Threonine is an essential amino acid for animals( Reference Habte-Tsion, Ren and Liu 1 ). Previous studies from our laboratory observed that dietary threonine deficiency caused poor percentage weight gain (PWG), decreased feed efficiency (FE) and reduced digestive and brush border enzyme activities in fish intestines( Reference Feng, Peng and Wu 2 , Reference Hong, Jiang and Kuang 3 ). As we all know, the digestive and absorptive capacities of animals are correlated with intestinal health, which is closely related to intestinal immune function( Reference Li, Tang and Hu 4 ). Intestinal immune function is dependent on its innate and adaptive immune responses( Reference Lan, Andriamihaja and Blouin 5 – Reference Lauriano, Pergolizzi and Capillo 8 ). However, there are just fragmentary reports about the effects of threonine on innate and adaptive immune responses in animal intestine. In animal intestine, it has been reported that threonine could promote the IgA production in broiler chicks( Reference Zhang, Chen and Eicher 9 ), up-regulate inflammatory cytokine IL-6 gene expression in piglets( Reference Ren, Liu and Wang 10 ) and down-regulate genes expression of IL-12 and interferon γ (IFN-γ) in broiler chicks( Reference Wils-Plotz, Jenkins and Dilger 11 ), IL-1β in piglets( Reference Ren, Liu and Wang 10 ) and TNF-α in blunt snout bream (Megalobrama amblycephala)( Reference Habte-Tsion, Ge and Liu 12 ). However, those researches still lack systematicness, and they did not investigate the involved mechanisms. Therefore, systematic attempts to investigate the relationship between threonine and intestinal immune function and depth examination to explore the molecular mechanisms in animals are required.
Intestinal innate immune responses are related to its innate immune components such as antimicrobial peptides (such as hepcidin, liver-expressed antimicrobial peptide (LEAP)-2A, LEAP-2B and β-defensin), lysozyme activities (LA), acid phosphatase (ACP) and complements (such as C3 and C4), and the adaptive immune response depends on Ig production and T lymphocytes( Reference Magnadóttir 13 , Reference Yang, Zou and Wu 14 ). However, except for the reported Ig, no evidence was demonstrated about the effects of threonine on those innate immune components in animals. In the hepatopancreas of blunt snout bream, threonine could up-regulate the peroxiredoxin II (Prx II) mRNA levels( Reference Habte-Tsion, Ren and Liu 15 ). It was confirmed that Prx II could increase the hepcidin expression in rat hepatocytes( Reference Mattè, De Falco and Federti 16 ). Meanwhile, threonine deficiency could depress the lymphocyte proliferation in the peripheral blood of piglets( Reference Ren, Liu and Wang 10 ). In addition, threonine deficiency could decrease the transcript abundance of Complement C1 subcomponent precursor (C1S) in the ileum of pigs( Reference Hamard, Mazurais and Boudry 17 ). Sarma & Ward( Reference Sarma and Ward 18 ) reported that C1S could prompt the generation of C3 in fish. As far as the above observations are concerned, we speculated that threonine might affect the intestinal immune function by modulating multiple innate immune components, which is worthy of systematic exploration.
Apart from the innate immune components, inflammatory cytokines also play a vital role in intestinal innate immune response, which consist of pro-inflammatory cytokines (such as TNF-α and IL-1β) and anti-inflammatory cytokines (such as transforming growth factor (TGF)-β and IL-10)( Reference Mattè, De Falco and Federti 19 , Reference Mia, Warnecke and Zhang 20 ). Besides the studied cytokines (TNF-α, IL-1β, IFN-γ, IL-6 and IL-12), other vital cytokines such as IL-17D, IL-10, IL-4, IL-8 and TGF-β also participated in the important immune regulation in animals( Reference Nilsen, Johansen and Jahnsen 21 ). However, there is scarce information about the impacts of threonine on those various inflammatory cytokines (except TNF-α, IL-1β, IFN-γ, IL-6 and IL-12) in animal intestine. In the lungs of weaned pigs, threonine could decrease relative mRNA abundance of Toll-like receptor 9 (TLR9)( Reference Mao, Lai and Yu 22 ). Studies showed that TLR9 could up-regulate the Treg IL-17 and down-regulate the THP1 cell IL-10 mRNA levels in humans ( Reference Adjobimey, Satoguina and Oldenburg 23 , Reference AK, G and A 24 ). Furthermore, pro-inflammatory cytokines could be regulated by NF-κB and the inhibitor of κB α (IκB α), as well as IκB kinase (IKK) complexes (IKK α, IKK β and IKK γ)( Reference Hong, Lee and Jung 25 ), whereas anti-inflammatory cytokines could be modulated by mammalian target of rapamycin (mTOR) in humans( Reference Weichhart, Haidinger and Katholnig 26 ). However, little evidence has been afforded about whether the effects of threonine on cytokines are related to the NF-κB (p65/p52/c-Rel)/IκB α/IKK and mTOR signalling regulation in animals. In blood monocytes of broilers, threonine deficiency up-regulated the nitric oxide level( Reference Corzo, Kidd and Dozier 27 ), which could induce the activation of NF-κB in rat heart( Reference Chen, Chuang and Liu 28 ). In addition, Shibata et al.( Reference Shibata, Imai and NAkATA 29 ) reported that dietary threonine supplement decreased the nicotinamide level in rat brains. In mice, it was noteworthy that nicotinamide could lead to the suppression of mTOR in C17·2 neural stem cells( Reference Wang, Cheng and Wang 30 ). The above observations indicate that there might be a potential relationship between threonine and multiple cytokines, as well as NF-κB and mTOR signalling, in animals, which needs to be investigated.
As reported, fish intestine displayed a regional immune specialisation in different intestines( Reference Rombout, Abelli and Picchietti 31 ). In the second gut segment of carp, more large resident intraepithelial Ig+ macrophages can be found with a strong Ig-binding capacity( Reference Rombout, Taverne-Thiele and Villena 32 ). Moreover, higher levels of T lymphocyte markers were observed in the posterior intestinal segment( Reference Vigneulle and Laurencin 33 ). To our knowledge, threonine could contribute to the proliferation of lymphocytes in the weaned pigs( Reference Ren, Liu and Wang 10 ). Thus, it is of great value to investigate the effect of threonine on the intestinal immune response.
Grass carp (Ctenopharyngodon idella), a herbivorous finfish without a stomach, is the third biggest contributor to the worldwide aquaculture production( Reference Wittmann, Jerde and Howeth 34 , Reference Zon 35 ). As a kind of economic fish species, it accounts for about 16 % of the global freshwater aquaculture( Reference Wang, Lu and Zhang 36 ). Recently, threonine requirement for juvenile grass carp was estimated on the basis of special growth rate (SGR)( Reference Gao, Yang and Liu 37 ). Nevertheless, nutrient requirements of fish may vary with different indices( Reference Feng, Li and Liu 38 – Reference Guardiola, Porcino and Cerezuela 42 ). Therefore, it is of great value to estimate the threonine requirements of juvenile grass carp based on different indicators.
Together, on the basis of our previous studies that threonine deficiency decreased growth and reduced digestive and brush border enzyme activities in fish intestines, first of all, this study was systematically conducted to investigate the effects of threonine on the innate and adaptive immune components, as well as cytokines, in animal intestines after infection with Aeromonas hydrophila. In addition, further study was conducted, for the first time, to delve into the relationship between threonine and immune-related signalling molecules (NF-κB (p65/p52/c-Rel)/IκBα/IKK and TOR/S6 kinase (S6K1) and eIF4E-binding protein 1 (4E-BP1)) with the aim of revealing the possible mechanism of threonine-regulating cytokines in animal intestines. Meanwhile, the threonine requirements of juvenile grass carp were also determined on the basis of different indicators, which may provide a reference for the commercial feed production of juvenile grass carp.
Methods
Experiments and designs
Composition of the basal diet is shown in Table 1. Fishmeal, casein and gelatin were used as dietary protein sources. Fish oil and soyabean oil were used as dietary lipid sources. The dietary protein level was fixed at 320·0 g/kg diet, as described by the National Research Council( 43 ). The overall composition of essential amino acids in the diets stimulated the amino acid pattern similar to that found in 320·0 g/kg crude protein from grass carp whole-body protein, excluding threonine, according to Wang et al.( Reference Wang, Liu and Tian 44 ). l-threonine was added to the basal diet to provide graded concentrations of 4·0 (un-supplemented diet), 7·5, 11·0, 14·5, 18·0 and 21·5 g threonine/kg diet. All diets were made iso-nitrogenous with graded glycine instead of incremental threonine according to Tang et al.( Reference Tang, Feng and Sun 45 ). Pellets were produced and stored at −20°C until use, as described by Hong et al.( Reference Hong, Jiang and Kuang 3 ). The threonine concentrations in the six experimental diets were analysed to be 3·99 (control), 7·70, 10·72, 14·10, 17·96 and 21·66 g threonine/kg diet according to Teshima et al.( Reference Teshima, Kanazawa and Yamashita 46 ) using the Agilent 1100 series HPLC (Agilent Technologies).
Table 1 Composition and nutrient content of basal diet.
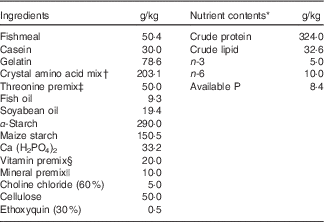
* Crude protein and crude lipid contents were measured. Available P, n-3 and n-6 contents were calculated according to the National Research Council(43).
† Crystal amino acid mix (g/kg diet): lysine, 11·42; methionine, 7·81; tryptophan, 2·88; arginine, 9·56; histidine, 7·86; leucine, 18·64; isoleucine, 10·30; phenylalanine, 10·22; tyrosine, 6·44; valine, 11·25; cysteine, 2·87; glutamic acid, 57·50; glycine, 46·35, respectively.
‡ Threonine premix was added to obtain graded levels of threonine, and the amount of glycine and maize starch was reduced to compensate. Per kg of threonine premix composition from diets 1 to 6 was as follows (g/kg): l-threonine 0·00, 71·40, 142·80, 214·20, 285·80, 357·20; glycine 222·80, 178·40, 133·80, 89·20, 44·60, 0·00 and maize starch 777·20, 750·20, 723·40, 696·60, 669·60, 642·80, respectively.
§ Per kg of vitamin premix (g/kg): retinyl acetate (172 mg/g), 2·10; cholecalciferol (172 mg/g), 0·40; dl-α-tocopheryl acetate (50 %), 12·58; menadione (22·9 %), 0·83; cyanocobalamin (1 %), 0·94; d-biotin (2 %), 0·75; folic acid (95 %), 0·42; thiamine nitrate (98 %), 0·11; ascorhyl acetate (95 %), 4·31; niacin (99 %), 2·58; meso-inositol (98 %), 19·39; calcium-d-pantothenate (98 %), 2·56; riboflavin (80 %), 0·63; pyridoxine hydrochloride (98 %), 0·62. All ingredients were diluted with maize starch to 1 kg.
|| Per kg of mineral premix (g/kg): MnSO4.H2O (31·8 %Mn), 1·8900; MgSO4.H2O (15·0 % Mg), 200·0000; FeSO4.H2O (30·0 % Fe), 24·5700; ZnSO4.H2O (34·5 % Zn), 8·2500; CuSO4.5H2O (25·0 % Cu), 0·9600; KI (76·9 % I), 0·0668 g; Na2SeO3 (44·7 % Se), 0·0168. All ingredients were diluted with maize starch to 1 kg.
Experimental facility and fish husbandry
Fish husbandry was conducted in the University of Sichuan Agricultural Animal Care Advisory Committee. Juvenile grass carps were obtained from local fisheries (Sichuan, China). Fish were acclimated to the experimental environment for 4 weeks, as described by Hong et al.( Reference Hong, Jiang and Kuang 3 ). Then, 1080 fish with mean initial weights of 9·53 (SD 0·02) g were randomly assigned to eighteen experimental cages (1·4 L×1·4 W×1·4 H (m)), resulting in sixty juveniles per cage. Each cage was equipped with a disc of 100 cm diameter at the bottom to collect the uneaten feed, according to our laboratory study( Reference Deng, Jiang and Liu 47 ). Each cage was randomly assigned to one of three replicates of the six dietary treatments, and fish were fed with the respective diet four times daily for 8 weeks, as described by Wen et al.( Reference Wen, Feng and Jiang 48 ). A period of 30 min after feeding, uneaten feed was collected, dried and weighed to calculate the feed intake, as previously described by Hong et al.( Reference Hong, Jiang and Kuang 3 ). During the experiment, water temperature was 28 (SD 2)°C. The pH and dissolved O2 levels were maintained at 7·0 (SD 0·2) mg/l and not <6·0 mg/l, respectively. Feeding trial was under natural light–dark cycle, similar to that described by Yue et al.( Reference Yue, Zou and Zhu 49 ).
Challenge trial and husbandry
According to our previous work, we used the successful model that was established by challenging with A. hydrophila and evaluating enteritis morbidity on the base of the severity of enteritis for estimating the enteritis resistance( Reference Xu, Wu and Jiang 50 , Reference Zhang, Feng and Jiang 51 ). After the growth trial, sixty fish of similar body weight were obtained from each treatment group and moved to labelled cages for acclimating to the experimental condition for 5 d according to our laboratory study( Reference Xu, Wu and Jiang 50 ). A. hydrophila was kindly provided by College of Veterinary Medicine, Sichuan Agricultural University, China. After the acclimatisation, fish were challenged with intraperitoneal injection of 1·0 ml of 2·5×105 colony-forming units/ml A. hydrophila for each individual. The injection concentration was determined with a nonlethal dosage that could induce inflammation efficiently according to our preliminary test (data not shown). The challenge test lasted for 14 d according to Xu et al.( Reference Xu, Wu and Jiang 50 ) and our preliminary test. The experimental conditions during the A. hydrophila exposure trial were similar to those in the growth trial.
Sample collection
At the initiation and termination of the feeding trial, fish from each cage were weighed and counted, respectively. Thirty fish from the same population before the experiment and six fish from each treatment group at the end of the feeding trial were used for the determination of initial and final carcass proximate composition, as described by Feng et al.( Reference Feng, Peng and Wu 2 ). After the growth trial, 6 h after the last feeding, the blood samples of six fish from each treatment were drawn from the caudal vein, and then the plasma was removed and stored for analysis of plasma ammonia content (PAC), similar to the study by Chen et al.( Reference Chen, Feng and Kuang 52 ). After that, forty-five fish from each treatment were randomly selected and anaesthetised in a benzocaine bath, as described by Geraylou et al.( Reference Geraylou, Souffreau and Rurangwa 53 ). The fish were then killed, and the muscle and hepatopancreas of fish were quickly removed and frozen in N2 and stored at −80°C, as described by Veiseth-Kent et al.( Reference Veiseth-Kent, Grove and Færgestad 54 ), for later assay of the glutamate-oxaloacetate transaminase (GOT) and glutamate-puruvate transaminase (GPT) activities.
At the end of the challenge trial, fish from each treatment were anaesthetised as the same process as the growth trial. Then, the intestines of fish were quickly removed, segmented (proximal intestine (PI), mid intestine (MI) and distal intestine (DI)) and the severity of intestinal inflammation of fish was evaluated based on the method of Song et al.( Reference Song, Zhao and Bo 55 ) and Refstie et al.( Reference Refstie, Baeverfjord and Seim 56 ); the intestines were then frozen in N2 and stored at −80°C for later analysis, as described by Deng et al.( Reference Deng, Jiang and Liu 47 ).
Biochemical parameter analysis
The approximate compositions of the feed and fish carcass were analysed according to the standard methods of the AOAC. PAC, GOT and GPT activities in hepatopancreas and muscle were assayed as described by Jiang et al.( Reference Jiang, Feng and Tang 57 ). The intestinal samples were homogenised on ice in 10 volumes (w/v) of ice-cold physiological saline and centrifuged at 6000 g at 4°C for 20 min, and then the collected supernatant was stored for the subsequent analysis of related parameters, as described by Chen et al.( Reference Chen, Feng and Kuang 52 ). The intestine LA and ACP activity were determined according to the method of El-Boshy et al. ( Reference El-Boshy, El-Ashram and Abdelhamid 58 ) and Molina et al. ( Reference Molina, Moreno and Pichardo 59 ), respectively. The contents of C3 and C4 were measured by using the immunoturbidimetry kit (Nanjing Jiancheng Bioengineering Institute), according to the method of Zhang et al.( Reference Zhang, Xia and Menkiszak 60 ). The IgM content was analysed by using the immunoturbidimetry kit (Nanjing Jiancheng Bioengineering Institute), according to the method of Li et al.( Reference Li, Liu and Zhang 61 ).
Real-time PCR analysis
The procedures of RNA isolation, reverse transcription and quantitative real-time PCR were similar to those descriptions conducted in the previous study in our laboratory(
Reference Wen, Feng and Jiang
48
). The total RNA of the samples was extracted from the PI, MI and DI using the RNAiso Plus kit (TaKaRa) according to the manufacturer’s instructions, followed by DNAse I treatment. The total RNA quality and quantity were assessed using agarose gel (1 %) electrophoresis and spectrophotometric (A260:280 nm ratio) analysis, respectively. Subsequently, RNA was reverse-transcribed into complementary DNA (cDNA) using the PrimeScript™ RT reagent Kit (TaKaRa) according to the manufacturer’s instructions. For quantitative real-time PCR, specific primers were designed according to the sequences cloned in our laboratory and the published sequences of grass carp in the National Center for Biotechnology Information (NCBI) (Table 2). According to the results of our preliminary experiment concerning the evaluation of internal control genes (data not shown), β-actin was used as a reference gene to normalise cDNA loading. The target and housekeeping gene amplification efficiencies were calculated according to the specific gene standard curves generated from 10-fold serial dilutions. The
![]() $$2^{{{\minus}\Delta \Delta C_{t} }} $$
method was used to calculate the expression results after verifying that the primers amplified with an efficiency of approximately 100 %, as described by Livak & Schmittgen(
Reference Livak and Schmittgen
62
).
$$2^{{{\minus}\Delta \Delta C_{t} }} $$
method was used to calculate the expression results after verifying that the primers amplified with an efficiency of approximately 100 %, as described by Livak & Schmittgen(
Reference Livak and Schmittgen
62
).
Table 2 Real-time PCR primer sequences

LEAP-2, liver-expressed antimicrobial peptide 2; IFN-γ2, interferon γ2; TGF-β, transforming growth factor β; IκBα, inhibitor of κBα; IKK, IκB kinase; TOR, target of rapamycin; S6K1, ribosomal protein S6 kinases 1; 4E-BP1, eIF4E-binding protein 1.
Western blot analysis
The protein homogenate preparation from intestines, antibodies and western blotting were processed as described in our previous studies( Reference Kai, Zhang and Lin 63 , Reference Jiang, Liu and Jiang 64 ). We determined the protein concentrations using a BCA assay kit (Beyotime Biotechnology Inc.). Protein samples (40 μg/lane) were separated by SDS-PAGE and transferred to a polyvinylidene fluoride (PVDF) membrane for western blot analysis. The membrane was blocked for 1 h at room temperature and then incubated with primary antibody overnight at 4°C. We used the same anti-total TOR, p-TOR Ser 2448 and β-Actin antibodies as those described in our previous studies( Reference Kai, Zhang and Lin 63 , Reference Jiang, Liu and Jiang 64 ). β-Actin was used as control proteins for total protein. After being washed, the PVDF membrane was incubated for 2 h with goat anti-rabbit horseradish peroxidase-conjugated secondary antibody (Santa Cruz Biotechnology) in TBST (Tris-buffered saline, with Tween-20). The immune complexes were visualised using ECL reagents (Beyotime Biotechnology Inc.). The western blot bands were quantified using the NIH Image 1.63 software. Different treatments were expressed relative to the level of the control group. This experiment was repeated at least three times, and similar results were obtained each time.
Calculations and statistical analysis
Growth performance parameters were calculated on the basis of the following formulas: growth performance was assessed in terms of PWG, SGR, feed intake (FI) and FE, protein efficiency ratio (PER) and protein retention value (PRV):
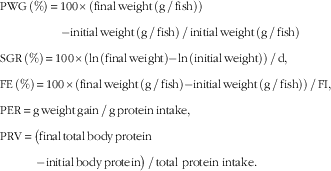 $$\eqalignno{ & {\rm PWG}\,\left( \% \right)\,{\equals}\,{\rm 1}00{\times}\left( {{\rm final}\,{\rm weight}\,\left( {{\rm g}\,/\,{\rm fish}} \right) } \right) \cr & \qquad \quad \qquad {\rm {\minus}{\rm initial}\,{\rm weight}\,\left( {{\rm g}\,/\,{\rm fish}} \right)\,/\,{\rm initial}\,{\rm weight}\,\left( {{\rm g}\,/\,{\rm fish}} \right)} \cr &{\rm SGR}\,\left( \% \right)\,{\equals}\,{\rm 1}00{\times}\left( {{\rm ln}\,\left( {{\rm final}\,{\rm weight}} \right){\minus}{\rm ln}\,\left( {{\rm initial}\,{\rm weight}} \right)} \right)\,/\,{\rm d,} \cr &{\rm FE}\,\left( \% \right)\,{\equals} \,{\rm 1}00{\times}\left( {{\rm final}\,{\rm weight}\,\left( {{\rm g}\,/\,{\rm fish}} \right){\minus}{\rm initial}\,{\rm weight}\,\left( {{\rm g}\,/\,{\rm fish}} \right)} \right)\,/\,{\rm FI,} \cr &{\rm PER}\,{\equals}\,{\rm g}\,{\rm weight}\,{\rm gain}\,/\,{\rm g}\,{\rm protein}\,{\rm intake,} \cr & {\rm PRV}\,{\equals}\,\big( {{\rm final}\,{\rm total}\,{\rm body}\,{\rm protein}}\cr &{\quad\qquad {\minus}{\rm initial}\,{\rm body}\,{\rm protein}} \big)\,/\,{\rm total} \ {\rm protein} \ {\rm intake}{\rm .} $$
$$\eqalignno{ & {\rm PWG}\,\left( \% \right)\,{\equals}\,{\rm 1}00{\times}\left( {{\rm final}\,{\rm weight}\,\left( {{\rm g}\,/\,{\rm fish}} \right) } \right) \cr & \qquad \quad \qquad {\rm {\minus}{\rm initial}\,{\rm weight}\,\left( {{\rm g}\,/\,{\rm fish}} \right)\,/\,{\rm initial}\,{\rm weight}\,\left( {{\rm g}\,/\,{\rm fish}} \right)} \cr &{\rm SGR}\,\left( \% \right)\,{\equals}\,{\rm 1}00{\times}\left( {{\rm ln}\,\left( {{\rm final}\,{\rm weight}} \right){\minus}{\rm ln}\,\left( {{\rm initial}\,{\rm weight}} \right)} \right)\,/\,{\rm d,} \cr &{\rm FE}\,\left( \% \right)\,{\equals} \,{\rm 1}00{\times}\left( {{\rm final}\,{\rm weight}\,\left( {{\rm g}\,/\,{\rm fish}} \right){\minus}{\rm initial}\,{\rm weight}\,\left( {{\rm g}\,/\,{\rm fish}} \right)} \right)\,/\,{\rm FI,} \cr &{\rm PER}\,{\equals}\,{\rm g}\,{\rm weight}\,{\rm gain}\,/\,{\rm g}\,{\rm protein}\,{\rm intake,} \cr & {\rm PRV}\,{\equals}\,\big( {{\rm final}\,{\rm total}\,{\rm body}\,{\rm protein}}\cr &{\quad\qquad {\minus}{\rm initial}\,{\rm body}\,{\rm protein}} \big)\,/\,{\rm total} \ {\rm protein} \ {\rm intake}{\rm .} $$
All data were subjected to a one-way ANOVA followed by the Duncan’s multiple-range test to evaluate significant differences among treatments at P<0·05 with SPSS 18.0 (SPSS Inc.), as described by Jiang et al.( Reference Jiang, Tang and Liu 65 ). On the basis of the means and standard deviations of growth and intestinal immune-related parameters, the minimum effect size was calculated to be 0·67 according to the method of Searcy-Bernal( Reference Searcy-Bernal 66 ). With the effect size of 0·67, a significance level of 0·05 and the six replicates in each treatment, the statistical power was calculated to be 0·82 using the R pwr package according to Grey et al. ( Reference Grey, Chiasson and Williams 67 ). On the basis of the SGR and intestinal health indicators, the threonine requirements were estimated by quadratic regression model according to the method of Ahmed et al.( Reference Ahmed, Khan and Jafri 68 ).
Results
Growth performance of juvenile grass carp, glutamate-oxaloacetate transaminase and glutamate-puruvate transaminase activities in the muscle and hepatopancreas, plasma ammonia content and enteritis morbidity
Effects of graded threonine levels on juvenile grass carp growth parameters are presented in Table 3. The growth performance (IBW, FBW, PWG, SGR, FI, FE, PER and PPV) was elevated significantly (P<0·05) with increased threonine levels up to 14·10 g threonine/kg diet, and then decreased significantly (P<0·05). As shown in Table 4, the GOT and GPT activities of the hepatopancreas were enhanced significantly with added threonine levels up to 14·10 g threonine/kg diet (P<0·05), and then decreased gradually. Meanwhile, with the addition of dietary threonine up to 14·10 g threonine/kg diet, increased muscle activities were obtained significantly in the GOT and GPT (P<0·05), and then decreased significantly (P<0·05) in the GOT but slightly in the GPT, respectively. Besides, the PAC decreased significantly with threonine level up to 14·10 g threonine/kg diet (P<0·05), and then increased significantly (P<0·05). In addition, with threonine level up to 14·10 g threonine/kg diet, enteritis morbidity after challenge with A. hydrophila was abated significantly (P<0·05) and then increased gradually (Fig. 1 and 2).

Fig. 1 The enteritis morbidity of juvenile grass carp (Ctenopharyngodon idella) fed diets containing graded levels of threonine after infection with Aeromonas hydrophila for 14 d. a,b,c,d Mean values with unlike letters were significantly different (P<0·05; ANOVA and Duncan’s multiple-range tests).

Fig. 2 Compared with optimal threonine supplementation, threonine deficiency led to obviously enteritis symptom of juvenile grass carp (Ctenopharyngodon idella) fed diets containing graded levels of threonine for 8 weeks after infection with Aeromonas hydrophila for 14 d. Visibly red and swollen and hyperaemia were observed for fish fed the threonine-deficient diet after infection with A. hydrophila.
Table 3 Initial body weight (IBW), final body weight (FBW), percentage weight gain (PWG), feed intake (FI), feed efficiency (FE), specific growth rate (SGR), protein efficiency ratio (PER) and protein retention value (PRV) of juvenile grass carp (Ctenopharyngodon idella) fed diets containing graded levels of threonine for 8 weeksFootnote * (Mean values and standard deviations)

a,b,c,d,e Mean values in the same row with unlike superscripts were significantly different (P<0·05; ANOVA and Duncan’s multiple-range tests).
* Values for IBW, FBW, PWG, SGR, FI and FE of three replicates groups, with sixty fish in each group. Values for PER and PRV of six replicates.
Table 4 Glutamate-oxaloacetate transaminase (GOT) and glutamate-pyruvate transaminase (GPT) activities in muscle and hepatopancreas; plasma ammonia contents (PAC) of juvenile grass carp (Ctenopharyngodon idella) fed with diets containing graded levels of threonine for 8 weeks (Mean values and standard deviations, six replicates)

a,b,c,d,e Means values in the same row with unlike superscripts are significantly different (P<0·05; ANOVA and Duncan’s multiple-range tests).
Intestine immune parameters
The intestinal activities of the LA, ACP and the contents of C3 and C4 in the three intestinal segments of juvenile grass carp after infection with A. hydrophila are presented in Table 5. With the addition of threonine up to 14·10 and 17·96 g threonine/kg diet individually, the LA enhanced significantly (P<0·05) in the PI but slightly in the MI, and then all decreased gradually. With the addition of threonine up to 14·10 g threonine/kg diet, the contents of C3 in the DI, IgM in the MI and DI increased gradually, and then all decreased smoothly. With the addition of threonine up to 14·10, 10·72 and 10·72 g threonine/kg diet, the ACP activities in the PI and the C3 contents in the PI and MI increased significantly (P<0·05), and then all plateaued (P>0·05). Compared with the dietary threonine deficiency, threonine supplementation increased the activities of the ACP in the MI and DI, and the C4 contents in the PI and MI. Meanwhile, fish fed 10·72 g threonine/kg diet showed the maximum C4 contents in the DI. On the contrary, the LA in the DI were diminished with threonine level up to 10·72 g threonine/kg diet (P<0·05), and then plateaued (P>0·05). However, dietary threonine had no effects on the IgM contents in the PI (P>0·05).
Table 5 Immune components in the intestine of juvenile grass carp (Ctenopharyngodon idella) fed diets containing graded levels of threonine for 8 weeks after injection with Aeromonas hydrophila for 14 d (Mean values and standard deviations)

PI, proximal intestine; MI, mid intestine; DI, distal intestine.
a,b,c,d Mean values in the same row with unlike superscripts were significantly different (P<0·05; ANOVA and Duncan’s multiple-range tests).
Gene expression in the intestine
Relative expressions of innate and adaptive components and inflammatory cytokines mRNA in fish intestines
The effects of dietary threonine on innate and adaptive components and inflammatory cytokines in the three intestinal segments of fish after infection with A. hydrophila are presented in Fig. 3 and 4. In the PI, with the addition of threonine up to 14·10, 14·10 and 14·10 g threonine/kg diet, the mRNA levels of hepcidin, LEAP-2A and IL-10 were up-regulated, respectively, and then all down-regulated gradually. Meanwhile, compared with threonine deficiency, optimal threonine supplementation up-regulated the LEAP-2B, IgZ, TGF-β 1, TGF- β2 and IL-4/13A mRNA levels significantly (P<0·05). However, there were no marked differences between the threonine-deficient diet group (3·99 g threonine/kg diet) and any other group added graded levels of threonine on β-defensin1 and IgM mRNA levels (P>0·05). In the MI, with the addition of threonine up to 10·72, 14·10, 14·10, 14·10, 14·10 and 14·10 g threonine/kg diet, the mRNA levels of hepcidin, LEAP-2A, β -defensin1, IgZ, TGF- β1 and TGF-β2 were elevated individually, and then all decreased gradually. The mRNA levels of LEAP-2B, IgM, IL-4/13A and IL-10 in the MI were lowest for juveniles fed a threonine-deficient diet (P<0·05), individually. In the DI, the mRNA levels of hepcidin, LEAP-2A, LEAP-2B, β-defensin1, IgM, IgZ, TGF- β2, IL-4/13A and IL-10 were up-regulated individually with increased threonine levels up to 14·10 g threonine/kg diet, and then all down-regulated gradually. Fish fed a threonine-deficient diet showed the minimum TGF-β1 level in the DI of juvenile grass carp (P<0·05). Surprisingly, no remarkable differences were found in the IL-4/13B mRNA levels in the three intestinal segments between fish fed graded levels of threonine (P>0·05).

Fig. 3 Relative expression of IgM, IgZ, hepcidin, liver-expressed antimicrobial peptide (LEAP)-2A, LEAP-2B and β-defensin1 and anti-inflammatory cytokines (TGF-β1, TGF-β2, IL-4/13A, IL-4/13B and IL-10) in the proximal intestine (PI, A), middle intestine (MI, B) and distal intestine (DI, C) of fish fed diets containing graded levels of threonine for 8 weeks after infection with Aeromonas hydrophila for 14 d. Values are means (six fishes per group), and standard deviations represented by vertical bars. ![]() , Thr 3·99;
, Thr 3·99; ![]() , Thr 7·70;
, Thr 7·70; ![]() , Thr 10·72;
, Thr 10·72; ![]() , Thr 14·10;
, Thr 14·10; ![]() , Thr 17·96;
, Thr 17·96; ![]() , Thr 21·66. a,b,c Mean values with unlike letters were significantly different (P<0·05; ANOVA and Duncan’s multiple-range tests).
, Thr 21·66. a,b,c Mean values with unlike letters were significantly different (P<0·05; ANOVA and Duncan’s multiple-range tests).

Fig. 4 Relative expression of pro-inflammatory cytokines (TNF-α, IL-1β, IFN-γ2, IL-6, IL-8, IL-12p35, IL-12p40, and IL-17D) in the proximal intestine (PI) (A), middle intestine (MI) (B) and distal intestine (DI) (C) of fish fed diets containing graded levels of threonine for 8 weeks after infection with Aeromonas hydrophila for 14 d. Values are means (six fishes per group), and standard deviations represented by vertical bars. ![]() , Thr 3·99;
, Thr 3·99; ![]() , Thr 7·70;
, Thr 7·70; ![]() , Thr 10·72;
, Thr 10·72; ![]() , Thr 14·10;
, Thr 14·10; ![]() , Thr 17·96;
, Thr 17·96; ![]() , Thr 21·66. a,b,c Mean values with unlike letters were significantly different (P<0·05; ANOVA and Duncan’s multiple-range tests).
, Thr 21·66. a,b,c Mean values with unlike letters were significantly different (P<0·05; ANOVA and Duncan’s multiple-range tests).
In addition, in the PI, the mRNA levels of TNF-α, IFN-γ2 and IL-17D were down-regulated individually with the addition of threonine up to 14·10, 14·10 and 10·72 g threonine/kg diet, and then all up-regulated, gradually. Compared with dietary threonine supplementation, threonine deficiency up-regulated the IL-1 β, IL-6 and IL-8 mRNA levels, significantly (P<0·05). In the MI, the mRNA levels of TNF-α, IL-1β, IFN-γ2 and IL-17D were down-regulated with the addition of threonine up to 14·10, 10·72, 10·72 and 10·72 g threonine/kg diet, respectively, and then all up-regulated gradually. Meanwhile, fish fed the threonine deficiency diet showed the highest IL-6 and IL-8 mRNA levels (P<0·05). In the DI, the mRNA levels of TNF-α, IL-8 and IL-17D were down-regulated individually with the addition of dietary threonine up to 10·72, 14·10 and 14·10 g threonine/kg diet, and then all up-regulated, gradually. Compared with threonine supplementation, threonine deficiency enhanced the IL-1β, IFN-γ2 and IL-6 mRNA levels significantly (P<0·05). Characteristically, dietary threonine had no effects on the mRNA levels of IL-12 p35 and IL-12 p40 in the three intestinal segments of juvenile grass carp (P>0·05).
Relative expressions of immune-related signal molecules in the intestines of fish
The effects of threonine on relative expressions of the NF-κ B p65, NF- κ B p52, c-Rel, IκBα, IKKα, β, γ, TOR, p70 S6K1 and 4E-BP1 after infection with A. hydrophila are presented in Fig. 5. In the PI, the addition of threonine up to 14·10, 10·72, 14·10, 14·10 and 14·10 g threonine/kg diet down-regulated mRNA levels significantly in the IKKβ (P<0·05) and slightly in the NF-κB p65, NF-κB p52, IKKγ and 4E-BP1, and then all up-regulated gradually. On the contrary, the addition of threonine up to 14·10 g threonine/kg diet up-regulated the mRNA levels in the TOR, I κBα and S6K1, and then all down-regulated gradually. In the MI, the mRNA levels of NF-κB p52, IKKβ, IKKγ and 4E-BP1 were down-regulated with threonine levels up to 14·10, 14·10, 14·10 and 17·96 g threonine/kg diet, respectively, and then all up-regulated gradually. Besides, the TOR mRNA levels were up-regulated with threonine levels added up to 14·10 g threonine/kg diet, and then down-regulated gradually. Besides, fish fed the threonine deficiency diet showed the maximum mRNA level in NF-κB p65 and the minimum in IκBα and S6K1 (P<0·05). In the DI, the addition of threonine up to 14·10 g threonine/kg diet down-regulated the mRNA levels slightly in the NF-κB p52, NF-κB p65, IKKβ, IKKγ and 4E-BP1, and then all up-regulated progressively. Meanwhile, IκBα and TOR mRNA levels were up-regulated individually with dietary threonine levels up to 14·10 and 17·96 g threonine/kg diet, and then all down-regulated gradually. Compared with threonine supplementation, threonine deficiency down-regulated S6K1 mRNA level significantly (P<0·05). Interestingly, no marked influences were detected in the IKKα and c-Rel mRNA levels in the three intestinal segments of juvenile grass carp (P>0·05).

Fig. 5 Relative expression of NF-κB p65, NF-κB p52, c-Rel, inhibitor of κBα (IκBα), IκB kinases (IKKα, IKKβ, IKKγ), target of rapamycin (TOR), S6 kinase (S6K1) and eIF4E-binding protein 1 (4E-BP1) in the proximal intestine (PI) (A), middle intestine (MI) (B) and distal intestine (DI) (C) of fish fed diets containing graded levels of threonine for 8 weeks after infection with Aeromonas hydrophila for 14 d. Values are means (six fishes per group), and standard deviations represented by vertical bars. ![]() , Thr 3·99;
, Thr 3·99; ![]() , Thr 7·70;
, Thr 7·70; ![]() , Thr 10·72;
, Thr 10·72; ![]() , Thr 14·10;
, Thr 14·10; ![]() , Thr 17·96;
, Thr 17·96; ![]() , Thr 21·66. a,b,c Mean values with unlike letters are significantly different (P<0·05; ANOVA and Duncan’s multiple-range tests).
, Thr 21·66. a,b,c Mean values with unlike letters are significantly different (P<0·05; ANOVA and Duncan’s multiple-range tests).
Protein levels and phosphorylation of target of rapamycin in the intestine of fish
The effects of threonine on the protein levels of total TOR (T-TOR) and phosphorylation of TOR on residue Ser2448 (p-TOR Ser2448) after infection with A. hydrophila are presented in Fig. 6. With the addition of threonine up to 17·96 g threonine/kg diet, the protein levels of the T-TOR increased gradually in the PI, MI and DI, and then decreased gradually in the DI and significantly in the PI and MI of juvenile grass carp (P<0·05). With the addition of threonine up to 17·96, 10·72 and 14·10 g threonine/kg, respectively, enhanced protein levels of the p-TOR Ser2448 were found in the PI, MI and DI, and then all decreased significantly in the PI (P<0·05) and gradually in the MI and DI of juvenile grass carp.

Fig. 6 Western blot analysis of total target of rapamycin (T-TOR) protein phosphorylation at Ser2448 (p-TOR Ser2448) in the proximal intestine (PI) (A), middle intestine (MI) (B) and distal intestine (DI) (C) of fish fed diets containing graded levels of threonine for 8 weeks after infection with Aeromonas hydrophila for 14 d. Values are means (three replicates per group), and standard deviations represented by vertical bars. ![]() , Thr 3·99;
, Thr 3·99; ![]() , Thr 7·70;
, Thr 7·70; ![]() , Thr 10·72;
, Thr 10·72; ![]() , Thr 14·10;
, Thr 14·10; ![]() , Thr 17·96;
, Thr 17·96; ![]() , Thr 21·66. a,b,c,d Mean values with unlike letters were significantly different between treatments (P<0·05; ANOVA and Duncan’s multiple-range tests).
, Thr 21·66. a,b,c,d Mean values with unlike letters were significantly different between treatments (P<0·05; ANOVA and Duncan’s multiple-range tests).
Discussion
Threonine deficiency decreased fish growth performance and the enteritis resistance
According to the study, dietary threonine deficiency decreased the growth performance of juvenile grass carp with poor PWG, FI, FE, SGR, PER and PRV. On the basis of the quadratic regression analysis of the SGR, the optimal dietary threonine level for juvenile grass carp was estimated to be 14·53 g threonine/kg diet (4·48 g threonine/100 g protein). Meanwhile, accelerated growth performance was associated with improvement of amino acid utilisation, which reflected in depressed PAC( Reference Costas, Aragão and Ruiz-Jarabo 69 ) and enhanced GOT and GPT levels in the hepatopancreas and muscle( Reference Borges, Medale and Dias 70 ). Our study displayed that dietary threonine deficiency increased the PAC and decreased the GOT and GPT activities in muscle and hepatopancreas of juvenile grass carp. In addition, it is well known that fish growth is related to intestine health, which is partly reflected in the enteritis resistance( Reference Romarheim, Hetland and Skrede 71 ). Previous studies of our laboratory demonstrated that the higher enteritis morbidity could reflect the weaker enteritis resistance after fish were challenged with A. hydrophila ( Reference Xu, Wu and Jiang 50 , Reference Zhang, Xia and Menkiszak 60 ). Thus, we next investigated the effect of threonine on the resistance to enteritis of fish after challenging them with A. hydrophila.
In the present study, we demonstrated for the first time that, compared with dietary threonine deficiency resulting in the maximum enteritis morbidity (25·5 %), the optimal threonine level significantly decreased the enteritis morbidity to be 1·5 % in juvenile grass carp after infection with A. hydrophila (P<0·05), indicating that dietary threonine deficiency attenuated the resistance ability against enteritis in fish. On the basis of the enteritis morbidity, the optimal threonine level to reinforce resistance against enteritis was recommended to be 15·05 g threonine/kg diet (4·64 g threonine/100 g protein) (Y=0·1841x 2−5·5399x+42·4060, R 2=0·897, P<0·05), which was slightly higher than that based on SGR (14·53 g threonine/kg diet). In addition, enteritis resistance is related to intestinal immune function, which is partly dependent on the innate and adaptive responses in fish( Reference Xu, Wu and Jiang 50 , Reference Romarheim, Hetland and Skrede 71 ). Thus, we next investigated the impacts of dietary threonine on innate and adaptive responses in the intestines of juvenile grass carp after infection with A. hydrophila.
Threonine deficiency impaired fish intestinal immunity by decreasing innate and adaptive immune component production after infection with Aeromonas hydrophila
In this study, for the first time, we investigated the effects of threonine on innate and adaptive immune components in fish intestines after infection with A. hydrophila. Current results displayed that dietary threonine deficiency decreased LA in PI and MI, IgM contents in MI and DI, ACP activities and contents of C3 and C4 in PI, MI and DI, mRNA levels of β-defensin1, IgM and IgZ in the MI and DI, Hepcidin, LEAP-2A and LEAP-2B in the three intestinal segments of juvenile grass carp after infection with A. hydrophila, indicating that threonine deficiency could depress the fish intestinal immunity.
Interestingly, the distinct effects of threonine deficiency on LA, IgM and β-defensin1 was first observed in the different intestinal segments of juvenile grass carp. The possible reasons for the diverse results are considered in the next discussion. First, threonine deficiency decreased the LA in the PI and MI but increased it in the DI of juvenile grass carp, which might be related to Cu/Zn-SOD and nitric oxide. Habte-Tsion et al.( Reference Habte-Tsion, Ge and Liu 12 ) demonstrated that threonine deficiency could enhance the Cu-Zn-SOD mRNA level in the distal part but suppress it in the proximal part of intestines in the blunt snout bream. In mice renal, Cu/Zn-SOD deletion could decrease the nitric oxide level( Reference Fujita, Fujishima and Takahashi 72 ), which could hinder the release of LA in rabbit neutrophils( Reference Vanuffelen, Vansteveninck and Elferink 73 ). Hence, we hypothesised that threonine deficiency down-regulated the Cu/Zn-SOD expression to decrease the nitric oxide level, thereby decreasing the LA in the PI and MI, while having adverse effects in the DI of fish. However, it warrants further investigation to support our hypothesis. Second, threonine deficiency down-regulated IgM mRNA levels and contents in the MI and DI (rather than PI) of juvenile grass carp, which might be related to the catabolite butyrate derived from threonine and different immunological relevance in the intestines per se. Smith et al.( Reference Smith and Macfarlane 74 ) confirmed that the production of butyrate decreased when threonine was insufficient in the distal parts of the intestine in humans. In bovine lymphocytes, insufficient butyrate decreased the synthesis of IgM ( Reference Nonnecke, Franklin and Young 75 ). Therefore, we suppose that threonine deficiency might decrease butyrate production to reduce IgM in the MI and DI (rather than PI) of fish, which needs further investigation. In the sea bass, a gradually increasing number of IgM+ cells were established from anterior and middle to the posterior part of the intestines, which suggested a higher immunological relevance for the posterior gut, as the same finding as in the Atlantic halibut( Reference Grove, Johansen and Reitan 76 , Reference Abelli, Picchietti and Romano 77 ). As we know, threonine could contribute to the proliferation of lymphocyte in the weaned pigs( Reference Ren, Liu and Wang 10 ). Thus, compared with the anterior part of the intestines, we hypothesised that threonine increased the IgM production only in the MI and DI of juvenile grass carp partly because of promoting larger amount of IgM+ cells, which were distributed predominantly in the posterior parts of intestines. However, the hypothesis remains to be investigated further. Third, threonine deficiency down-regulated β-defensin1 mRNA level in the MI and DI (not PI) of juvenile grass carp, which is likely to be relevant to TGFβ1. In this study, we observed that threonine deficiency down-regulated TGFβ1 mRNA levels in the intestines of juvenile grass carp. Leppäranta et al.( Reference Leppäranta, Pulkkinen and Koli 78 ) reported that suppression of TGFβ1 could inhibit the transcription factor GATA-6 expression in A549 cells. In mature mice, inhibited GATA-6 was able to down-regulate the Apoa1 mRNA levels in the posterior but not in the anterior parts of the intestine( Reference Beuling, Baffour–Awuah and Stapleton 79 ). In humans, it was reported that the absence of Apoa1 decreased the PGE2 production, which could down-regulate β-defensin mRNA levels( Reference Kwang Dong, Ho Yong and Hee Gu 80 , Reference Zhang, Case and Bowler 81 ). Therefore, we presumed that threonine deficiency could decline TGF-β1 mRNA levels to down-regulate GATA-6 expression, thus decreasing Apoa1 and PGE2 mRNA levels only in the MI and DI (not PI) to decrease β-defensin1 mRNA levels only in the MI and DI (not PI) of fish. However, the hypothesis needs further verification.
In addition, fish intestinal immune function is also associated with its inflammatory responses, which are primarily mediated by cytokines( Reference Nilsen, Johansen and Jahnsen 21 ) and related signalling molecules( Reference Hong, Lee and Jung 25 , Reference Weichhart, Haidinger and Katholnig 26 ). Therefore, we next examined the effects of threonine on inflammatory cytokines and explored the possible mechanism by investigating the actions of threonine on NF-κB- and TOR-related signalling molecules in the intestines of juvenile grass carp after infection with A. hydrophila.
Threonine deficiency aggravated intestinal inflammation associated with NF-κB and target of rapamycin signalling pathways in fish after infection with Aeromonas hydrophila
Threonine deficiency triggered intestinal inflammation by up-regulating pro-inflammatory cytokines partly through the NF-κB signalling pathway in fish after infection with A. hydrophila
Generally, it is noteworthy that intestinal immune function can be activated by mediating pro-inflammatory cytokines such as TNF-α and IL-1β, the up-regulation of which can trigger intestinal inflammation in fish( Reference Zuo, Ai and Mai 19 ). In this study, we systematically examined the effects of threonine on multiple pro-inflammatory cytokine responses in fish intestines after infection with A. hydrophila. Our study showed that, compared with optimal threonine supplementation, threonine deficiency up-regulated the pro-inflammatory cytokines TNF-α, IL-1β, IFN-γ2, IL-6, IL-8 and IL-17D mRNA levels in three intestinal segments of juvenile grass carp after infection with A. hydrophila, suggesting that threonine deficiency aggravated the inflammatory responses in fish intestines. Furthermore, studies confirmed that cytokine production could be modulated by the signalling regulators of NF-κB in humans( Reference Hong, Lee and Jung 25 ). Therefore, we next investigated the effects of threonine on NF-κB signalling pathways in the intestines of juvenile grass carp after infection with A. hydrophila.
NF-κB is a transcription factor containing three subunits (p65/p52/c-Rel), which was traps activated by its inhibitor IκBα that could be degraded by IKK complex (including IKKα, IKKβ and IKKγ), and the activation of which could up-regulate pro-inflammatory cytokine (such as IL-1β and IL-8) expression in mammals( Reference Hoesel and Schmid 82 ). In this study, compared with threonine supplementation, threonine deficiency induced the up-regulation of NF-κB (p65 and p52 (not c-Rel)), IKK (β and γ (not α)) and the down-regulation of IκBα mRNA levels in the three intestinal segments of juvenile grass carp after infection with A. hydrophila. Correlation analysis (Table 6) indicated that the mRNA levels of pro-inflammatory cytokines (TNF-α, IFN-γ2, IL-6, IL-8 and IL-17D) were positively related to NF-κBp65 or NF-κBp52 in the three intestinal segments of fish and that the mRNA levels of IKKβ and IKKγ in the PI and DI were present the inverse correlation with IκBα which were negatively correlated with NF-κBp65 and NF-κBp52, respectively. Meanwhile, the mRNA levels of IKKβ showed the adverse tendency to IκBα, which was negatively correlated to NF-κBp65 in the MI of juvenile grass carp. The results given above implied that threonine deficiency up-regulated pro-inflammatory cytokine mRNA levels, which was partially attributed to IKK (β and γ (not α))/IκBα/NF-κB (p65 and p52 (not c-Rel)) signalling, thus triggering inflammation in fish.
Table 6 Correlation coefficients of genes relative expression in the intestine

PI, proximal intestine; MI, mid intestine; DI, distal intestine; IKK, IκB kinase; TGF, transforming growth factor; S6K1, ribosomal protein S6 kinases 1; 4E-BP1, eIF4E-binding protein 1; TOR, target of rapamycin.
Surprisingly, no significant differences were found in the IL-12p35, IL-12p40, c-Rel and IKKα mRNA levels of three intestinal segments for fish fed graded levels of threonine diets after infection with A. hydrophila. The potential reasons might be explained as follows. First, the fact that threonine deficiency did not affect the intestinal IL-12 (p35 and p40) mRNA levels might be related to the unchanged c-Rel. In mice, it was reported that c-Rel was essential for the activation of the IL-12 p35 and IL-12p40 expressions in dendritic cells and macrophages( Reference Grumont, Hochrein and O’Keeffe 83 ), respectively. However, our study displayed that threonine deficiency had no impact on c-Rel mRNA levels in the three intestinal segments of fish, supporting our hypothesis. As for the unchanged c-Rel mRNA levels by threonine, it might be related to glutamate. Hamard et al.( Reference Hamard, Sève and Floc’H 84 ) demonstrated that threonine deficiency could accumulate the glutamate levels in the plasma of pigs. A study has shown that glutamate had no impact on c-Rel binding activities in mice( Reference Fan and Cooper 85 ). Thus, the fact that threonine deficiency did not alter the c-Rel transcript abundances might be partially related to the enhanced glutamate levels in fish intestines. However, the speculated reason still remains to be elucidated further. Second, the reason that threonine deficiency elevated IKKβ and IKKγ but not IKKα mRNA levels in the three intestinal segments of juvenile grass carp might be related to IFN-γ altering PKCζ. In this study, we observed that threonine deficiency up-regulated IFN-γ mRNA levels in the intestines of juvenile grass carp. In mice, it was found that IFN-γ could enhance the PKCζ levels( Reference Wieteska-Skrzeczyńska, Grzelkowska-Kowalczyk and Rejmak 86 ), which could up-regulate IKKβ and IKKγ but ignore IKKα expression( Reference Peng, Sigua and Karsonovich 87 ). Hence, we hypothesised that threonine deficiency up-regulated the IFN-γ mRNA levels, partly resulting in increasing PKCζ levels, thus leading to the up-regulation of IKKβ and IKKγ (not IKKα) in fish intestines. However, our hypothesis still needs further investigation. In addition, except for up-regulation of pro-inflammatory cytokines, down-regulation of anti-inflammatory cytokines can also initiate the inflammation process( Reference Byrne and Viney 88 ). Then, we next examined the effects of threonine on anti-inflammatory cytokines in the intestines of juvenile grass carp after infection with A. hydrophila.
Threonine deficiency induced intestinal inflammation via down-regulating the anti-inflammatory cytokines partly associated with target of rapamycin signalling pathway in fish after infection with Aeromonas hydrophila
In humans, it was confirmed that the down-regulation of anti-inflammatory cytokines (such as TGF-β, IL-4/13 and IL-10) could aggravate the inflammation process( Reference Mia, Warnecke and Zhang 20 ), which could be regulated by TOR signalling via inhibiting ribosomal protein S6K1 and activating 4E-BP1 ( Reference Zhao, Benakanakere and Hosur 89 ). In this study, for the first time, we probed the effects of threonine on anti-inflammatory cytokines in animal intestines after infection with A. hydrophila. Current results showed that, compared with optimal threonine supplementation, threonine deficiency down-regulated the anti-inflammatory cytokines TGF-β1, TGF-β2, IL-4/13A (not IL- 4/13B) and IL-10 transcript abundances in the PI, MI and DI of juvenile grass carp after infection with A. hydrophila, suggesting that threonine deficiency triggered the inflammatory responses via down-regulating anti-inflammatory cytokines in fish intestines. Furthermore, our data displayed that, compared with optimal threonine supplementation, threonine deficiency down-regulated TOR and S6K1 mRNA levels, decreased TOR protein and its phosphorylation levels and up-regulated 4E-BP1 mRNA levels in the three intestinal segments of juvenile grass carp. Correlation analyses (Table 6) indicated that those anti-inflammatory cytokine mRNA levels (TGF-β1, TGF-β2, IL-4/13A, IL-10 and S6K1) were positively related to TOR, which were negatively related to 4E-BP1 in the three intestinal segments of juvenile grass carp. The above observations manifested that threonine deficiency down-regulated anti-inflammatory cytokines partially because of the abridgement of the signal cascades ((TOR/(S6K1 and 4E-BP1)) in fish intestines.
Nevertheless, threonine deficiency down-regulated IL-4/13A (rather than IL-4/13B) mRNA levels in the three intestinal segments of juvenile grass carp, which might be explained by TOR and transcription factor GATA-3. In this study, threonine deficiency down-regulates TOR mRNA levels in the three intestinal segments of juvenile grass carp. It was confirmed that inhibition of TOR could down-regulate the GATA-3 expression in mice CD4+ T cells( Reference Cook and Miller 90 ). In Fugu, GATA-3 could precede binding to presumable promoter region for sharing a motif of TATA box in IL-4/13A gene but not IL-4/13B ( Reference Ohtani, Hayashi and Hashimoto 91 ). Therefore, we speculated that threonine deficiency down-regulated TOR to diminish the GATA-3 expression, thus leading to attenuating the binding to IL-4/13A (not IL-4/13B) to down-regulate the IL-4/13A (not IL-4/13B) mRNA levels in fish intestines. However, the possible supposition still remains to be elucidated.
Comparison of optimal threonine levels for juvenile grass carp based on different indices
In the context, threonine deficiency decreased growth performance, exaggerated enteritis morbidity, attenuated intestinal immunity and impaired intestinal inflammation response in juvenile grass carp after infection with A. hydrophila. On the basis of SGR, the threonine requirement was estimated to be 14·53 g threonine/kg diet (4·48 g threonine/100 g protein), which is a little higher than that recommended by Gao et al. ( Reference Gao, Yang and Liu 37 ) with 13·7 g threonine/kg diet (3·61 g threonine/100 g protein). To our knowledge, the higher the growth rate, the more adequate nutrients should be required in fish( Reference Morais and Conceição 92 ). In this study, fish fed optimal threonine level showed a slightly higher SGR than that described by Gao et al. ( Reference Gao, Yang and Liu 37 ). Besides, based on the protecting fish against enteritis morbidity and IgM content, threonine requirements were estimated to be 15·05 g threonine/kg diet (4·64 g threonine/100 g protein) and 15·17 g threonine/kg diet (4·68 g threonine/100 g protein), respectively (Fig. 7 and 8). Comparatively, the requirements for against enteritis morbidity and improving immune index were close to or slightly higher than those for the growth performance, suggesting that a little more threonine supplementation is required for assuring intestinal health of fish.

Fig. 7 Quadratic regression analysis of specific growth rate for the fish fed diets containing graded threonine levels for 8 weeks.

Fig. 8 Quadratic regression analysis of IgM for the fish fed diets containing graded threonine levels for 8 weeks after infection with Aeromonas hydrophila for 14 d. MI, middle intestine.
Conclusions
In summary (Fig. 9), on the basis of the previous study about threonine on the growth performance of juvenile grass carp, for the first time, we systematically demonstrated that dietary threonine deficiency depressed the intestine immune function in fish by regulating immune-related signalling molecules after infection with A. hydrophila, as displayed in the following aspects: (1) threonine deficiency decreased the resistance against enteritis and attenuated intestinal immunity by reducing innate and adaptive immune components including LA (not in DI), ACP, IgM (not in PI), C3, C4 and antimicrobial peptide transcript abundances including hepcidin, LEAP-2A, LEAP-2B, IgZ, IgM and β-defensin1 (not in PI); (2) threonine deficiency aggravated intestinal inflammation response by up-regulating pro-inflammatory cytokine TNF-α, IL-1β, IFN-γ2, IL-6, IL-8 and IL-17D (not IL-12p35 and IL-12p40) gene expression partly associated with NF-κB signalling pathway ((IKKβ, IKKγ but not IKKα)/IκBα/(NF-κB p65, NF-κB p52 but not c-Rel)) and anti-inflammatory cytokines TGF-β1, TGF-β2, IL-4/13A (not IL-4/13B) and IL-10 mRNA transcript levels partly through TOR signalling (TOR/(S6K1 and 4E-BP1)). In addition, based on quadratic regression for SGR, protecting fish against the enteritis morbidity and IgM content, dietary threonine requirements for juvenile grass carp were estimated to be 14·53 g threonine/kg diet (4·48 g threonine/100 g protein), 15·05 g threonine/kg diet (4·64 g threonine/100 g protein) and 15·17 g threonine/kg diet (4·68 g threonine/100 g protein), respectively.

Fig. 9 Potential action pathways of dietary threonine regulating intestinal immunity and inflammation response of fish. LA, lysozyme activities; DI, distal intestine; IKK, IκB kinase; TOR, target of rapamycin; ACP, acid phosphatase; C3 and C4, complements; PI, proximal intestine; S6K1, ribosomal protein S6 kinases 1; 4E-BP1, eIF4E-binding protein 1; LEAP, liver-expressed antimicrobial peptide; IFN-γ, interferon γ; TGF, transforming growth factor.
Acknowledgements
The authors would like to thank the personnel of these teams for their kind assistance.
This research was financially supported by National Natural Science Foundation of China (L. F., grant no. 31572632), the National Basic Research Program of China (973 Program) (Y.-A. Z., grant no. 2014CB138600), Outstanding Talents and Innovative Team of Agricultural Scientific Research (Ministry of Agriculture), Science and Technology Support Programme of Sichuan Province of China (X.-Q. Z., grant no. 2014NZ0003), Major Scientific and Technological Achievement Transformation Project of Sichuan Province of China (X.-Q. Z., grant no. 2013NC0045), the Demonstration of Major Scientific and Technological Achievement Transformation Project of Sichuan Province of China (X.-Q. Z., grant no. 2015CC0011) and Foundation of Sichuan Youth Science and Technology Innovation Research Team (L. F., grant no. 2017TD0002). The funding agencies had no role in the design and analysis of the study or in the writing of the article.
The author’s contributions are as follows: X.-Q. Z. and L. F. designed the study; Y.-W. D., W.-D. J. and L. F. conducted the study and analysed the data; Y. L., P. W., J. J., S.-Y. K., L. T., W.-N.T. and Y.-A. Z. participated in the interpretation of the results; Y.-W. D. and W.-D. J. wrote the manuscript; X.-Q. Z. had primary responsibility for the final content of the manuscript. All authors read and approved the final manuscript.
The authors declare that there are no conflicts of interest.

















