Environmentally sustainable diets, that are rich in plant foods, are advocated as a solution to feeding the growing human population and reducing the impact of our food systems on the planet(1). Today, vegetarian and vegan diets are widely supported and adopted throughout the population, particularly among young people and women(Reference Martinelli and Berkmanienė2–Reference Allès, Baudry and Méjean4). Vegans do not consume any animal products, whereas vegetarians exclude only meat and fish(Reference Phillips5). The inclusion of fish in a vegetarian diet is often called a pescatarian diet(Reference Phillips5). There are also growing numbers of people in Western countries reducing their animal product consumption who define as flexitarian(Reference Phillips5,6) .
The rising interest in plant-based eating has increased demand for a diversity of commercially available alternative products (e.g. plant-based milk) suitable for vegetarians, vegans or those wishing to reduce their intake of animal products(7). Fast-food chains have similarly designed animal-free products targeting consumers(7,Reference Dunn, Soto and Hua8) , and restaurants have expanded menu options to suit all dietary requirements(7,Reference Dunn, Soto and Hua8) . Individuals now have a more diverse pool of foods suitable for their dietary preferences than before, permitting huge variations in food and nutrient intake. However, although the breadth of choice is beneficial for diet diversity, there continue to be difficulties in achieving adequate quantities of some micronutrients, most notably iodine(Reference Gallagher, Hanley and Lane9).
Iodine is essential for the synthesis of the thyroid hormones (triiodothyronine (T3) and thyroxine (T4)) and is dependent on adequate supply via the diet(Reference Ahad and Ganie10). The WHO recommends a daily intake of 150 μg/d for adults (18+ years) and 250 μg/d for pregnant and lactating women to maintain thyroid hormones synthesis and prevent the development of associated ‘iodine deficiency disorders’ (IDD)(Reference Ahad and Ganie10,11) . However, iodine recommendations are often country-specific, in the UK a Reference Nutrient Intake (RNI) of 140 µg/d is advised for adults (19–50 years)(12). The UK currently has no RNI set for pregnant/lactating women(12). The thyroid hormones have a vital role in the regulation of metabolism, growth and fetal neurological development(Reference Zimmermann13–Reference Hess and Zimmermann15). IDD include hypothyroidism, goitre, the formation of thyroid nodules and cretinism (as a consequence of gestational exposure)(Reference Ahad and Ganie10,Reference Zimmermann14,Reference Eastman and Zimmermann16) . Iodine excess (consumption of > 1000 µg/d iodine) can also have health repercussions, including hypo- and hyperthyroidism(Reference Leung and Braverman17,Reference Bürgi18) .
Iodine deficiency remains a public health issue worldwide(Reference Zimmermann and Andersson19), although the number of countries with adequate iodine intake has nearly doubled over the past 20 years(20). Improvements in global iodine intake and status have largely been due to the effective implementation of universal salt iodisation (USI)(Reference Zimmermann and Andersson19,20) . However, not all countries currently facilitate USI programmes, and other factors (e.g. low household coverage, low usage, low or no accessibility, and inadequate quality) may limit its effectiveness(Reference Zimmermann and Andersson19). Currently in industrialised countries, the predominant dietary iodine sources are iodised salt, cows′ milk, dairy products, fish and seafood, eggs and fortified foods (e.g. bread)(Reference Zimmermann21). Plant foods are generally low in iodine, unless bio-fortified, with seaweed being an exception(Reference Golubkina, Moldovan and Kekina22,Reference Bath and Rayman23) . The iodine content of seaweed is highly variable and can be linked to iodine excess in certain populations(Reference Smyth and Karger24–Reference Zava and Zava27).
Individuals who restrict iodine-rich foods from their diet or are solely dependent on iodine provision from plant foods are at risk of iodine deficiency(Reference Eveleigh, Coneyworth and Avery28). The increased risk of iodine deficiency in vegans and vegetarians was highlighted by the Scientific Advisory Committee on Nutrition (SACN) in 2014(29). Since its publication, several studies have assessed iodine nutrition to be poorer in these groups(Reference Allès, Baudry and Méjean4,Reference Kristensen, Madsen and Hansen30–Reference Eveleigh, Coneyworth and Zhou34) . Innovation in the vegan and vegetarian food sector has resulted in a rapid proliferation of products. Many items, such as meat alternatives, are not often fortified with iodine(Reference Pointke and Pawelzik35,Reference Thomas, Nicol and Bath36) . Alternative dairy products such as alternative ‘milks’ and ‘yogurts’ are now regularly fortified with vitamin B12, D and Ca(Reference Thomas, Nicol and Bath36–Reference Vanga and Raghavan40), but rarely with iodine(Reference Thomas, Nicol and Bath36,Reference Dineva, Rayman and Bath37,Reference Bath, Hill and Infante39) . Additionally, iodine supplements are still not universally consumed by individuals following vegan and vegetarian diets(Reference Eveleigh, Coneyworth and Zhou34).
We previously reviewed the literature on iodine intake and the status of vegans and vegetarians living in industrialised countries in 2020(Reference Eveleigh, Coneyworth and Avery28). Our study concluded that those individuals’ not consuming seaweed or iodine-containing supplements were at risk of poor iodine nutrition. However, half of the studies included in our review were published before meat-free diets entered the mainstream in many countries (2010) and, therefore, may not represent the diversity of ‘modern-day’ vegan and vegetarian diets observed in today’s society(Reference Eveleigh, Coneyworth and Avery28). In the past, traditional vegan diets were reliant on the consumption of wholegrains, pulses, fruit and vegetables(Reference Gallagher, Hanley and Lane9). Whereas, modern-day vegan dietary patterns conflict with traditional vegan dietary patterns due to the improved availability of foods that are often designed for convenience and are ultra-processed (e.g. snacks foods, alternative milks and meats, etc.)(Reference Gallagher, Hanley and Lane9). Greater food availability and choice have permitted a larger number of dietary patterns to be followed within the standard definitions of vegan and vegetarian(Reference Gallagher, Hanley and Lane9).
Following the publication of our review, many new studies have examined iodine in the diet of vegans and vegetarians. Iodine deficiency is still a major public health issue and given that sustainable diets are being promoted in many countries worldwide, a re-evaluation of the literature is needed to monitor iodine nutrition and deficiency in those who select to follow a vegan or vegetarian diet in the modern day.
This review aims to assess the iodine intake and status in adults following a vegan or vegetarian diet in the modern day. The objectives included (1) determination of the iodine intake and food consumption in vegan and vegetarian adults; (2) assessment of the iodine status and prevalence of iodine deficiency using urinary iodine concentration (UIC); (3) comparison of the iodine intake, status, and prevalence of deficiency between modern-day vegans, vegetarians, and omnivores; and (4) completion of a meta-analysis to provide a more precise estimate of the effect size results of individual studies. We hypothesise that vegans and vegetarians will continue to be a subgroup at risk of iodine deficiency; however, given the increase in the availability of iodine fortified foods suitable for individuals following these diets, iodine deficiency may be less severe.
Methods
Search strategy
The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) and the Meta-analyses of Observational Studies (MOOSE) checklist were used to complete this systematic review(Reference Stroup, Berlin and Morton41,Reference Moher, Liberati and Tetzlaff42) . A systematic search of the literature was performed from 5 October 2020 to 16 December 2022. Electronic databases (Ovid MEDLINE, Web of Science, PubMed and Scopus) were explored. Searches were completed with appropriate text terms with truncation and medical subject headings. Our search strategy is the same as that described in our previous systematic review(Reference Eveleigh, Coneyworth and Avery28) (online Supplementary Table 1). All database searches were refined by ‘humans, adults (aged > 18 years), English language, full-papers, and publications between January 2020–December 2022’. Search lists were exported into EndNoteTM before the removal of duplicates. Additional studies were identified via reference lists of relevant published materials and citation searching. A review protocol has not been published prior to our literature search.
Population and outcomes
The population–intervention–comparison–outcome (PICOS) formulation was used to assess study eligibility (Table 1)(Reference Eriksen and Frandsen43).
Table 1. Population–intervention–comparison–outcome (PICOS) criteria for study inclusion and exclusion(Reference Eriksen and Frandsen43)

Data extraction
Extraction forms identified study information including author name, journal, publication date, study location, participant characteristics, dietary groups addressed and grouping method, relevant outcome measures (length of diet, iodine intake, status and supplement use), and any key findings or study considerations (limitations, etc.). Where possible, gendered data were considered separately. Data extraction was completed by the first author.
Classification of outcomes
The nomenclature defining dietary preferences can vary; therefore, dietary groups were compared according to the following classifications (Table 2). We also recorded the method of dietary grouping used and the length of dietary adherence for each study. For articles where individuals are following vegan/vegetarian/etc. diets as part of an intervention, post-intervention data were used.
Table 2. Definition of common vegetarian and vegan diet types

* Diet definition includes descriptions of omnivore (basic) and omnivore (regular) from Kowalska et al. 2020(Reference Kowalska, Brodowski and Pokorska-Niewiada57). Omnivore (basic) includes all food groups including meat, poultry, fish, dairy products and eggs; omnivore (regular) describes data collected from 24-h dietary recalls from ten Polish adults that were used to make standard ‘menus’ for omnivorous participants.
† Diet definitions from Phillips 2005(Reference Phillips5).
‡ Diet definition from Abraham et al. 2022(Reference Abraham, Trefflich and Gauch53).
The WHO criteria were used to assess iodine status using UIC(11,44) . Therefore, a median UIC > 100 μg/l was considered ‘sufficient’ and a median UIC < 100 μg/l was considered ‘deficient’, with severity of IDD based on UIC being: 50–99 μg/l mild, 20–49 μg/l moderate and > 20 μg/l severe(11,44) . Recommended adequate iodine intake varies across countries, so the WHO RDA for adults and adolescents of 150 μg/d was used to assess iodine intake between studies(11,44) .
Quality assessment
The quality of observational cohort, cross-sectional and case–control (matched pairs) studies was assessed using the Newcastle–Ottawa scale (good, fair or poor)(Reference Wells, Shea and O’Connell45). The quality of modelling studies was not assessed (n 1). Quality assessment was completed by the first author and was reviewed by the study team (online Supplementary Table 2).
Statistical analysis
We performed meta-analysis and subgroup analysis to compare iodine intake and status in vegan and vegetarian diets compared with omnivores using the software RevMan (version 5) designed by Cochrane(46). We assumed that the studies are heterogenous; therefore, we selected a random effects mode. For studies reporting data as medians (IQR), we estimated the sample mean and standard deviation using calculations as described by Wan et al (Reference Wan, Wang and Liu47).We separated comparisons of vegan and vegetarian diets into different forest plots to avoid arbitrary omission of relevant groups and double counting of participants. If the test for overall effect were P = < 0·05, the following covariables for subgroup analysis for both meta-analyses were used: sex (female v. mixed sex), USI status and iodine status by country. Countries were categorised into iodine-deficient or -sufficient according to the Iodine Global Network (IGN) Scorecard(20).
Results
The initial literature search yielded 1208 articles. Following screening and exclusion, twenty-eight reports were assessed for eligibility. The PRISMA 2020 flow diagram outlines the study selection process for this review (Fig. 1)(Reference Page, McKenzie and Bossuyt48). A total of eleven articles were identified as eligible for review inclusion (Table 3).

Fig. 1. PRISMA 2020 flow diagram of the study selection process(Reference Page, McKenzie and Bossuyt48). PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses.
Table 3. Studies investigating iodine among vegans, vegetarians and omnivores published after January 2020

NA, not assessed.
* Median and IQR.
Urinary iodine status
Iodine status by UIC was investigated in five studies with a total of 700 participants (vegan; 271, vegetarian; 135, pescatarian; 35, omnivore; 189, flexitarian; 70)(Reference Eveleigh, Coneyworth and Zhou34,Reference Dawczynski, Weidauer and Richert49–Reference Whitbread, Murphy and Clifton52) (Fig. 2). Three studies assessed UIC using single-spot urine samples(Reference Eveleigh, Coneyworth and Zhou34,Reference Groufh-Jacobsen, Hess and Aakre50,Reference Whitbread, Murphy and Clifton52) ; the remaining two studies used 24-h urinary samples(Reference Dawczynski, Weidauer and Richert49,Reference Menzel, Abraham and Stangl51) . We were unable to separate UIC estimates by sex, as all included studies provided either mixed-sex estimates or estimates in females alone.
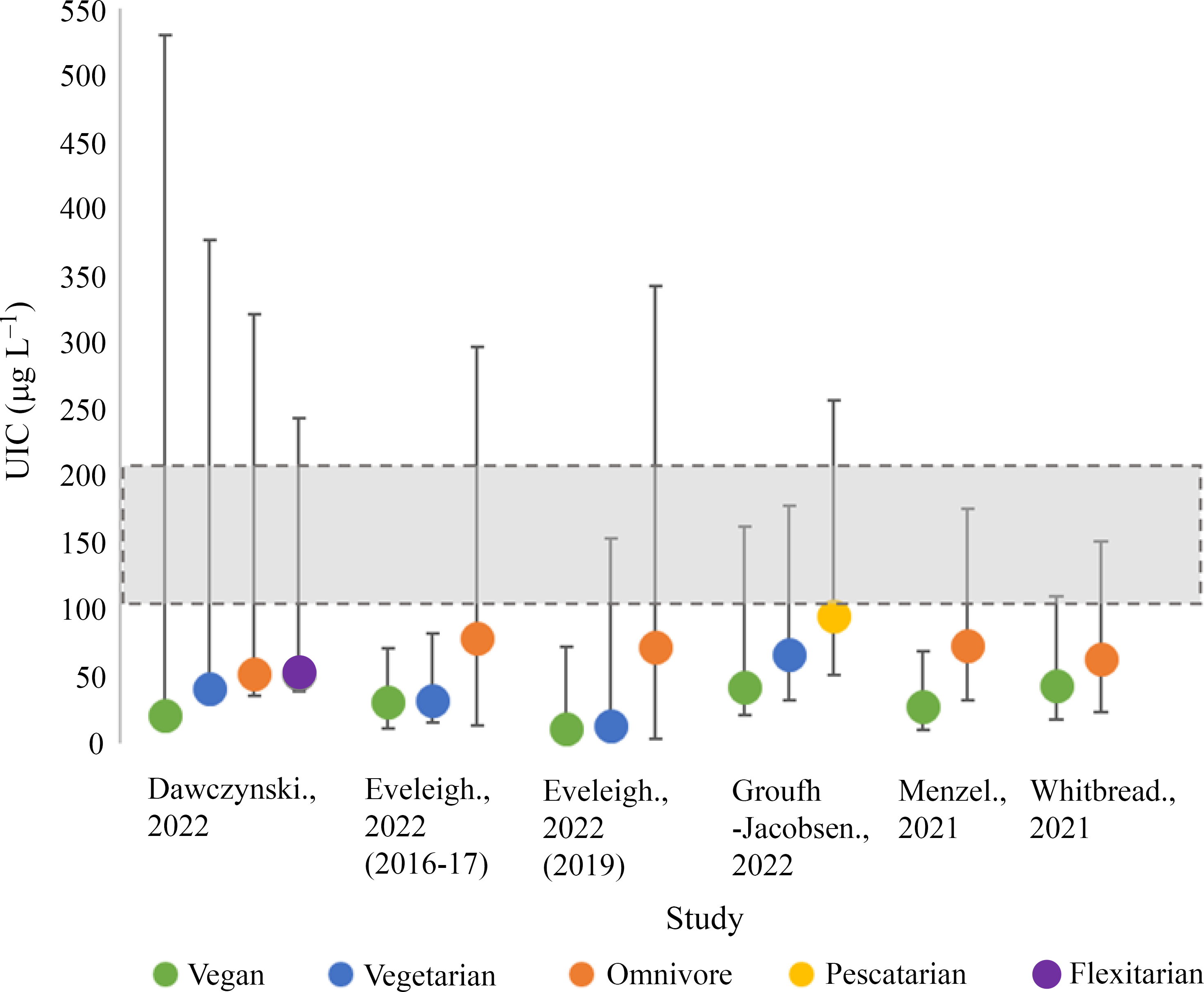
Fig. 2. Iodine status measurements of included studies by median urinary iodine concentration (UIC). Shaded grey areas illustrate the WHO criteria for optimal iodine status (100–200 µg/l)(11).
Vegan participants had the lowest median UIC in all five studies (12·2–44·0 µg/l)(Reference Eveleigh, Coneyworth and Zhou34,Reference Dawczynski, Weidauer and Richert49–Reference Whitbread, Murphy and Clifton52) , with the lowest value recorded by Eveleigh et al. in UK vegans (12·2 µg/l)(Reference Eveleigh, Coneyworth and Zhou34). In all studies, vegans had significantly lower median UIC compared with omnivores (P < 0·05)(Reference Eveleigh, Coneyworth and Zhou34,Reference Dawczynski, Weidauer and Richert49–Reference Whitbread, Murphy and Clifton52) . Vegetarians in the included studies tended to have median UIC values greater than vegans but lower than omnivores, flexitarians and pescatarians(Reference Dawczynski, Weidauer and Richert49,Reference Groufh-Jacobsen, Hess and Aakre50) . Only one study reported the median UIC of vegetarians to be significantly lower than omnivores (P < 0·05)(Reference Eveleigh, Coneyworth and Zhou34). Pescatarians in Norway experienced the greatest median UIC of 96·0 µg/l(Reference Groufh-Jacobsen, Hess and Aakre50). The median UIC of flexitarians was reported in one study (52·0 µg/l) and was slightly lower than the omnivores in the same study (53·0 µg/l)(Reference Dawczynski, Weidauer and Richert49). The included studies showed considerable variation in UIC recorded within dietary groups, for example, the upper range for median UIC for vegans in Dawcynski et al. study was 509 µg/l(Reference Dawczynski, Weidauer and Richert49).
None of the dietary groups included in this review had median UIC values within the optimal range for iodine status (100–200 µg/l) according to WHO criteria and would be classified as iodine-deficient (mild–severe; 50–99 µg/l–> 20 µg/l)(Reference Eveleigh, Coneyworth and Zhou34,Reference Dawczynski, Weidauer and Richert49–Reference Whitbread, Murphy and Clifton52) . Severe iodine deficiency (> 20 µg/l) was observed in one study of vegans living in the UK(Reference Eveleigh, Coneyworth and Zhou34). Moderate deficiency (20–49 µg/l) was observed in all vegan diets and 75 % (3/4) of vegetarian diets(Reference Eveleigh, Coneyworth and Zhou34,Reference Dawczynski, Weidauer and Richert49–Reference Whitbread, Murphy and Clifton52) . Omnivores, flexitarians and pescatarians had median UIC values within the range of mild iodine deficiency (50–99 µg/l)(Reference Eveleigh, Coneyworth and Zhou34,Reference Dawczynski, Weidauer and Richert49–Reference Whitbread, Murphy and Clifton52) . None of the dietary groups in our current review had median UIC values relating to excessive iodine intake (> 300 µg/l)(Reference Eveleigh, Coneyworth and Zhou34,Reference Dawczynski, Weidauer and Richert49–Reference Whitbread, Murphy and Clifton52) .
None of the studies investigating iodine status used creatinine correction(Reference Eveleigh, Coneyworth and Zhou34,Reference Dawczynski, Weidauer and Richert49–Reference Whitbread, Murphy and Clifton52) .
Dietary iodine intake
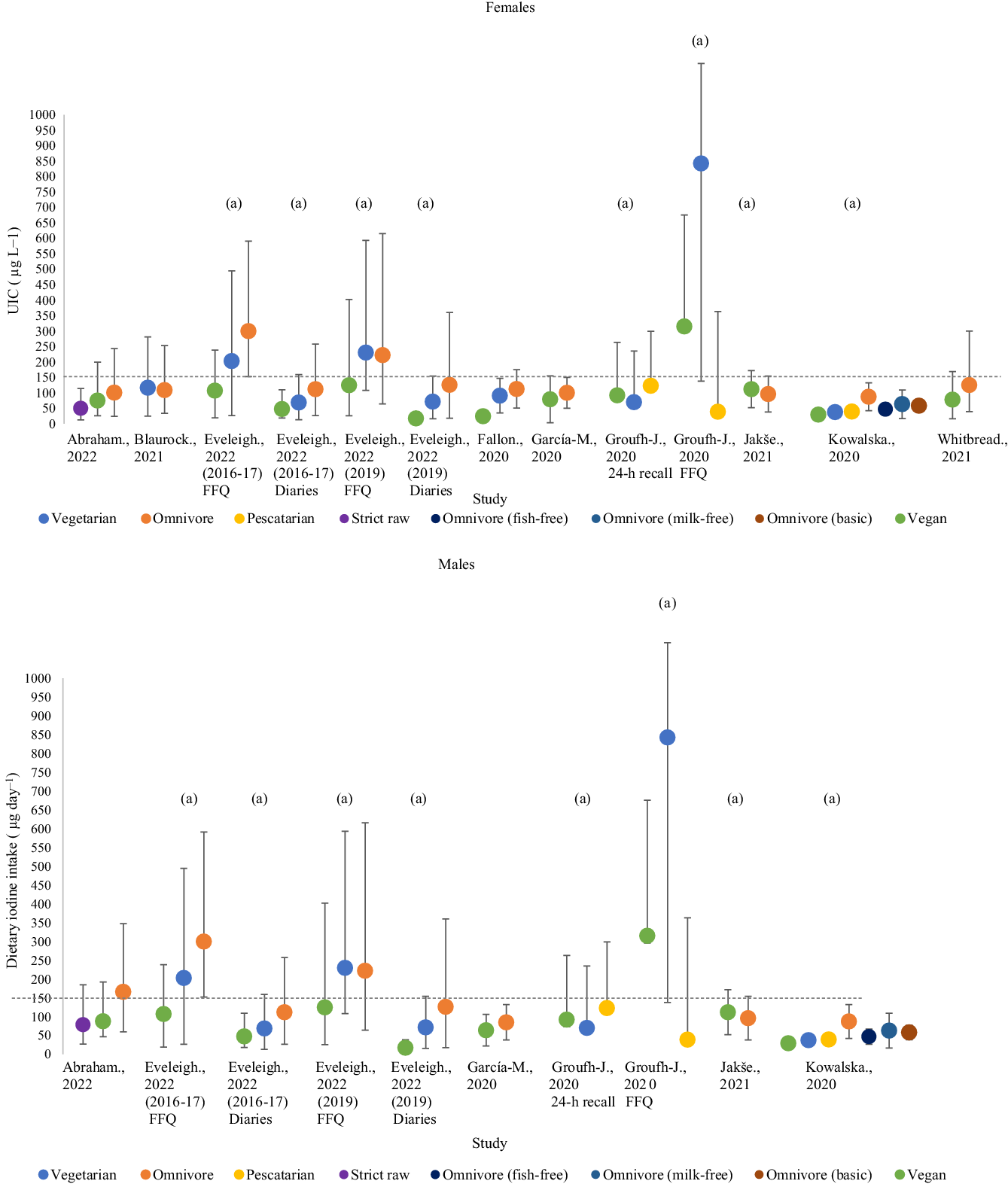
Dietary iodine intakes were recorded in nine studies and included 678 participants (strict raw; 16, vegan; 280, vegetarian; 117, pescatarian; 35, omnivore; 230; Table 4; Fig. 3)(Reference Fallon and Dillon33,Reference Eveleigh, Coneyworth and Zhou34,Reference Groufh-Jacobsen, Hess and Aakre50,Reference Whitbread, Murphy and Clifton52–Reference Kowalska, Brodowski and Pokorska-Niewiada57) . All studies evaluated iodine intake between vegans and one or more dietary groups(Reference Fallon and Dillon33,Reference Eveleigh, Coneyworth and Zhou34,Reference Groufh-Jacobsen, Hess and Aakre50,Reference Whitbread, Murphy and Clifton52–Reference Kowalska, Brodowski and Pokorska-Niewiada57) . Most studies reported mixed-sex estimates of dietary iodine. Only two studies separated dietary iodine intake by sex(Reference Abraham, Trefflich and Gauch53,Reference García-Morant, Cortés-Castell and Palazón-Bru55) , and three studies only recruited females to study(Reference Fallon and Dillon33,Reference Whitbread, Murphy and Clifton52,Reference Blaurock, Kaiser and Stelzl54) . Sixty-seven per cent of studies (6/9) were conducted in countries considered ‘adequate’ according to national data from the Global Scorecard of Iodine Nutrition (2021)(20,Reference Fallon and Dillon33,Reference Eveleigh, Coneyworth and Zhou34,Reference Whitbread, Murphy and Clifton52,Reference García-Morant, Cortés-Castell and Palazón-Bru55–Reference Kowalska, Brodowski and Pokorska-Niewiada57) .
Table 4. Assessment of dietary iodine intake for vegans, vegetarians and omnivores in included studies

* Median (Q1–Q3).
† Mean ± sd.
‡ According to WHO criteria of 150 ug/d.
Food diaries > 3 d (44 %, 4/9) and FFQ (44 %, 4/9) were the most commonly used dietary method to measure iodine intake(Reference Fallon and Dillon33,Reference Eveleigh, Coneyworth and Zhou34,Reference Abraham, Trefflich and Gauch53,Reference Blaurock, Kaiser and Stelzl54,Reference Jakše, Godnov and Pinter56) . Other dietary methods included 24-h food recalls (33 %, 3/9)(Reference Groufh-Jacobsen, Hess and Aakre50,Reference Whitbread, Murphy and Clifton52,Reference García-Morant, Cortés-Castell and Palazón-Bru55) . Three studies addressed iodine intake using two dietary methods to assess both short-term and longer-term dietary intake(Reference Eveleigh, Coneyworth and Zhou34,Reference Groufh-Jacobsen, Hess and Aakre50,Reference Whitbread, Murphy and Clifton52) .
Vegan participants living in the UK in 2019 had the lowest average iodine intake of 17·3 µg/d (17·2–21·4 µg/d; mixed-sex estimates)(Reference Eveleigh, Coneyworth and Zhou34). The greatest iodine intake was recorded in vegetarians in Oslo, Norway, at 843·0 µg/d (705·0–1590·0 µg/d; mixed-sex estimates)(Reference Groufh-Jacobsen, Hess and Aakre50). Omnivores had the greatest dietary iodine intake in 70 % (7/10) of included cohorts(Reference Fallon and Dillon33,Reference Eveleigh, Coneyworth and Zhou34,Reference Whitbread, Murphy and Clifton52,Reference Abraham, Trefflich and Gauch53,Reference García-Morant, Cortés-Castell and Palazón-Bru55,Reference Kowalska, Brodowski and Pokorska-Niewiada57) , whereas vegans had the lowest iodine intake in 73 % of cohorts (8/11)(Reference Fallon and Dillon33,Reference Eveleigh, Coneyworth and Zhou34,Reference Whitbread, Murphy and Clifton52,Reference García-Morant, Cortés-Castell and Palazón-Bru55,Reference Kowalska, Brodowski and Pokorska-Niewiada57) . There was substantial variation in iodine intake within dietary groups with the greatest variation being observed by Groufh-Jacobsen et al in FFQ estimates of pescatarians (705·0–1590·0 µg/d)(Reference Groufh-Jacobsen, Hess and Aakre50).
Seven studies recorded a significant difference in iodine intake between one or more dietary group (P = < 0·05)(Reference Fallon and Dillon33,Reference Eveleigh, Coneyworth and Zhou34,Reference Groufh-Jacobsen, Hess and Aakre50,Reference Whitbread, Murphy and Clifton52,Reference Abraham, Trefflich and Gauch53,Reference García-Morant, Cortés-Castell and Palazón-Bru55,Reference Jakše, Godnov and Pinter56) . Four studies found the iodine intake of vegans to be significantly lower than omnivores (P = < 0·001)(Reference Fallon and Dillon33,Reference Eveleigh, Coneyworth and Zhou34,Reference Whitbread, Murphy and Clifton52,Reference García-Morant, Cortés-Castell and Palazón-Bru55) . Vegans and vegetarians had significantly lower habitual iodine intake compared with pescatarians in one study(Reference Groufh-Jacobsen, Hess and Aakre50). Raw food eaters were recorded to have significantly lower iodine intake than vegans and omnivores(Reference Abraham, Trefflich and Gauch53). The study conducted by Kowalska et al. (2020) did not statistically evaluate the difference in iodine intake between groups. There were differences in significance between groups according to methods of assessing iodine intake. In the study by Groufh-Jacobsen et al. (2020), a significant difference in iodine intake between vegans, vegetarians and pescatarians was only observed in FFQ estimates and not 24-h recalls. However, Eveleigh et al. did not observe a significant difference in iodine intake by FFQ in their 2019 cohort but did see differences when measured using food diaries(Reference Eveleigh, Coneyworth and Zhou34).
The WHO recommended iodine intake of 150 µg/d was used for the assessment of adequate dietary intake(11). None of the included studies recorded estimates above the adequate range for all dietary groups. Iodine inadequacy was reported in all dietary groups in 75 % (9/12) of the cohorts assessed(Reference Fallon and Dillon33,Reference Eveleigh, Coneyworth and Zhou34,Reference Groufh-Jacobsen, Hess and Aakre50,Reference Whitbread, Murphy and Clifton52–Reference Kowalska, Brodowski and Pokorska-Niewiada57) . Only two studies reported at least one dietary group to have adequate average iodine intake(Reference Eveleigh, Coneyworth and Zhou34,Reference Groufh-Jacobsen, Hess and Aakre50) .
Five studies addressed the types of foods consumed by different dietary groups(Reference Eveleigh, Coneyworth and Zhou34,Reference Whitbread, Murphy and Clifton52–Reference Blaurock, Kaiser and Stelzl54,Reference Jakše, Godnov and Pinter56) , of which three estimated the possible contribution of specific food groups to total iodine intake(Reference Eveleigh, Coneyworth and Zhou34,Reference Groufh-Jacobsen, Hess and Aakre50,Reference Whitbread, Murphy and Clifton52) . Overall, the vegan and vegetarian groups consumed greater quantities of plant-based food groups (fruit, vegetables, legumes, tubers, cereals and grains)(Reference Eveleigh, Coneyworth and Zhou34,Reference Whitbread, Murphy and Clifton52–Reference Blaurock, Kaiser and Stelzl54,Reference Jakše, Godnov and Pinter56) . Two studies recorded alternative milk intake to be significantly greater in vegan and vegetarian groups(Reference Eveleigh, Coneyworth and Zhou34,Reference Whitbread, Murphy and Clifton52) . Within these two studies, dairy products and eggs were the greatest source of iodine for individuals not restricting these foods. Kowalska et al. (2020) created model diets for each dietary group and used foods that were typically consumed according to dieticians’ advice and current research(Reference Kowalska, Brodowski and Pokorska-Niewiada57). Foods present in the models of vegan diets included cereal, fruit, vegetables, nuts, mushrooms, legumes, oils and alternative milk.
Mandatory or voluntary USI was present in seven of the included studies, and investigations conducted in the UK had no USI programme(Reference Fallon and Dillon33,Reference Eveleigh, Coneyworth and Zhou34) . Only two studies addressed the relative consumption of iodised salt to total iodine intake(Reference Eveleigh, Coneyworth and Zhou34,Reference Whitbread, Murphy and Clifton52) . Iodised salt was only consumed by vegan (n 4; 4/31)(Reference Whitbread, Murphy and Clifton52) and omnivorous participants (n 5; 1/34 & 4/26)(Reference Eveleigh, Coneyworth and Zhou34,Reference Whitbread, Murphy and Clifton52) . Whitbread et al. (2021) was the only study to monitor the intake of bread fortified with iodine, an average quantity of 28 g/d and 36 g/d for vegans and omnivores consumed(Reference Whitbread, Murphy and Clifton52). Consumption of iodised bread was similar between dietary groups and was acknowledged as a good source of iodine. The consumption of iodine-containing supplements to total iodine intake was recorded in five studies(Reference Eveleigh, Coneyworth and Zhou34,Reference Groufh-Jacobsen, Hess and Aakre50,Reference Blaurock, Kaiser and Stelzl54–Reference Jakše, Godnov and Pinter56) . Two of the studies that reported supplement use did not provide data on the actual contribution of iodine-containing supplements to total intake(Reference Abraham, Trefflich and Gauch53,Reference Blaurock, Kaiser and Stelzl54) . One study prevented supplement intake during the study(Reference Whitbread, Murphy and Clifton52). Four studies addressed the consumption of seaweed and microalgae in different dietary groups(Reference Eveleigh, Coneyworth and Zhou34,Reference Groufh-Jacobsen, Hess and Aakre50,Reference Whitbread, Murphy and Clifton52,Reference Blaurock, Kaiser and Stelzl54) ; however, in two of these studies, the relative contribution of seaweed to iodine was not discussed(Reference Whitbread, Murphy and Clifton52,Reference Abraham, Trefflich and Gauch53) . Vegans (n 29) were most likely to consume seaweed as part of their diet. Seaweed intake was also reported in strict raw food eaters (n 1), vegetarians (n 8), pescatarians (n 4) and omnivores (n 2)(Reference Eveleigh, Coneyworth and Zhou34,Reference Groufh-Jacobsen, Hess and Aakre50,Reference Whitbread, Murphy and Clifton52,Reference Abraham, Trefflich and Gauch53) .
Meta-analysis of iodine status and intake in vegans and vegetarians compared with omnivores
There was a strong trend for vegan or vegetarian diets to be associated with reduced iodine status as measured by UIC, but this did not quite reach significance (Fig. 4 and 5; P = 0·06 & P = 0·18), so no subgroup analysis was completed. There was, however, an overall significant negative effect of vegan diets on iodine intake (P = < 0·001; Fig. 6), with no effect observed for vegetarian diets (P = 0·12; Fig. 7). Subgroup analysis for iodine intake vegan v omnivore is shown in Supplementary Fig. 1. Significant effects were shown for female-only intake (P = 0·007), mixed-sex intake (P = 0·01), living in a country with voluntary (P = 0·01) or no USI programme (P = P < 0·001), and living in a country with national iodine intake considered as adequate (P = <0·001). In countries with mandatory salt iodisation, there was no effect of vegan diet on iodine intake (P = 0·60).

Fig. 4. Meta-analysis forest plot comparing the effect of a vegan v. omnivorous diet on iodine status. Data presented is female and mixed-sex estimates only.

Fig. 5. Meta-analysis forest plot comparing the effect of a vegetarian v. omnivorous diet on iodine status. Data presented is female and mixed-sex estimates only.

Fig. 6. Meta-analysis forest plot comparing the effect of vegan v. omnivorous diet on iodine intake. Data presented is female and mixed-sex estimates only.

Fig. 7. Meta-analysis forest plot comparing the effect of vegetarian v. omnivorous diet on iodine intake. Data presented is female and mixed-sex estimates only.
Discussion
This systematic review is an update of our previous work, investigating the evidence for iodine intake and status among adults following vegan and vegetarian diets, to reflect dietary consumption in the modern day. The publication of eleven studies eligible for inclusion in the past 2 years since our previous review demonstrates that there is an increased interest and awareness in this area. Our systematic review confirms that individuals following vegan and vegetarian diets, living in countries without mandatory salt iodisation, and not consuming seaweed or iodine-containing supplements, still have an increased risk of low iodine status, iodine deficiency, and inadequate iodine intake compared with less restrictive dietary groups(Reference Eveleigh, Coneyworth and Avery28). Our review also highlights that iodine nutrition is poor in many subgroups of the global population and that difficulties achieving iodine recommendations are not unique to those following restrictive diets.
In our previous review(Reference Eveleigh, Coneyworth and Avery28), we found that the degree of vulnerability in all dietary groups appeared to be impacted by the standing of national iodine estimates, whereby the median UIC of omnivorous participants studied tended to reflect a country’s national data. In our present review, we found that on average urine samples provided by omnivorous participants were lower in iodine than national estimates(Reference Eveleigh, Coneyworth and Zhou34,Reference Dawczynski, Weidauer and Richert49–Reference Whitbread, Murphy and Clifton52) . Only in studies conducted in Germany were omnivores within the same bracket of the WHO criteria as national data (89 μg/l; 50–99 μg/l mild)(11,20,Reference Dawczynski, Weidauer and Richert49,Reference Menzel, Abraham and Stangl51) . However, there is still a general trend in which vegans are likely to have median UIC corresponding to a lower bracket of the WHO criteria than omnivores recruited in the same study(Reference Eveleigh, Coneyworth and Zhou34,Reference Dawczynski, Weidauer and Richert49–Reference Whitbread, Murphy and Clifton52) . Although, the median UIC of all dietary groups studied in this review fell below the WHO criteria for optimal iodine status (100–200 µg/l)(11,Reference Eveleigh, Coneyworth and Zhou34,Reference Dawczynski, Weidauer and Richert49–Reference Whitbread, Murphy and Clifton52) .
We found that 67 % (6/9) of studies investigating iodine intake were conducted in countries considered ‘adequate’ according to national data from the Global Scorecard of Iodine Nutrition (2021)(Reference Fallon and Dillon33,Reference Eveleigh, Coneyworth and Zhou34,Reference Whitbread, Murphy and Clifton52,Reference García-Morant, Cortés-Castell and Palazón-Bru55–Reference Kowalska, Brodowski and Pokorska-Niewiada57) . But, none of the included studies recorded estimates above the adequate range for all dietary groups(Reference Fallon and Dillon33,Reference Eveleigh, Coneyworth and Zhou34,Reference Groufh-Jacobsen, Hess and Aakre50,Reference Whitbread, Murphy and Clifton52–Reference Kowalska, Brodowski and Pokorska-Niewiada57) . Equally, iodine inadequacy was reported in 75 % (9/12) of the cohorts assessed(Reference Fallon and Dillon33,Reference Eveleigh, Coneyworth and Zhou34,Reference Groufh-Jacobsen, Hess and Aakre50,Reference Whitbread, Murphy and Clifton52–Reference Kowalska, Brodowski and Pokorska-Niewiada57) . The global scorecard population monitoring uses the WHO recommendation of median UIC from school-aged children or women of childbearing age as a proxy for assessing adequate iodine intake on a national level(20). The use of median UIC from school-aged children as an indicator of iodine intake has been criticised for masking particular subgroups with varying diets and/or iodine sources(Reference Zimmermann and Andersson19). Women are a subgroup more vulnerable to the consequences of iodine deficiency and are twice as likely as men to adopt a vegan or vegetarian diet(Reference Modlinska, Adamczyk and Maison58). Three studies assessed iodine intake specifically in women of childbearing age(Reference Fallon and Dillon33,Reference Whitbread, Murphy and Clifton52,Reference Blaurock, Kaiser and Stelzl54) . Iodine intake was found to be below the WHO recommendation of 150 µg/d in all studies of women in our review regardless of dietary choice(Reference Fallon and Dillon33,Reference Whitbread, Murphy and Clifton52,Reference Blaurock, Kaiser and Stelzl54) . Moderate iodine deficiency pre-conception increases the risk of infant mortality by stillbirth and miscarriage(Reference Sheila59,Reference Zimmermann60) . Furthermore, iodine deficiency in utero may disrupt normal fetal neurodevelopment and cause irreversible neurological damage(Reference Sheila59,Reference Zimmermann60) . Our present review highlights that iodine intake must be assessed among different subsets of the population to identify groups at risk of deficiency.
Vegan participants living in the UK were recorded to have the lowest average iodine intake of 17·3 µg/d and 24·4 µg/(Reference Fallon and Dillon33,Reference Eveleigh, Coneyworth and Zhou34) . Dietary intake estimates recorded by Eveleigh et al (2022) and Fallon et al. (2020) were much lower than past data collected on British vegans(Reference Sobiecki, Appleby and Bradbury32,Reference Lightowler61,Reference Lightowler and Davies62) , suggesting that iodine intake has reduced over time. Vegans living in the UK may find it more challenging to meet their iodine requirements because along with restricting rich sources of iodine from animal sources, individuals selecting these diets also live in a country with no current USI programme. Salt iodisation is an effective and sustainable way of improving iodine intake(Reference Zimmermann and Andersson19). Currently, 145 countries globally have enrolled in either mandatory (124/145) or voluntary (21/145) USI(Reference Zimmermann and Andersson19). Apart from the UK, all other included studies were conducted in a country with USI(Reference Groufh-Jacobsen, Hess and Aakre50,Reference Whitbread, Murphy and Clifton52–Reference Kowalska, Brodowski and Pokorska-Niewiada57) . Iodised salt can be purchased in the UK, but its availability is low and tends to be more expensive than non-iodised household varieties(Reference Bath, Button and Rayman63). Eveleigh et al (2022) found that only one omnivorous participant recorded consuming iodised salt equating to 61 % of their total dietary iodine. However, it is very difficult to quantify the amount of salt added to meals and used or lost during cooking(Reference Zimmermann and Andersson19). Additionally, the iodine content of fortified salt can vary considerably(Reference Zimmermann and Andersson19). Whitbread et al (2021) was the only study, conducted in a location with USI, to estimate the relative contribution of iodised salt and foods containing iodised salt such as bread(Reference Whitbread, Murphy and Clifton52). Hence, dietary iodine intake may be lower in many of the included studies because the involvement of iodised salt was not estimated.
Bread has been selected as a vehicle for iodine fortification in countries, including Australia, New Zealand (NZ) and Denmark(Reference Whitbread, Murphy and Clifton52). In most countries, bread is a staple food product that can be consumed by nearly everyone in the population including those following vegan and/or vegetarian diets making it a practical method of iodine fortification. In the study by Whitbread et al (2021), iodised bread was the main iodised food source in the diets of Australian vegans and greater bread intake (g/d) correlated significantly with improved UIC(Reference Whitbread, Murphy and Clifton52). The mandatory fortification of bread in Australia is estimated to be about 53–70 μg per 100 g(64). The British Dietetics Association (BDA) denotes a portion of bread is equivalent to one slice or 34–36 g(65), if bread was fortified in the UK to the same level as in Australia, one portion would provide an average of 22 μg which could potentially boost iodine intake in all dietary groups.
Alternative milk has also been identified and promoted as a possible vehicle for iodine fortification for vegans and/or those who do not consume cows’ milk(Reference Govindji66). A recent study investigating the iodine fortification status of plant-based dairy and fish alternatives in the UK found that only 28 % of milk alternatives and 6 % of yogurt alternatives were fortified with iodine(Reference Thomas, Nicol and Bath36). Similarly, no cheese or fish alternatives were found to be currently fortified with iodine(Reference Thomas, Nicol and Bath36). Other studies have identified that individuals who consume alternative milk exclusively tend to have significantly lower iodine intake than cows’ milk consumers (94 v. 129 µg/d)(Reference Dineva, Rayman and Bath37). Two studies in our review recorded alternative milk intake to be significantly greater in vegan and vegetarian groups(Reference Eveleigh, Coneyworth and Zhou34,Reference Whitbread, Murphy and Clifton52) , and the displacement of cows’ milk may have contributed to lower iodine intake. In May 2021, the BDA’s England Board and the Maternal & Fertility Nutrition Specialist Group completed a project to consider the case for the wider iodine fortification of alternative milk to improve iodine intake(67). However, at present, no UK policy has been implemented for the mandatory iodine fortification of any foods; therefore, individuals following a vegan and/or vegetarian diet are responsible for planning their diet to include appropriate sources of iodine (e.g. iodine supplements, occasional seaweed consumption and/or iodine fortified alternative milk).
Although mandatory USI measures are considered to be the optimal approach, most countries with voluntary fortification are iodine-sufficient(Reference Zimmermann and Andersson19). We identified that individuals following a vegan diet living in countries with no mandatory USI programme were significantly more likely to have lower iodine intake. Our findings match the global experience; in that voluntary fortification may not benefit all subgroups of the population(68). There is an urgent need to investigate the usefulness of mandatory iodine fortification to address the emergence of iodine deficiency in those selecting vegan or vegetarian diets.
The modelling study conducted by Kowalska et al. (2020) in Poland demonstrates the difficulty in achieving adequate iodine intake in a number of different diet types if iodine-fortified foods (including salt) or supplements are not available. In this study, modelled diet menus of vegan, vegetarian, pescatarian and variations of the omnivorous diet (fish free, milk free, basic and regular) were prepared by qualified dieticians with the use of methods avoiding nutritional deficiencies. Even though these diets had been appropriately planned by dieticians, all of the diets were characterised by insufficient iodine intake. In Poland, the main sources of dietary iodine are iodised salt and white fish including cod and pollock(Reference Kowalska, Brodowski and Pokorska-Niewiada57). Therefore, it is unsurprising that iodine intake was observed to be lowest in individuals following diets that exclude fish (vegans, vegetarians and fish-free omnivores) when contribution of iodised salt was not added to intake estimates.
The greatest average iodine intake and status were recorded in Norwegian pescatarians (123·0 µg/d and UIC of 96·0 µg/l)(Reference Groufh-Jacobsen, Hess and Aakre50). Pescatarians do not consume meat but may eat fish, milk or eggs in varying quantities(Reference Phillips5). In a typical Norwegian diet, lean fish like cod, haddock and saithe are the richest sources of iodine along with cows’ milk and dairy products(Reference Groufh-Jacobsen, Hess and Aakre50). However, the iodine content of fish can vary significantly between species and geographical locations(Reference Nerhus, Markhus and Nilsen69). The Norwegian Directorate of Health recommends one portion of fish two or three times a week; however, annual fish intake has dropped in Norway across all age groups apart from the elderly; this decline is particularly noticeable in young people (18–34 years of age)(70). The authors suggest that greater iodine intake in pescatarians in this study was more due to consumption of iodine-containing supplements rather than that of fish(Reference Groufh-Jacobsen, Hess and Aakre50), as this significantly improves iodine status(Reference Bath, Walter and Taylor71,Reference Delange and Lecomte72) . A larger proportion of vegans and vegetarians consumed dietary supplements (inclusive of non-iodine-containing varieties) compared with omnivores, and in two studies, iodine-containing supplements provided between 140 and 150 µg/d to total iodine(Reference Eveleigh, Coneyworth and Zhou34,Reference Groufh-Jacobsen, Hess and Aakre50) . However, despite their consumption, the majority of participants had inadequate UIC and below-recommended iodine intake. The WHO recommends a daily iodine supplement dose of 150 µg/d for women of childbearing age (15–19 years) living in areas with insufficient access to iodised salt or vulnerable groups(11). Considering the vulnerability of individuals in the included studies, the effect of iodine-containing supplements in vegan and vegetarian populations warrants further investigation.
We found previously that the greatest iodine intake was recorded for females following vegan diets, living in London (1448·0 ± 3879·0 µg/d), whose regular consumption of seaweed increased intakes to over six times the RNI(Reference Lightowler and Davies62). In our present review, four studies addressed the consumption of seaweed and microalgae in different dietary groups(Reference Eveleigh, Coneyworth and Zhou34,Reference Groufh-Jacobsen, Hess and Aakre50,Reference Whitbread, Murphy and Clifton52,Reference Abraham, Trefflich and Gauch53) ; however, not all studies considered their contribution to total iodine. Vegans were the most frequent consumers of seaweed which provided iodine intake close to the maximum tolerable level of 1000 µg/d. The iodine content of seaweed is high and varies considerably according to the species consumed(Reference Bouga and Combet25,Reference Yeh, Hung and Lin26) , with kombu having the greatest iodine content (2523·5 mg/kg) and so is not recommended to improve iodine intake due to the significant risk of excess(Reference Bouga and Combet25,Reference Yeh, Hung and Lin26) .
In our previous review, we discussed key issues with the methodology selected to record intake from vegan and vegetarian groups including accurately defining diets(Reference Eveleigh, Coneyworth and Avery28), the use of outdated food tables or databases, and variation in techniques used to measure iodine in the diet (e.g. 24-h food recalls v. FFQ). These issues are still relevant to the present review and may reduce the accuracy of dietary intake estimates. Further limitations of this study include the relatively small number of included studies, oversimplifying of dietary practice to enable comparison of studies and a lack of studies that were well populated or well represent the general public.
Conclusions
This review agrees with findings from our previous systematic assessment on this topic(Reference Eveleigh, Coneyworth and Avery28). We found that vegans and vegetarians consuming seaweed or iodine-containing supplements continue to have increased risk of low iodine status, iodine deficiency and inadequate iodine intake compared with adults following less restrictive diets living in countries lacking mandatory iodisation of salt. Similarly to our previous review, there is a relationship between national iodine deficiency and the degree of vulnerability to vegans and vegetarians. However, we also conclude that iodine nutrition is inadequate in many subgroups, and that complications achieving iodine recommendations are universal. There is an urgent need to monitor iodine intake and status in at risk populations including young women and those following a vegan or vegetarian diet. In addition to research into safe routes of improving iodine intake in vegan and vegetarian populations living in regions where staple foods are not fortified or USI coverage is not present or is not mandatory. Further awareness of how to appropriately plan a vegan or vegetarian diet to achieve iodine recommendations is required.
Acknowledgements
The Biotechnology and Biological Sciences Research Council (BBSRC) for funding and the Division of Food, Nutrition, and Dietetics at The University of Nottingham for backing.
This research was supported by a BBSRC doctoral training programme studentship, grant number BB/M008770/1. The BBSRC had no role in the design, analysis or writing of this article.
Conceptualisation: E. R. E., L. C. and S. J. M. W.; methodology: E. R. E., L. C. and S. J. M. W.; formal analysis: E. R. E. and L. C.; investigation: E. R. E., L. C. and S. J. M. W.; writing – original draft preparation: E. R. E.; writing – review and editing: E. R. E., L. C. and S. J. M. W.; visualisation: E. R. E.; supervision: L. C. and S. J. M. W.; funding acquisition: E. R. E., L. C. and S. J. M. W. All authors have read and agreed to the published version of the manuscript.
There are no conflicts of interest.
Supplementary material
For supplementary material/s referred to in this article, please visit https://doi.org/10.1017/S000711452300051X