Oxidative stress describes an imbalance of oxidant/antioxidant networks that results in the disruption of redox signalling and/or molecular damage( Reference Katiyar and Mukhtar 1 – Reference Roncevic 3 ). In humans, persistent oxidative stress can lead to oxidation of lipids, alteration of protein function and mutation of DNA( Reference Vaziri and Rodriguez-Iturbe 4 – Reference Coluzzi, Colamartino and Cozzi 7 ). These disruptions may contribute to the pathogenesis of cancer, diabetes and neurodegenerative disease( Reference Sosa, Moline and Somoza 8 – Reference Wu, Tang and Chen 10 ). To detect increases in oxidative stress, oxidation products of lipids are often used as biomarkers( Reference Milne, Musiek and Morrow 11 ). Urinary 8-iso-PGF2α (F2-IsoP) and its primary metabolite, 2,3-dinor-5,6-dihydro-15-F2t-isoprostane (15-F2t-IsoP-M), are stable biomarkers of lipid peroxidation( Reference Dorjgochoo, Gao and Chow 12 , Reference Van’t Erve, Lih and Jelsema 13 ). Several studies have reported higher levels of specific urinary F2-IsoP in medical conditions including cancer and CVD( Reference Rossner, Gammon and Terry 14 – Reference van ‘t Erve, Kadiiska and London 16 ).
Tea is a popular and accessible beverage worldwide( Reference Lin, Liang and Lin-Shiau 17 ) and may have beneficial health effects for diabetes, CVD and cancer( Reference Yang, Wang and Fan 18 – Reference Arab, Khan and Lam 20 ). Green tea and black tea contain polyphenols( Reference Chen, Wan and Yang 21 ) that have potential antioxidant properties( Reference Scalbert, Johnson and Saltmarsh 22 ). For example, the polyphenol epigallocatechin-3-gallate (EGCG) is a natural antioxidant found in green and black tea( Reference Cavet, Harrington and Vollmer 23 , Reference Tipoe, Leung and Hung 24 ). To experimentally study the anti-oxidative effects of tea (green tea, black tea and green tea extract)( Reference Basu, Sanchez and Leyva 25 – Reference Hakim, Harris and Cordova 28 ), participants were assigned to high levels of tea consumption for a short period of time (e.g. 4 cups of green tea/d for 8 weeks or placebo beverage). Participants in the intervention group drinking green tea had lower levels of blood malondialdehyde (MDA), a biomarker of oxidative stress( Reference Basu, Sanchez and Leyva 25 ). Participants assigned to receiving one capsule (379 mg) of green tea extract per d for 3 months had a higher total antioxidant status compared with placebo( Reference Bogdanski, Suliburska and Szulinska 26 ). A third study reported that black tea consumption (4 cups/d for 6 months) reduced lipid peroxidation among thirty-four female former smokers( Reference Hakim, Harris and Cordova 28 ). These findings support a potential inverse association between tea consumption and oxidative stress.
However, in the general population, tea consumption patterns may reflect longer durations of lower consumption levels. It is unknown whether these patterns also translate to benefits for oxidative stress. Here, we conducted a cross-sectional analysis of 889 premenopausal women to examine the association between tea consumption and oxidative stress.
Methods
Data for this analysis come from the National Institute of Environmental Health Sciences (NIEHS) Sister Study. Study participants (n 50 884) aged 35–74 years were enroled between 2003 and 2009 across the USA and Puerto Rico( Reference Sandler, Hodgson and Deming-Halverson 29 ). To be eligible, participants had at least one sister who had been diagnosed with breast cancer, but no personal history of breast cancer. All participants provided written consent at enrolment. Study protocols were approved by the Institutional Review Board of the NIEHS, the National Institutes of Health and the Copernicus Group.
Population for analysis
Within the Sister Study, 1367 women were identified (456 cases, 911 controls) for a nested case–control study investigating oxidative stress and breast cancer risk among premenopausal women. To be eligible for the nested case assigned control study, participants had to meet the following criteria: aged 54 and younger, premenopausal status (at least one menstrual cycle in the previous 12 months or hysterectomy with ≥1 ovary conserved) and have an available blood and urine sample from enrolment. Controls were matched to cases with a ratio of 2:1 on the basis of age and enrolment year and were free of breast cancer at the time of their matched case’s diagnosis. For this analysis, information from the 911 controls was used. We further excluded women missing data of black and green tea consumption levels (n 22), which yielded 889 participants for this study.
Oxidative stress measurement
At Sister Study enrolment, participants self-collected approximately 60 ml of first-morning void urine in a study-provided collection cup( Reference Sandler, Hodgson and Deming-Halverson 29 ). Participants refrigerated the samples without preservative until they were picked up by study examiners who shipped the samples on ice to the study repository( Reference Parks, Miller and McCanlies 30 ). On receipt, urine samples were aliquoted and stored at –80°C. In 2012, samples were retrieved and urinary concentrations of F2-IsoP and 15-F2t-IsoP-M were measured using GC/negative ion chemical ionisation MS at the Eicosanoid Core Laboratory at Vanderbilt University Medical Center. The mean storage time of urinary samples was 8·3 years. Protocols for chemical analysis and procedures have been described in detail( Reference Milne, Sanchez and Musiek 31 – Reference Milne, Gao and Terry 34 ). A total of seventy-seven batches were run; each batch contained eighteen samples from study subjects (twelve controls and six cases) and two quality control (QC) samples. The CV for QC duplicates was 16·0 and 12·5 % for F2-IsoP and 15-F2t-IsoP-M, respectively( Reference Anderson, Milne and Sandler 35 ). Urinary levels of F2-IsoP and 15-F2t-IsoP-M were adjusted for creatinine (ng/mg creatinine) to correct for urine diluteness.
Exposure and covariate measurement
During an enrolment home visit, trained examiners measured height and weight without shoes. These measurements were taken three times and values were rounded to the nearest quarter inch for height and whole pound for weight( Reference Lin, DeRoo and Jacobs 36 ). BMI was calculated as weight (kg)/height (m)2. Black and green tea consumption during the past 12 months was measured by the self-administered Block 98 FFQ at study enrolment( Reference Block, Hartman and Dresser 37 ). Within the FFQ, participants reported their frequency of tea consumption and the cups consumed each time( Reference Sandler, Hodgson and Deming-Halverson 29 ). Frequency was reported at nine levels, ranging from ‘never’ to ‘everyday’. Participants reported how many cups of tea they consumed each time as ‘1 cup, 2 cups, 3–4 cups or 5 or more cups’. Regular (non-decaffeinated) coffee consumption was measured using the same methods described above. Healthy eating index, dietary fruit and vegetable intake and dietary β-carotene, vitamin C, and vitamin E intake were obtained via information collected in self-administered FFQ. Total energy intake and caffeine from beverages (soda and black tea) and dietary sources were calculated from the FFQ by NutritionQuest( 38 ). We assigned caffeine levels to coffee (regular and decaffeinated) and green tea on the basis of data from USDA Food Composition Databases( Reference Mitchell, Knight and Hockenberry 39 , 40 ). Each cup of regular coffee was assigned 95·2 mg caffeine, each cup of decaffeinated coffee 2 mg caffeine, and each cup of green tea 24·8 mg caffeine. On average, one cup of black tea contains 47·2 mg caffeine( Reference Mitchell, Knight and Hockenberry 39 ).
A validated series of questions were used for measurement of physical activity( Reference Ainsworth 41 , Reference Craig, Marshall and Sjostrom 42 ). Weekly energy expenditures at enrolment were calculated as metabolic equivalents (MET), and total physical activity was calculated by summing the MET-h/week of all sports, physical exercise and daily activity self-reported at enrolment. Participants were also asked for information about the total annual income from all household members and the highest level of school they had completed as well as their age and race/ethnicity.
Statistical analysis
Tea consumption was categorised into four levels (0, <1, 1–<5 and ≥5 cups/week). The consumption value was obtained by multiplying the frequency of consumption (times/week) and serving size (cups consumed each time) together. Cut-off points were determined based on the distribution of tea consumption among women who reported drinking black tea to approximate tertiles. Non-consumers were identified a priori as the reference group. The same cut-off points were used for green tea consumption for consistency. Tea consumers with missing serving size information (twenty-two for black tea, twenty-four for green tea) were assigned a serving size of one cup per serving (the most common serving size for black (56·7 %) and green (76·0 %) tea consumption). For coffee consumption, one and two cups per serving were about equally common (37·0 and 38·5 %, respectively), and coffee consumers with missing serving size information (n 13) were also assigned as drinking one cup per serving. Coffee consumption was categorised into four levels: 0, <10, 10–<15 and ≥15 cups/week. Caffeine intake was categorised based on approximate quartiles (<33·9, 33·9–<111·2, 111·2–<205·2 and ≥205·2 mg/d). BMI categories were defined based on WHO guidelines as underweight/normal weight (<24·9 kg/m2), pre-obesity (25·0–29·9 kg/m2), obesity class I (30·0–34·9 kg/m2), obesity class II (35·0–39·9 kg/m2) and obesity class III (≥40 kg/m2)( 43 ). The healthy eating index, dietary fruit and vegetable intake and dietary β-carotene, vitamin C, vitamin E, total energy intake and physical activity were categorised to approximate quartiles.
Geometric means and 95 % CI of urinary F2-IsoP and 15-F2t-IsoP-M were calculated for each level of tea consumption and by other covariates. The distribution of urinary F2-IsoP and 15-F2t-IsoP-M concentrations was right skewed; thus, a natural log transformation was applied for these biomarkers to approximate normality. Univariate geometric mean difference and adjusted geometric mean difference (aGMD) and 95 % CI of urinary F2-IsoP and 15-F2t-IsoP-M were calculated using linear regression of the natural log-transformed values. To calculate aGMD of F2-IsoP or 15-F2t-IsoP-M across tea consumption levels, the linear regression model adjusted for age (35–<40, 40–<45, 45–<50 and ≥50 years), race (non-Hispanic white, non-Hispanic black and other), BMI (BMI, <25, 25–<30, 30–<35, 35–<40 and ≥40 kg/m2), education (high school or less, some college or undergraduate and graduate school), annual income (0–<$50 000, $50 000–<$100 000 and ≥$100 000), smoking status (never, former and current), healthy eating index (<53, 53–<63, 63–<72 and ≥72), dietary fruit (<0·6, 0·6–<1·1, 1·1–<2 and ≥2 servings/d) and vegetable intake (<1·6, 1·6–<2·7, 2·7–<4·3 and ≥4·3 servings/d), dietary β-carotene (<2427·2, 2427·2–<4106·1, 4106·1–<6942·3 and ≥6942·3 μg/d), vitamin C (<55·3, 55·3–<84·1, 84·1–<121·9 ≥121·9 mg/d) and vitamin E intake (<5·6, 5·6–<7·6, 7·6–<10·1 ≥10·1 mg/d), total energy intake (<5148·0, 5148·0–<6396·1, 6396·1–<8263·0 and ≥8263·0 kJ/d) and physical activity (<28·11, 28·11–<44·16, 44·16–<65·99, ≥65·99 MET-h/week) were used as potential confounders( Reference Willett, Howe and Kushi 44 ). We also evaluated the impact of additional adjustment for caffeine (<33·9, 33·9–<111·2, 111·2–<205·2 and ≥205·2 mg/d)( Reference Devasagayam, Kamat and Mohan 45 , Reference Shi, Dalal and Jain 46 ). The assumptions of the linear regression (linearity, independence, multivariate normality and homoscedasticity) were examined by scatterplots of urinary F2-IsoP or 15-F2t-IsoP-M v. tea consumption and plots of the residuals v. fitted values of the regression model; results did not suggest that assumptions were violated.
Subgroup analyses were conducted to address potential effect modification of associations between tea and F2-IsoP or 15-F2t-IsoP-M according to overweight (BMI <25 kg/m2 v. BMI ≥25 kg/m2) and regular coffee consumption (drinker v. non-drinker). Interaction terms between tea consumption and the covariates were included in multivariable linear regressions, and log-likelihood ratio tests were used to assess whether the interaction terms were statistically significant.
We did not adjust for multiple comparisons as our analysis was hypothesis driven( Reference Nikitina, Chen and Vallis 47 – Reference Savitz and Olshan 49 ). Two-sided P values <0·05 were considered to be statistically significant. All statistical analyses were conducted with Sister Study data release 6.0 using Stata 13.0 (StataCorp, LLP).
Results
Of the 889 participants in our analysis, the average age at baseline was 47·28 (sd 4·45) and the majority were non-Hispanic white (87·3 %). The geometric means of urinary F2-IsoP and 15-F2t-IsoP-M were 1·44 (95 % CI 1·39, 1·49) and 0·71 (95 % CI 0·69, 0·73) ng/mg creatinine, respectively. Table 1 presents geometric means and mean differences of urinary F2-IsoP and 15-F2t-IsoP-M according to participant characteristics. Both F2-IsoP and 15-F2t-IsoP-M decreased slightly as age increased, but the differences were not statistically significant. Average F2-IsoP and 15-F2t-IsoP-M levels among non-Hispanic black women were lower compared with white women. Levels of both F2-IsoP and 15-F2t-IsoP-M were positively associated with BMI and inversely associated with higher income and physical activity. Current smokers had higher levels of both F2-IsoP and 15-F2t-IsoP-M compared with never smokers, but associations were statistically significant only for 15-F2t-IsoP-M. Inverse but non-significant associations with education were also observed for 15-F2t-IsoP-M but not F2-IsoP. High-level coffee consumption (≥15 cups/week) was not associated with F2-IsoP or 15-F2t-IsoP-M. Total energy intake was positively associated with F2-IsoP or 15-F2t-IsoP-M. Associations with the healthy eating index, vegetable intake and vitamin C were not statistically significant for F2-IsoP and 15-F2t-IsoP-M. Higher fruit intake and dietary β-carotene were inversely associated with F2-IsoP and 15-F2t-IsoP-M, respectively, but not with both markers. Dietary vitamin E appeared inversely associated with both biomarkers, but only estimates of 15-F2t-IsoP-M were statistically significant.
Table 1 Characteristics of study participants and estimates of urinary 8-iso-PGF2α (F2-IsoP) or 2,3-dinor-5,6-dihydro-15-F2t-isoprostane (15-F2t-IsoP-M) by covariatesFootnote * (Numbers and percentages; geometric means (GM) and 95 % confidence intervals)
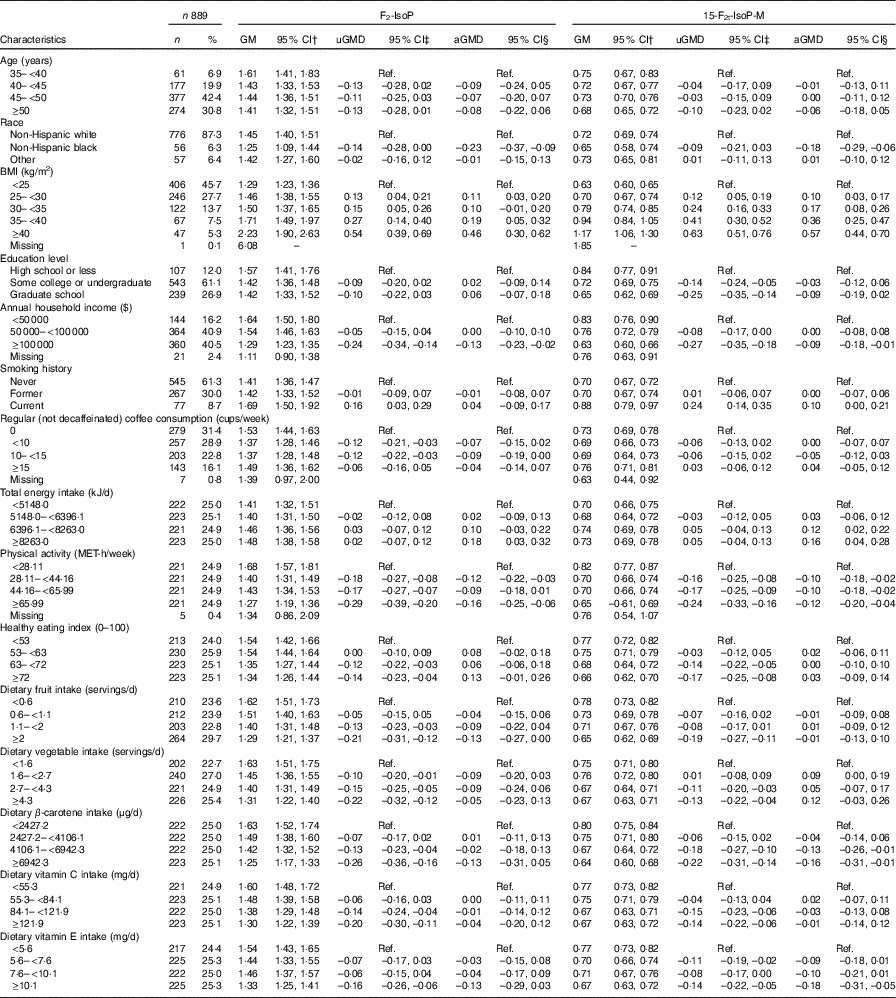
uGMD, univariate geometric mean difference; aGMD, adjusted geometric mean difference; Ref., reference; MET, metabolic equivalent of task.
* Geometric mean difference and 95 % CI were calculated on the basis of natural logarithm of F2-IsoP and 15-F2t-IsoP-M.
† GM was calculated using the whole sample (n 889).
‡ Univariate models were restricted to participants without missing values of the covariates and had the same size as adjusted models (n 856).
§ aGMD was calculated in multivariable model adjusting for all variables in Table 1.
Associations between black and green tea consumption and caffeine intake with urinary oxidative stress measures are shown in Table 2. Black tea consumption was more common than green tea consumption; 18·6 % of women reported never drinking black tea, while 45·9 % of women reported never drinking green tea. The highest level of consumption, ≥5 cups/week, was reported by 24·9 and 7·6 % of women for black and green tea consumption, respectively. Overall, black and green tea consumption were not associated with F2-IsoP levels (Table 2). However, mean concentrations of 15-F2t-IsoP-M were higher for black tea consumption of 5 cups/week or more compared with 0 cups/week (aGMD=0·10, 95 % CI 0·02, 0·19). High-level green tea consumption (≥5 cups/week compared with 0) was not significantly associated with 15-F2t-IsoP-M (aGMD=0·09, 95 % CI –0·02, 0·20).
Table 2 Association between tea consumption or caffeine intake and urinary 8-iso-PGF2α (F2-IsoP) or 2,3-dinor-5,6-dihydro-15-F2t-isoprostane (15-F2t-IsoP-M)Footnote * (Numbers and percentages; geometric means (GM) and 95 % confidence intervals)
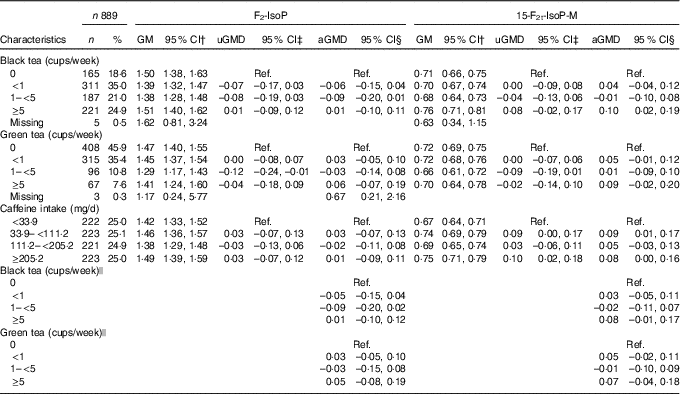
uGMD, univariate geometric mean difference; aGMD, adjusted geometric mean difference; Ref., reference.
* Geometric mean difference and 95 % CI were calculated on the basis of natural logarithm of F2-IsoP and 15-F2t-IsoP-M.
† GM was calculated using the whole sample (n 889).
‡ Univariate models were restricted to participants without missing values of the covariates (black tea: n 858, green tea: n 861, caffeine: n 863).
§ The multivariable model adjusted for age, race, smoking status, BMI, physical activity, household income, education level, energy intake, healthy eating index, dietary fruit, vegetable, β-carotene, vitamin C and vitamin E intake (black tea: n 858, green tea: n 861, caffeine: n 863).
|| The multivariable model additionally adjusted for caffeine intake.
Caffeine intake was not associated with F2-IsoP. As compared with the lowest quartile (<33·9 mg/d), higher levels of caffeine intake were positively associated with 15-F2t-IsoP-M, but there was no consistent increase across quartiles. Additional adjustment for caffeine intake attenuated the association between black tea and 15-F2t-IsoP-M towards the null (Table 2). Associations between tea consumption and urinary F2-IsoP or 15-F2t-IsoP-M were not modified by overweight (online Supplementary Table S1) or regular coffee consumption (online Supplementary Table S2).
Discussion
Our analysis did not provide support for an inverse association between dietary tea consumption and urinary F2-IsoP or 15-F2t-IsoP-M, high-quality biomarkers of oxidative stress. Green tea consumption was not associated with either F2-IsoP or 15-F2t-IsoP-M. Black tea consumption was not associated with F2-IsoP; however, drinking at least 5 cups of black tea/week (compared with none) was associated with higher 15-F2t-IsoP-M concentrations before adjustment for caffeine.
Clinical studies have found an inverse association between tea consumption and oxidative stress( Reference Basu, Sanchez and Leyva 25 – Reference Klaunig, Xu and Han 27 , Reference Stote, Clevidence and Novotny 50 ). For example, by observing nineteen people in a 5-d experimental study, Stote et al. ( Reference Stote, Clevidence and Novotny 50 ) found that green tea consumption could lower plasma levels of F2-IsoP. However, subjects in this study consumed a higher level of tea (e.g. 2 servings of green tea/d for 5 d) than was commonly consumed in our population-based sample of the US women. In addition, this study enroled only nineteen obese people at high risk of insulin resistance, which may have compromised the generalisability of their outcomes.
A cross-sectional epidemiological study( Reference Dorjgochoo, Gao and Chow 12 ) of 845 Chinese women observed an almost null association between any tea drinking and urinary levels of F2-IsoP (geometric mean: never drinker: 1·62, ever drinker: 1·65, P=0·72) and 15-F2t-IsoP-M (geometric mean: never drinker: 0·56, ever drinker: 0·61, P=0·06) after adjustment for age, education, occupation, smoking, BMI, multivitamin supplement use, fruit and vegetable intakes, plasma total carotenoids, tocopherols and retinol, assay batch and urinary tea polyphenols. However, the ever/never analysis did not consider level of consumption or potential difference between black and green tea( Reference Dorjgochoo, Gao and Chow 12 ). Green and black tea differ in concentrations of polyphenols (e.g. EGCG) and caffeine( Reference Henning, Niu and Lee 51 , Reference Stoner and Mukhtar 52 ). For example, green tea has a higher level of EGCG compared with black tea( Reference Lee, Lee and Lee 53 ), while black tea contains more caffeine( Reference Henning, Niu and Lee 51 ). In addition, a previous study measuring total phenol levels and antioxidant capacity of tea products sold in the USA found green tea had a higher antioxidant capacity than black tea of the same volume (436 v. 239 mg vitamin C equivalents per serving)( Reference Lee, Lee and Lee 54 ). These suggest analysis pooling all types of tea in to one category may obscure meaningful variation.
15-F2t-IsoP-M is the metabolite of F2-IsoP under β-oxidation( Reference Dorjgochoo, Gao and Chow 12 ). Both black and green tea contain EGCG and caffeine which have been found to facilitate β-oxidation on the basis of laboratory evidence( Reference Rains, Agarwal and Maki 55 , Reference Sinha, Farah and Singh 56 ). The suggested positive associations between black tea and caffeine with 15-F2t-IsoP-M, but not F2-IsoP, may be due, in part, to related increases in β-oxidation pathways. We did not observe an association between green tea and 15-F2t-IsoP-M; however, there were few high-level green tea consumers in our analysis.
Our results regarding the association between caffeine and 15-F2t-IsoP-M were similar to an experimental study( Reference Olcina, Timon and Munoz 57 ) that assigned twenty participants caffeine (5 mg/kg) or placebo before physical exercise and observed a positive association between caffeine and plasma MDA using blood samples collected immediately after exercise. However, other experimental studies have reported inverse associations between caffeine intake and biomarkers of oxidative stress using other caffeine dosages or different biomarkers of antioxidant activity (e.g. plasma glutathione) or oxidative stress (e.g. 8-hydroxydeoxyguanosine)( Reference Metro, Cernaro and Santoro 58 , Reference Zeraatpishe, Malekirad and Nik-Kherad 59 ). Due to the different study design, biomarkers used for analysis and divergent findings to date, the association between caffeine and oxidative stress deserves further investigation.
Strengths of our study included the use of a general population sample, the clear categorisation of tea type and detailed information on socio-demographic and lifestyle factors for statistical adjustment. Particularly, using a population-based sample can better reflect real-world tea consumption pattern as compared with high-level tea assignment in experimental studies. The use of urinary F2-IsoP provided a stable biomarker of lipid peroxidation. A previous biochemical study has shown that plasma stored appropriately for at least 10 years has F2-IsoP levels similar to freshly prepared samples( Reference Van’t Erve, Lih and Jelsema 13 ). Given that artefactual generation of F2-IsoP through autoxidation of lipids only occurs in plasma and not in urine( Reference Dorjgochoo, Gao and Chow 12 , Reference Van’t Erve, Lih and Jelsema 13 ), our samples were likely to be equally or more stable. In some previous studies, MDA was used as the biomarker of oxidative stress( Reference Basu, Sanchez and Leyva 25 , Reference Klaunig, Xu and Han 27 ); however, MDA is more affected by dietary lipid consumption( Reference Richelle, Turini and Guidoux 60 ) and can be generated from non-lipid sources such as bile pigments( Reference Janero 61 ), which may cause measurement error. 8-Hydroxy-2’-deoxyguanosine (8-OHdG) is another biomarker of oxidative stress which is an end product of non-enzymatic DNA oxidation( Reference Il’yasova, Scarbrough and Spasojevic 62 ). However, levels of 8-OHdG can be influenced by DNA repair capacity which makes 8-OHdG an indicator of the combined effects of oxidative stress-associated damage and DNA repair capability. Furthermore, a previous study of ten healthy volunteer suggests that there is not a significant diurnal variation in urinary F2-IsoP( Reference Helmersson and Basu 63 ), whereas many studies suggest that diurnal variation in urinary 8-OHdG is substantial( Reference Il’yasova, Scarbrough and Spasojevic 62 , Reference Sabatini, Barbieri and Tosi 64 , Reference Pilger, Ivancsits and Germadnik 65 ). These characteristics make urinary F2-IsoP a more desirable biomarker of oxidative stress.
Our study also has some limitations. Our sample included only premenopausal women, which may compromise the external validity for men, older women or individuals with specific medical conditions. Also, tea consumption was measured by retrospective self-report, and non-differential measurement error could be introduced. Duration of tea consumption was not available for analysis, and the antioxidant potential of green and black tea sources was not directly measured. Finally, our study is a cross-sectional analysis with one-time urinary sampling and two biomarkers of oxidative, which makes it inappropriate for casual interpretation.
Our study contributes real-world data regarding associations between tea consumption and oxidative stress. In our study, we did not observe an inverse association between green or black tea consumption and urinary F2-IsoP. Additional studies with detailed information on the timing and duration of tea consumption, and additional measures of oxidative stress, may be warranted to inform the use of antioxidant products.
Acknowledgements
The authors appreciate the helpful comments of Dr Thomas Joost Van't Erve.
This work was supported, in part, by the National Institute of Environmental Health Sciences (Z01 ES044005) and the Avon Foundation (02-2012-085).
D. Z., H. B. N. and D. P. S. designed the study. D. P. S. and G. L. M. conducted the study. D. Z. conducted statistical analysis. All the authors contributed to manuscript writing and revision. All the authors read and approved the final version of the manuscript.
None of the authors had any conflict of interest.
Supplementary material
For supplementary material/s referred to in this article, please visit https://doi.org/10.1017/S0007114518003732





