Strong inhibition of Pseudomonas aeruginosa by LB, guar galactomannans and acacia gum has been observed(Reference Zinger-Yosovich and Gilboa-Garber13). In the same line, Hermes et al. (Reference Hermes, Manzanilla and Martin-Orue14) showed the anti-adhesive response against ETEC K88 in porcine intestinal epithelial cells using LB, whereas, more recently, Badia et al. (Reference Badia, Brufau and Guerrero-Zamora40) have reported the anti-adhesive effects of a highly rich β-galactomannan purified from the carob bean gum against Salmonella. The chemical organisation and ramification of sugars of these leguminous plants seem to be essential for the disruption of pathogen receptor recognition(Reference Badia, Zanello and Chevaleyre41).
It is intriguing why the positive effects observed were associated with LB, but not with LG or with the purified d-mannose sugar. The LB used in the present study is from a mixture of seeds and pods where the percentage of endosperm per one pod is about 6 %(Reference Albanell, Caja and Plaixats42), while LG is processed from seed milling, where 52 % of the weight of the seed corresponds to the endosperm (where galactomannans are also present). Therefore, galactomannan content is much lower in LB than in LG. On this basis, LB composition is more heterogeneous. The locust pod is especially rich in insoluble dietary fibre and diverse polyphenolic compounds (83 mg/kg)(Reference Papagiannopoulos, Wollseifen and Mellenthin43). Phenolic compounds have been reported to be involved in the blockage of bacterial adhesion(Reference Huttunen, Toivanen and Arkko44–Reference Riihinen, Ryynanen and Toivanen46) and specifically in the recognition of ETEC K88(Reference Wittschier, Lengsfeld and Vorthems37). Thus, it could be hypothesised that the anti-adhesive response exhibited by LB could have been mediated by the phenolic compounds interfering with F4 recognition rather than by galactomannans. Liu et al. (Reference Liu, Gallardo-Moreno and Pinzon-Arango47) demonstrated that proanthocyanidins obtained from cranberry juice can compress the P fimbriae of uropathogenic E. coli and interfere with bacterial adhesion.
With regard to the fractions obtained from LB, the first fraction after enzymatic digestion, WEM, exhibited the same anti-adhesive capacity as did the sonicated extract, whereas the dialysed products did not. This result could suggest that the digestion process did not modify the responsible moiety. Based on this point of view, soluble galactomannans and/or phenolic compounds could be responsible for the recognition of the type 1 fimbriated E. coli (Reference Zinger-Yosovich and Gilboa-Garber13, Reference Swanson, Grieshop and Flickinger39), as none of the three enzymes included in the digestion process were expected to break down their linkages.
Exopolysaccharides
EPS are long-chain polysaccharides consisting of branched, repeating units of sugars or sugar derivatives, mainly glucose, galactose and rhamnose, in different ratios(Reference Welman and Maddox48). They are exocellular molecules excreted during bacterial growth. EPS from Lactobacillus and Bifidobacteria have been shown to play an important role in the formation of biofilms(Reference Lebeer, Verhoeven and Perea Velez49). The product tested in this work was obtained from the natural fermentation process of green olives in which Lactobacillus pentosus and yeasts have had a relevant role(Reference Garrido-Fernández, Fernández-Díaz and Adams50, Reference Arroyo-López, Querol and Bautista-Gallego51). The results demonstrated the ability of ETEC K88 to adhere to this substrate, but it was not able to promote significant reductions of the adhesion of ETEC K88 to the intestinal mucus. It seems as if the adhesion of fimbria F4 to EPS would not be enough to interfere with the blocking process. We can hypothesise that bacterial EPS, as complex chemical structures, could offer a variable number of complementary molecules to bacterial and mucus lectins. If EPS could attach not only to fimbria F4 but also to other molecular structures in the mucus, EPS could even favour the attachment of the bacteria. In this regard, the potential of different EPS to adhere to probiotics or enteropathogens in the mucus have been described previously by other authors(Reference Ruas-Madiedo, Gueimonde and de los Reyes-Gavilan16, Reference Ruas-Madiedo, Gueimonde and Margolles17). The lack of ability, as had been found by us, to reduce the attachment to the mucus does not fully exclude the potential of EPS to interfere with the adhesion of bacteria to the epithelial cells. The adhesion to epithelial cells is a requisite for infection; nevertheless, adhesion to the mucus can be considered as a way to maintain bacteria far from the epithelium borderline(Reference Variyam52). In vitro studies carried out with epithelial cells could confirm this hypothesis. In this regard, different works describing how EPS can reduce the adhesion of E. coli to Caco-2 cells(Reference Alp, Aslim and Suludere53) and to porcine erythrocytes can be found(Reference Wang, Gänzle and Schwab54).
Other ingredients
All the ingredients used in the present study were chosen based on previously reported properties interfering with bacterial adhesion and/or on their chemical composition, particularly regarding a carbohydrate profile. However, several of them exhibited limited or non-significant effects in the present study.
LG and guar gum seemed to be of interest for testing because of their reported high galactomannan content(Reference Mathur55) and due to the reduction of bacterial numbers observed in the digesta of piglets(Reference Van Nevel, Decuypere and Dierick56). However, the present results did not indicate any effect on adhesion or blocking activity. Soyabean meal oligosaccharides, mainly composed of fructose, galactose and glucose, have been reported to promote the competitive exclusion of potential pathogens(Reference Qiang, YongLie and QianBing57). Studies carried out by van der Meulen et al. (Reference van der Meulen, Panneman and Jansman58) have demonstrated the effects of different legume seeds and their hull fractions as preventives of ETEC K88 colonisation. Nonetheless, the present in vitro tests were not able to evaluate competitive exclusion effects; rather, they only evaluated the pathogen–mucus interaction.
SBP is an important by-product of the sugar extraction industry rich in heterogeneous pectins such as rhamnogalacturonan, among others(Reference Guillon and Thibault59). The inclusion of SBP in swine diets has been shown to modify the intestinal microbiota and the fermentative pattern by a bifidogenic effect(Reference Hermes, Molist and Ywazaki33) related to the presence of long-chain arabino-oligosaccharides(Reference Holck, Lorentzen and Vigsnaes60). However, SBP did not exhibit adhesive and/or anti-adhesive effects in these in vitro microtitration-based models.
With regard to CRA, MOS, A. oryzae, fructo-oligosaccharides and inulin, no significant effect either in the adhesion test or in the BT was found, despite the previous evidence found in the literature. Cranberry extract is known to prevent human urinary tract infections by disrupting bacterial ligand-uroepithelial receptors and by changing the physico-chemical surface properties of E. coli (Reference Liu, Gallardo-Moreno and Pinzon-Arango47). The MOS is an extract from yeast cell walls that has already been shown to exhibit beneficial effects in piglets by reducing the jejunal number of enterobacteria(Reference Castillo, Martin-Orue and Taylor-Pickard61) and by reducing faecal coliform numbers after an ETEC K88 challenge(Reference White, Newman and Cromwell62). MOS had been described by many authors as being able to specifically interfere with the adhesion of pathogens, such as E. coli (Reference Baurhoo, Phillip and Ruiz-Feria18, Reference Baurhoo, Letellier and Zhao19) and Salmonella (Reference Fernández, Hinton and van Gils63), to the intestine. Similar properties have been described for A. oryzae (Reference Becker and Galleti64). The fermented extract from A. oryzae also presents a very unique composition based upon primary fermentation containing high amounts of mannoproteins. Even though the anti-adhesive properties of oligosaccharides such as fructo-oligosaccharides and inulin against bacterial pathogens have been proposed(Reference Gibson, McCartney and Rastall65), no interference was found against ETEC K88 in the present in vitro study.
In summary, the use of two microtitration-based in vitro models has allowed for the identification of certain FI to specifically adhere to and to inhibit the adhesion ETEC K88 to the mucus. However, it should be stated that the absence of effects observed in the present study for some of the tested ingredients does not completely exclude the possibility that they could have a potential role in intestinal colibacillosis through other mechanisms not evaluated by the present in vitro models, such as the blockage of adhesion to the epithelial cells. Among all the FI screened in the present study, WB, CGMP and LB exhibited anti-adhesive properties against ETEC K88, representing the most promising FI. The results obtained from the fractionation of WB suggest that the responsible molecule may be a glycoprotein or a complex of different proteins that specifically recognises ETEC K88. By contrast, galactomannans or phenolic compounds could be the responsible antiadhesive in LB. Despite the fact that molecules responsible for the anti-adhesive response have not been elucidated, the positive effects demonstrated in the present study make them candidates suitable to be included in the diet of piglets with supported functional activity. Even though the purpose of the present study was to evaluate the anti-adhesive properties of ‘intact’ natural products, it should be noted that some of these FI could be digested in the small intestine of piglets(Reference Kiarie, Nyachoti and Slominski66, Reference Gdala, Johansen and Bach Knudsen67) and then activities may be modified. In this sense, further studies are needed to check their anti-adhesive activity throughout the intestinal tract as well as their efficacy to prevent the attachment of pathogens under field conditions.
Acknowledgements
The authors thank Professor Calvo of the Universitat Autònoma de Barcelona for her kind assistance throughout the whole experiment. The authors also acknowledge the companies that kindly provided the FI used in both in vitro screenings: Armegol Hermanos S.A, Arla Foods, Cran Max and Molimen, S.L. This manuscript was proofread by Mr Chuck Simmons, a native English-speaking instructor of English of the Universitat Autònoma de Barcelona.
The present study was supported by the public research project of the Spanish Ministry of Education and Science (Project AGL 2009-07328). The Spanish Ministry of Education and Science had no role in the design and analysis of the study or in the writing of this article.
All the co-authors participated in the research and the writing of this article equally.
None of the authors has any conflicts of interest.








