The protein digestibility-corrected amino acid score (PDCAAS) has been used for more than 20 years to evaluate protein quality in human foods( 1 ), but the PDCAAS procedure has limitations because values are calculated from the total tract digestibility of crude protein (CP) and calculations for PDCAAS are based on the assumption that all amino acids (AA) have the same digestibility as CP. It is, however, recognised that digestibility of AA is most correctly determined at the end of the small intestine (the ileum), because AA are absorbed only from the small intestine and because hindgut fermentation can affect faecal AA excretion( Reference Sauer and Ozimek 2 ). Therefore, ileal digestibility is a more accurate estimate of AA bioavailability than total tract digestibility in both humans and pigs( Reference Stein, Seve and Fuller 3 , Reference Cervantes-Pahm, Liu and Stein 4 ). In addition, the digestibility of CP is not representative of the digestibility of all AA( Reference Stein, Seve and Fuller 3 ), because individual AA are digested with different efficiencies( Reference Stein, Seve and Fuller 3 ). Other criticisms of the PDCAAS procedure have been recently reviewed and include use of truncation to avoid having values >1, use of a scoring pattern that is based on AA requirements for children and use of metabolic faecal N to correct for endogenous losses of AA( Reference Schaafsma 5 – Reference Rutherfurd, Fanning and Miller 7 ). It was also recently concluded that PDCAAS generally underestimates the value of high-quality proteins and overestimates the value of low-quality proteins( Reference Rutherfurd, Fanning and Miller 7 ).
To avoid the flaws of the PDCAAS procedure, the Food and Agriculture Organization (FAO)( 8 ) now recommends an AA evaluation procedure called digestible indispensable amino acid score (DIAAS). To calculate DIAAS, it is necessary to determine the digestibility of individual AA at the end of the small intestine (the ileum), and the pig has been recognised as an appropriate model for estimating CP and AA digestibility in foods for humans( 8 – Reference Deglaire, Bos and Tomé 10 ). In contrast, PDCAAS values according to the original definition are determined in rats( 1 ). The apparent ileal digestibility of AA is defined as the net disappearance of ingested dietary AA from the digestive tract before the distal ileum( Reference Stein, Seve and Fuller 3 ). If values for apparent ileal digestibility are corrected for the basal endogenous losses of AA, the resulting values are described as standardised ileal digestibility (SID)( Reference Stein, Seve and Fuller 3 ). Values for SID of AA are additive in mixed diets( Reference Stein, Pedersen and Wirt 11 ) and may be used to calculate DIAAS in proteins used in human nutrition( Reference Cervantes-Pahm, Liu and Stein 4 , 8 ).
Research in our laboratory estimated DIAAS in eight cereal grains by calculating SID values for all indispensable AA in pigs( Reference Cervantes-Pahm, Liu and Stein 4 ). Results indicated that to meet dietary requirements for AA in humans, diets based on sorghum, wheat, rye or maize require more AA supplementation than diets based on polished rice or dehulled oats. However, in human nutrition, protein is usually supplied by either animal-based proteins or plant-based proteins. Animal proteins include a number of dairy products, and commonly used dairy proteins include whey protein concentrate (WPC), whey protein isolate (WPI), milk protein concentrate (MPC) and skimmed milk powder (SMP). Commonly used plant proteins include soya protein isolate (SPI), soya flour and pea protein concentrate (PPC). To our knowledge, there are no published values for DIAAS for these proteins that have been determined in pigs and it is not known how values for DIAAS determined in pigs compare with PDCAAS-like values determined in pigs. Therefore, the aim of this experiment was to compare PDCAAS-like values determined in pigs and values for DIAAS in eight commonly used proteins and test the hypothesis that values for DIAAS are more appropriate to quantify protein quality than values for PDCAAS.
Methods
The protocol for the experiment was reviewed and approved by the Institutional Animal Care and Use Committee at the University of Illinois (protocol no. 13354). Four dairy proteins (WPI, WPC, MPC and SMP) were procured from Cereal Byproducts Company. SPI and soya flour were obtained from Archer Daniels Midland Company and PPC was obtained from AGT Foods. Wheat was obtained from Siemers (Table 1). Each ingredient was included in one diet as the only source of CP and AA with the exception that wheat was included in combination with soya flour (Tables 2 and 3). A N-free diet was also formulated to measure basal endogenous losses of CP and AA. Vitamins and minerals were included in all diets to meet or exceed current requirement estimates for growing pigs( 12 ). All diets also contained 0·4 % chromic oxide as an indigestible marker and all diets were provided in meal form.
Table 1 Analysed nutrient composition of ingredients (as-fed basis)Footnote *

WPI, whey protein isolate; WPC, whey protein concentrate; MPC, milk protein concentrate; SMP, skimmed milk powder; PPC, pea protein concentrate; SPI, soya protein isolate.
* The trypsin inhibitor units in soya flour and SPI were 8·06 and 2·75 units/mg, respectively.
Table 2 Ingredient composition of experimental diets (as-is basis)Footnote *

WPI, whey protein isolate; WPC, whey protein concentrate; MPC, milk protein concentrate; SMP, skimmed milk powder; PPC, pea protein concentrate; SPI, soya protein isolate.
* All diets were formulated to contain approximately 17 % crude protein, 0·70 % Ca and 0·33 % standardised total tract digestible P.
† The vitamin–micromineral premix provided the following quantities of vitamins and micro minerals per kg of complete diet: vitamin A as retinyl acetate, 3·83 mg; vitamin D3 as cholecalciferol, 0·06 mg; vitamin E as dl-α-tocopheryl acetate, 48·53 mg; vitamin K as menadione dimethylprimidinol bisulfite, 1·42 mg; thiamin as thiamine mononitrate, 0·24 mg; riboflavin, 6·59 mg; pyridoxine as pyridoxine hydrochloride, 0·24 mg; vitamin B12, 0·03 mg; d-pantothenic acid as d-calcium pantothenate, 23·5 mg; niacin, 44·1 mg; folic acid, 1·59 mg; biotin, 0·44 mg; Cu, 20 mg as copper sulfate and copper chloride; Fe, 126 mg as ferrous sulfate; I, 1·26 mg as ethylenediamine dihydriodide; Mn, 60·2 mg as manganese sulfate; Se, 0·3 mg as sodium selenite and Se yeast; and Zn, 125·1 mg as zinc sulfate.
Table 3 Analysed nutrient composition of experimental diets (as-fed basis)

WPI, whey protein isolate; WPC, whey protein concentrate; MPC, milk protein concentrate; SMP, skimmed milk powder; PPC, pea protein concentrate; SPI, soya protein isolate.
Nine growing barrows (initial body weight: 26·25 (sd 1·48) kg) were equipped with a T-cannula in the distal ileum using procedures adapted from Stein et al.( Reference Stein, Shipley and Easter 13 ). Pigs were allowed a 7-d recovery after the surgery and they were then allotted to a 9×9 Latin square design with nine diets and nine 9-d periods. No pig received the same diet more than once during the experiment and there was, therefore, nine replicate pigs per treatment. With nine replicates we expected to be able to detect differences in SID values among ingredients of 2·5–4 percentage units (depending on the AA). Pigs were housed in individual pens (0·9×1·8 m) in an environmentally controlled room. Pens had smooth sides and fully slatted concrete floors. A feeder and a nipple drinker were installed in each pen. At the conclusion of the experiment, pigs were approximately 19 weeks of age and had a body weight of 84·70 (sd 6·48) kg.
All pigs were fed their assigned diets in a daily amount of three times the estimated energy requirement for maintenance (i. e. 824 kJ metabolisable energy/kg0·60)( 12 ). The daily feed allotment was provided every day at 08.00 hours. Water was available at all times. Pig weights were recorded at the beginning of each period and at the conclusion of the experiment. The amount of feed supplied each day was recorded as well. The initial 5 d of each period were considered an adaptation period to the diet. Faecal samples were collected on days 6 and 7 and immediately frozen at −20°C. Ileal digesta were collected for 8 h (from 08.00 to 16.00 hours) on days 8 and 9 using standard operating procedures( Reference Stein, Shipley and Easter 13 ). In brief, cannulas were opened and cleaned, a plastic bag was attached to the cannula barrel and digesta flowing into the bag were collected. Bags were removed whenever they were filled with digesta or at least once every 30 min, and immediately frozen at −20°C to prevent bacterial degradation of the AA in the digesta. Individual pig weights recorded at the conclusion of each period were used to calculate the feed provision for the subsequent period.
At the conclusion of the experiment, faecal samples were dried in a forced air oven and finely ground through a 1-mm screen in a Wiley Mill (model 4; Thomas Scientific) before analysis. Ileal samples were thawed, mixed within animal and diet, and a sub-sample was collected for analysis. A sample of each source of protein and of each diet was collected at the time of diet mixing. Digesta samples were lyophilised and finely ground before chemical analysis. Diets, ingredients, faecal samples and ileal digesta samples were analysed for DM (method 927·05)( 14 ) and CP by combustion (method 990·03)( 14 ) on an Elementar Rapid N-cube protein/N apparatus (Elementar Americas Inc.). Aspartic acid was used as a calibration standard and CP was calculated as N×6·25. Samples were analysed in duplicate, but analyses were repeated if the analysed values were >5 % apart. Diets, faecal samples and ileal digesta were also analysed in duplicate for Cr (method 990·08)( 14 ) and all diets, ingredients and ileal digesta samples were analysed for AA on a Hitachi Amino Acid Analyzer (model L8800; Hitachi High Technologies America Inc.) using ninhydrin for post-column derivatisation and norleucine as the internal standard. Samples were hydrolysed with 6 N-HCl for 24 h at 110°C before analysis, but methionine and cysteine were analysed as methionine sulfone and cysteic acid after cold performic acid oxidation overnight before hydrolysis and tryptophan was determined after NaOH hydrolysis for 22 h at 110°C (method 982·30 E (a, b, c))( 14 ).
Calculations
Values for apparent ileal digestibility of CP and AA, basal endogenous losses of CP and AA, and SID of CP and AA were calculated for all diets as previously explained( Reference Stein, Seve and Fuller 3 ). For all ingredients except wheat, the SID for CP and AA in the diets also represented the SID of the ingredient, but for wheat, the SID of CP and AA were calculated using the difference procedure( Reference Rojas and Stein 15 ). Values for the standardised total tract digestibility (STTD) of CP were calculated as explained for the calculation of SID of CP.
The concentration of SID AA (g/kg) in each ingredient was calculated by multiplying the SID value (%) for each AA by the concentration (g/kg) of that AA in the ingredient, and this value was then divided by the concentration of CP in the ingredient to calculate digestible indispensable AA content (mg) in 1 g protein( Reference Cervantes-Pahm, Liu and Stein 4 ). The digestible indispensable AA reference ratios were calculated for each ingredient using the following equation( 8 ): digestible indispensable AA reference ratio=digestible indispensable AA content in 1 g protein of food (mg)/mg of the same dietary indispensable AA in 1 g of the reference protein. The reference proteins were based on FAO( 8 ) and separate ratios were calculated using the reference protein for infants less than 6 months old, children from 6 to 36 months old and children older than 36 months, adolescents and adults( 8 ). The DIAAS values were then calculated using the following equation( 8 ):
Values for STTD of CP were used to calculate PDCAAS-like values using the following equation( Reference Schaafsma 16 ):
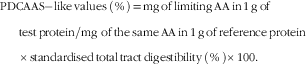 $$\eqalignno{ & {\rm PDCAAS{\minus}like}\,{\rm values}\,\left( {\rm \,\%\,} \right){\rm \,{\equals}\,mg}\,{\rm of}\,{\rm limiting}\,{\rm AA}\,{\rm in}\,{\rm 1}\,{\rm g}\,{\rm of}\, \cr & \;\;\quad{\rm test}\,{\rm protein/mg}\,\,{\rm of}\,{\rm the}\,{\rm same}\,{\rm AA}\, {\rm in}\,{\rm 1}\,{\rm g}\,{\rm of}\,{\rm reference}\,{\rm protein} \cr & \;\;\quad{\times}{\rm standardised}\,{\rm total}\,{\rm tract}\,{\rm digestibility}\,\left( {\rm \,\%\,} \right){\times}{\rm 100}. $$
$$\eqalignno{ & {\rm PDCAAS{\minus}like}\,{\rm values}\,\left( {\rm \,\%\,} \right){\rm \,{\equals}\,mg}\,{\rm of}\,{\rm limiting}\,{\rm AA}\,{\rm in}\,{\rm 1}\,{\rm g}\,{\rm of}\, \cr & \;\;\quad{\rm test}\,{\rm protein/mg}\,\,{\rm of}\,{\rm the}\,{\rm same}\,{\rm AA}\, {\rm in}\,{\rm 1}\,{\rm g}\,{\rm of}\,{\rm reference}\,{\rm protein} \cr & \;\;\quad{\times}{\rm standardised}\,{\rm total}\,{\rm tract}\,{\rm digestibility}\,\left( {\rm \,\%\,} \right){\times}{\rm 100}. $$
Calculation of PDCAAS-like values used the reference protein for 2–5 year-old children as recommended if values are calculated from STTD of CP in rats( 1 ). However, to allow for a direct comparison between PDCAAS-like values and values for DIAAS, PDCAAS-like values were also calculated using the three reference proteins that were used to calculate DIAAS values( 8 ).
Statistical analyses
Normality of data was verified and outliers were identified using the UNIVARIATE and BOXPLOT procedures, respectively (SAS Inst. Inc.). Data were analysed by ANOVA using the MIXED procedure of SAS (SAS Institute Inc.) in a randomised complete block design with the pig as the experimental unit. The statistical model to determine differences in SID of AA values among ingredients included diet as the main effect and pig and period as random effects. The model to compare values for SID and STTD of CP within each ingredient included calculation procedure (SID or STTD) as main effect and pig and period as random effects. The model to compare values for DIAAS and PDCAAS used calculation procedure (DIAAS or PDCAAS) as main effect and pig and period as random effects. Treatment means were calculated using the LSMEANS statement, and if significant, means were separated using the PDIFF option of the MIXED procedure. Significance and tendency was considered at P<0·05 and 0·05≤P<0·10, respectively.
Results
All pigs remained healthy throughout the experiment and readily consumed their diets. Gross chemical composition of all ingredients was generally in agreement with published values( 12 ). The concentration of CP in ingredients ranged from 11·67 to 92·66 %.
With the exception of tyrosine, the SID of all AA was not different between WPI and WPC (Table 4). The SID of isoleucine, cysteine and serine was less (P<0·05) in MPC than in WPI and WPC, and the SID of valine and glutamic acid was less (P<0·05) in MPC than in WPI, but for all other AA, no differences among MPC, WPI and WPC were observed. However, the SID of most AA was greater (P<0·05) in WPI, WPC and MPC than in SMP, PPC, soya flour and wheat, but for SPI, many AA had SID values that were not different from those in WPI, WPC and MPC. With the exception of arginine, tryptophan, alanine and glycine, the SID of all AA was greater (P<0·05) in SPI than in soya flour. The SID of methionine, tryptophan and cysteine was less (P<0·05) in PPC than in soya flour and the SID of aspartic acid and glutamic acid was greater (P<0·05) in PPC than in soya flour, but for all other AA, no difference between these two ingredients was observed. The SID of all indispensable AA and of alanine and tyrosine was less (P<0·05) in wheat than in all other ingredients.
Table 4 Standardised ileal digestibility of amino acids in ingredientsFootnote * (Pooled standard errors)

WPI, whey protein isolate; WPC, whey protein concentrate; MPC, milk protein concentrate; SMP, skimmed milk powder; PPC, pea protein concentrate; SPI, soya protein isolate.
a,b,c,d,e,f Mean values within a row with unlike superscript letters are different (P<0·05).
* Standardised ileal digestibility values were calculated by correcting values for apparent ileal digestibility for the basal ileal endogenous losses. Endogenous losses of amino acids were calculated from pigs fed the N-free diet as follows (g/kg DM intake): arginine, 0·59; histidine, 0·20; isoleucine, 0·29; leucine, 0·49; lysine, 0·40; methionine, 0·08; phenylalanine, 0·29; threonine, 0·49; tryptophan, 0·10; valine, 0·40; alanine, 0·62; aspartic acid, 0·72; cysteine, 0·17; glutamic acid, 0·94; glycine, 1·50; serine, 0·43; tyrosine, 0·23.
The SID of CP was greater (P<0·05) than the STTD of CP for WPI, WPC and wheat (Table 5). In contrast, the STTD of CP was greater (P<0·05) than the SID of CP in MPC, SMP and SPI, whereas no difference between SID and STTD of CP was observed for PPC and soya flour.
Table 5 Standardised ileal digestibility (SID) and standardised total tract digestibility (STTD) of crude protein (CP) in ingredients

WPI, whey protein isolate; WPC, whey protein concentrate; MPC, milk protein concentrate; SMP, skimmed milk powder; PPC, pea protein concentrate; SPI, soya protein isolate.
The protein digestibility-corrected AA reference ratios calculated according to the recommendations from FAO/WHO( 1 ) but using pigs instead of rats and based on the scoring pattern for preschool children (2–5 years old) are presented in the online Supplementary Table SA. However, the protein digestibility-corrected AA reference ratios calculated from STTD values of CP in pigs were also calculated according to FAO( 8 ) and based on requirements of infants (birth to 6 months of age), children (6 months to 3 years of age) and older children (older than 3 years of age), adolescents and adults, and these values are presented in the online Supplementary Table SB. Likewise, the digestible indispensable AA reference ratios calculated according to FAO( 8 ) and based on the same three age groups are presented in the online Supplementary Table SC.
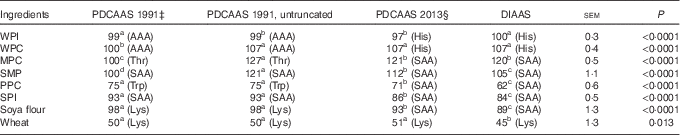
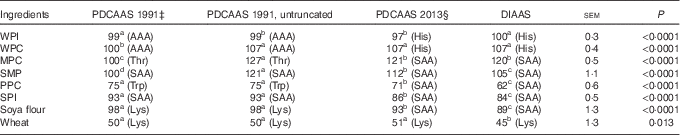
If PDCAAS-like values calculated according to FAO/WHO( 1 ) were truncated as recommended, values for WPC, MPC, SMP were less (P<0·05) than values for DIAAS, whereas PDCAAS-like values for PPC, SPI, soya flour and wheat were greater (P<0·05) than for DIAAS (Table 6). However, if PDCAAS-like values were not truncated, the PDCAAS-like value for WPC was not different from DIAAS, but PDCAAS-like values for MPC and SMP were greater (P<0·05) than DIAAS. If PDCAAS-like values were calculated according to the same scoring pattern as DIAAS( 8 ), PDCAAS-like values for SMP, PPC, SPI, soya flour and wheat were greater (P<0·05) than values for DIAAS, whereas the PDCAAS-like value for WPI was less (P<0·05) than the DIAAS for WPI.
Table 6 Comparison of protein digestibility corrected amino acid scores (PDCAAS) and digestible indispensable amino acid scores (DIAAS) based on different requirement patterns* †
WPI, whey protein isolate; AAA, aromatic amino acids (phenylalanine+tyrosine); WPC, whey protein concentrate; MPC, milk protein concentrate; SAA, sulfur amino acids (methionine+cysteine); SMP, skimmed milk powder; PPC, pea protein concentrate; SPI, soya protein isolate.
a,b,c,d Mean values within a row with unlike superscript letters are different (P<0·05).
* Values for PDCAAS were calculated from the total tract digestibility of crude protein in pigs and values for DIAAS were calculated from the ileal digestibility of amino acids in pigs.
† First-limiting amino acid is in parenthesis.
‡ PDCAAS were calculated using the recommended amino acid scoring pattern for preschool children (2–5 years). The indispensable amino acids reference patterns are expressed as mg amino acid/g protein: histidine, 19; isoleucine, 28; leucine, 66; lysine, 58; sulfur amino acids, 25; aromatic amino acids, 63; threonine, 34; tryptophan, 11; valine, 35( 1 ).
§ PDCAAS and DIAAS were calculated using the recommended amino acid scoring pattern for a child (6 months to 3 years). The indispensable amino acid reference patterns are expressed as mg amino acid/g protein: histidine, 20; isoleucine, 32; leucine, 66; lysine, 57; sulfur amino acids, 27; aromatic amino acids, 52; threonine, 31; tryptophan, 8·5; valine, 40( 8 ).
For values for DIAAS, the first-limiting AA in WPI and WPC was histidine, but for MPC, SMP, PPC, SPI and soya flour, the sulfur AA were first limiting, and lysine was first liming in wheat. If PDCAAS-like values were calculated using the same scoring patterns as used to calculate DIAAS, the first-limiting AA in the proteins was not different from those identified for DIAAS. However, if PDCAAS-like values were calculated using the original scoring patterns( 1 ), either truncated or not truncated, the first-limiting AA for whey proteins was the aromatic AA and threonine was first limiting in MPC and the sulfur AA were first limiting in SMP and SPI. However, the first-limiting AA in PPC was tryptophan, whereas lysine was first limiting in soya flour and wheat.
Calculated PDCAAS-like values for infants were greater (P<0·05) than values for DIAAS for SMP, PPC, SPI and wheat, whereas the value for DIAAS for WPI tended (P=0·062) to be greater than the PDCAAS-like value (Table 7). For children older than 3 years, adolescents and adults, PDCAAS-like values for SMP, PPC and SPI were greater (P<0·05) than DIAAS, and the PDCAAS-like value for soya flour tended (P=0·053) to be greater than DIAAS. In contrast, the DIAAS for WPI was greater (P<0·05) than the PDCAAS-like value.
Table 7 Comparison of protein digestibility-corrected amino acid scores (PDCAAS) and digestible indispensable amino acid scores (DIAAS)* †

WPI, whey protein isolate; WPC, whey protein concentrate; MPC, milk protein concentrate; SMP, skimmed milk powder; PPC, pea protein concentrate; SPI, soya protein isolate; AAA, aromatic amino acids (phenylalanine+tyrosine); SAA, sulfur amino acids (methionine+cysteine).
* Values for PDCAAS were calculated from the total tract digestibility of crude protein in pigs and values for DIAAS were calculated from the ileal digestibility of amino acids in pigs.
† First-limiting amino acid is in parenthesis.
‡ PDCAAS and DIAAS were calculated using the recommended amino acid scoring pattern for an infant (birth–6 months). The indispensable amino acid reference patterns are expressed as mg amino acid/g protein: histidine, 21; isoleucine, 55; leucine, 96; lysine, 69; sulfur amino acids, 33; aromatic amino acids, 94; threonine, 44; tryptophan, 17; valine, 55( 8 ).
§ PDCAAS and DIAAS were calculated using the recommended amino acid scoring pattern for children older than 3 years, adolescents and adults. The indispensable amino acid reference patterns are expressed as mg amino acid/g protein: histidine, 16; isoleucine, 30; leucine, 61; lysine, 48; sulfur amino acids, 23; aromatic amino acids, 41; threonine, 25; tryptophan, 6·6; valine, 40( 8 ).
The first-limiting AA for DIAAS calculated for infants were the aromatic AA for the whey proteins, tryptophan for MPC and PPC, threonine for SMP, the sulfur AA for SPI, leucine for soya flour and lysine for wheat. The first-limiting AA for PDCAAS-like values calculated for infants in SMP was tryptophan, but for all other ingredients, the first-limiting AA in the calculation of DIAAS was also first limiting for PDCAAS-like values. For children >3 years old, adolescents and adults, the first-limiting AA for both DIAAS and PDCAAS-like values for all proteins were the same as those identified for children from 6 months to 3 years old.
Discussion
The amount and quality of protein consumed throughout the world varies depending on protein availability, AA composition of proteins and digestibility of AA( Reference Schaafsma 16 ). In many parts of the world, plant proteins are the primary sources of AA in the diet( Reference Cervantes-Pahm, Liu and Stein 4 , Reference Schonfeldt and Hall 17 , Reference Swaminathan, Vaz and Kurpad 18 ), whereas animal proteins are the primary sources of AA in other parts of the world( Reference Swaminathan, Vaz and Kurpad 18 ). However, the composition and digestibility of both of these types of proteins differ( Reference Cervantes-Pahm, Liu and Stein 4 , Reference Gilani and Sepehr 19 ), and both plant and animal proteins, therefore, need to be evaluated. In the present experiment we attempted to do that, but it is acknowledged that all proteins were fed as raw ingredients without the processing that these ingredients most often go through before consumption by humans. If processing changes the digestibility of the protein, results may be different. Other limitations of the experiment include the assumption that AA digestibility in growing castrated male pigs are representative of values obtained in both male and female humans of all ages.
In the current experiment, values for AA digestibility calculated from the total tract digestibility of CP were estimated from pigs although the rodent is the recommended model in the definition of PDCAAS( 1 ). However, it was the objective to determine if total tract digestibility values for CP can be used to accurately estimate ileal digestibility values of individual AA and if we had used a rodent to calculate PDCAAS values and the pig to calculate DIAAS values, any differences would have been confounded by using the two different animal models. It is, therefore, important that the comparison is done within the same animal and because the pig has been recommended as the preferred animal model to calculate DIAAS values( 8 ), we chose to use the pig to also calculate PDCAAS-like values in this study.
As expected, dairy proteins had greater SID values than the plant proteins and they are, therefore, considered high-quality proteins for humans( Reference James, Mattin and Aldiss 20 – Reference Stanstrup, Schou and Holmer-Jensen 22 ). Protein quality in WPC, SMP and SPI or soya protein concentrate have been studied in rats, and results indicated that WPC had greater PDCAAS than SMP, SPI and soya protein concentrate( Reference Rutherfurd, Fanning and Miller 7 , Reference Gilani and Sepehr 19 ). Results of this experiment agree with previous results and also indicate that the PDCAAS-like value for WPC is greater than for SMP and that the whey proteins have a more balanced AA profile compared with whole milk protein. The major protein in SMP is casein, which has a low concentration of cysteine, and this may be the reason for the reduced PDCAAS-like value for SMP compared with WPC.
According to the FAO recommended AA patterns for older children, adolescents and adults and recommendations for nutrient claims, all dairy proteins tested in this experiment can be considered ‘excellent/high’ quality sources of protein, with DIAAS ≥100( 8 ). By the same guidelines, SPI and soya flour qualify as ‘good’ sources of protein, with a score ≥75 and <100. In contrast, proteins with DIAAS <75 are recommended to make no claims regarding protein quality( 8 ), and PPC and wheat tested in this experiment fall into this category. However, it is recognised that the cut-off values for protein quality assessments that were proposed were arbitrarily chosen and not based on documented research( 8 ).
The N-free diet was used to estimate endogenous AA losses. Values obtained using this procedure are estimates for the basal endogenous losses that are independent of the diet and secreted only in response to DM being present in the small intestine( Reference Stein, Seve and Fuller 3 ). In addition to the basal endogenous losses, diet-specific endogenous losses may also occur, but these losses will not be included in the values obtained from the N-free diet, and therefore, diet-specific losses are debited against the ingredients in the calculations of SID values. Thus, if a specific diet or ingredient induces diet-specific endogenous losses because of high concentrations of dietary fibre or anti-nutritional factors, the SID values for that diet or ingredient will be reduced compared with values for a diet or ingredient that does not induce specific endogenous losses. However, because endogenous losses are really lost from the body, values for SID will give a better estimate of the AA that are available for metabolism than if values for diet-specific endogenous losses had not been debited against the ingredient or diet. The calculated values for the SID of glycine in several ingredients exceeded 100 % in the current experiment, which is not biologically possible, but these values are an artifact that is caused by an overestimation of endogenous glycine, which often happens when the N-free procedure is used to determine endogenous losses of AA( Reference Stein, Seve and Fuller 3 ).
For all proteins, SID values were different among both indispensable and dispensable AA indicating that one single value cannot be used to estimate the digestibility of individual AA as is assumed in the calculation of PDCAAS( 1 ). For all ingredients used in this experiment with the exception of wheat, threonine had a lower SID value than lysine, which is usually the case for proteins that are not heat damaged. This is a result of the greater concentrations of threonine than of lysine and other indispensable AA in mucin protein secreted into the small intestine( Reference Stein, Trottier and Bellaver 23 ). Mucin protein is resistant to protease digestion, and therefore is included in the endogenous protein fraction that reaches the distal end of the ileum in pigs without being hydrolysed. We are not aware of data for the AA composition of mucin in humans, but it has been reported that the ileal digestibility of threonine in humans is less than that of other indispensable AA, which indicates that mucin in humans also may have a high concentration of threonine( Reference Rowan, Moughan and Wilson 9 , Reference Deglaire, Bos and Tomé 10 ). The observation that both lysine and tryptophan in wheat had a lower SID value than threonine may indicate that the wheat used in this experiment had been heat damaged during drying or grinding.
The differences between values for SID and STTD of CP that were observed are in agreement with reports indicating that the apparent ileal digestibility of CP is different from the apparent total tract digestibility of CP( Reference Sauer and Ozimek 2 , Reference Knabe, LaRue and Gregg 24 ). In most cases, the total tract digestibility of CP is greater than the ileal digestibility because of absorption of ammonia from the hindgut( Reference Hendriks, van Baal and Bosch 25 , Reference Boye, Wijesinha-Bettoni and Burlingame 26 ), but as illustrated in this experiment, in some cases, N may be secreted into the hindgut resulting in a reduced value for STTD compared with SID. However, because N exchange in the hindgut does not contribute to the AA balance in humans and monogastric animals and because AA are absorbed only in the small intestine, the differences between STTD and SID values illustrate why values for STTD do not always represent absorption of AA. Thus, the use of STTD of CP to estimate the digestibility of all AA in the PDCAAS system will result in inaccuracies of estimates for AA digestibility, which has also been previously illustrated( Reference Rutherfurd, Fanning and Miller 7 , Reference McAllan, Skuse and Cotter 21 ).
In addition to the lack of digestibility values for individual AA, a major limitation of the PDCAAS system is that all scores are truncated to 100 % with the rationale that any amount of AA beyond the requirement pattern confers no additional benefit to the individual consuming the protein( 8 , Reference Schaafsma 16 , Reference Boye, Wijesinha-Bettoni and Burlingame 26 , Reference Sarwar 27 ). This assumption, however, neglects the complementary effect that excess AA may have in combination with AA from other proteins( Reference Boye, Wijesinha-Bettoni and Burlingame 26 , Reference Sarwar 27 ), and as a consequence, PDCAAS values do not give credit for extra indispensable AA that a protein may add to a diet( Reference Boye, Wijesinha-Bettoni and Burlingame 26 , 28 ). In contrast to the PDCAAS system, values for DIAAS are not truncated to 100 %, and therefore, give credit to a protein based on its value as a complementary source of AA with other sources of proteins in a mixed diet( Reference Rutherfurd, Fanning and Miller 7 ).
Despite the challenges with the PDCAAS procedures, which have been previously reviewed( Reference Schaafsma 5 , Reference Boye, Wijesinha-Bettoni and Burlingame 26 , Reference Sarwar 27 ), it is important to recognise that criticism related to the scoring patterns that were originally suggested( 1 ) can be easily overcome by adopting different scoring patterns. Indeed, in a later report from WHO/FAO, scoring patterns for several age groups of children, teenagers and adults were suggested( 28 ). Likewise, the problems associated with truncation can also be easily corrected by using untruncated values( Reference Boye, Wijesinha-Bettoni and Burlingame 26 ). As a consequence, the principal methodological difference between values calculated for PDCAAS and values calculated for DIAAS is related to the assumption that the small intestinal absorption of individual AA can be predicted from the total tract digestibility of CP. As was clearly illustrated in this experiment, differences in the ileal digestibility among individual AA in all proteins exist with the digestibility of threonine being the least for most proteins. As a consequence, the ileal digestibility of AA cannot be accurately predicted from a single value obtained for the total tract digestibility of CP. It is also clearly illustrated that both STTD and SID of CP overestimate the ileal digestibility of AA for proteins with lower AA digestibility and as a consequence, values for PDCAAS that are predicted from the STTD of CP are expected to be less accurate for proteins with low AA digestibility than for proteins with greater AA digestibility. These principles are illustrated by the data in Table 6 where PDCAAS-like values are calculated according to the original recommendation( 1 ) with scoring patterns for 2–5-year-old children and all values are truncated to 100. The observation that the PDCAAS-like values for WPC, MPC and SMP are much less than values for DIAAS is a consequence of truncation. However, if values are not truncated, none of these proteins have PDCAAS-like values that are less than values for DIAAS. Indeed, removing the truncation resulted in PDCAAS-like values that were greater than values for DIAAS for six of the eight protein sources, indicating an overestimation of protein quality by using PDCAAS-like values. Values for DIAAS were calculated based on the scoring pattern for children from 6 to 36 months( 8 ), and because this scoring pattern is different from the original PDCAAS scoring pattern( 1 ), this will influence the calculations. However, even if the PDCAAS-like values were calculated using the DIAAS scoring pattern, PDCAAS-like values for five of the eight proteins were greater than values for DIAAS. This observation is a consequence of the fact that total tract digestibility of CP is usually greater than the ileal digestibility of AA as discussed above, and as expected, the difference between PDCAAS-like values and DIAAS is greater for proteins with lower AA digestibility than for proteins with greater digestibility. Thus, it appears that the major inaccuracies in the calculation of PDCAAS are a consequence of the incorrect assumption that the ileal digestibility of all indispensable AA can be predicted from the total tract digestibility of CP. This inaccuracy will have greater impact on evaluation of proteins used in developing countries than in developed countries, because foods typically consumed in many developing countries have lower digestibility of CP than food typically consumed in developed countries( Reference Gilani, Cockell and Sepehr 29 ).
If PDCAAS-like values and DIAAS values were calculated for children older than 6 months or for adults and if the same scoring pattern was used, no differences between the two methodologies in terms of predicting the first-limiting AA were observed with lysine being first limiting in wheat, histidine being first limiting in the whey proteins and the sulfur AA being first limiting in the whole milk proteins and the soya and pea proteins. However, if the original scoring pattern for PDCAAS was used, the predicted first-limiting AA were different for all proteins except SMP, PPC and wheat, which illustrates that the choice of scoring pattern will influence, which AA is predicted to be first limiting in a specific protein.
The observation that PDCAAS-like values and values for DIAAS were much less if the scoring pattern for infants (i. e. <6 months old) was used instead of scoring patterns for older children or adults illustrate the high-protein quality that is needed in proteins by infants. The fact that some of the proteins such as PPC and wheat, have very low DIAAS and PDCAAS-like values for infants is likely of minor consequence because these proteins are not expected to be used to a great extent in the feeding of infants.
In conclusion, data from this experiment indicate that PDCAAS-like values calculated from the total tract digestibility of CP in pigs and DIAAS values for dairy proteins are greater than for proteins obtained from soyabeans, peas or wheat. Data also indicate that for most proteins, significant differences between PDCAAS-like values and DIAAS were observed. Whereas some of the flaws in the calculation of PDCAAS can be corrected by using different scoring patterns, the fundamental problem with values for PDCAAS is that they are calculated using the incorrect assumption that the ileal digestibility of all AA can be predicted from the total tract digestibility of CP. Because of this assumption, PDCAAS values do not accurately predict ileal AA digestibility and it appears that specifically for low-quality proteins, values for PDCAAS overestimate the protein quality. Thus, to better meet protein requirements of humans, specifically for individuals consuming diets that are low or marginal in digestible AA, values for DIAAS should be used to estimate protein quality of ingredients and diets.
Acknowledgements
Financial support for this experiment was provided by the National Dairy Institute, Rosemont, Illinois, USA (project no. DMI 2014-06352). The National Dairy Institute did not contribute to the design of the experiment, analysis of data or writing of this article.
The contributions of the authors were as follows: J. K. M. conducted the animal work and laboratory work, analysed the data and wrote the majority of the manuscript. Y. L. prepared the experiment proposal, secured approval from appropriate animal welfare regulatory bodies and contributed to calculations, data analysis and interpretation of data. H. H. S. was the principal investigator. He designed the experiment, oversaw the development of the experiment and wrote the final version of the manuscript.
The authors declare no conflicts of interest.
Supplementary materials
For supplementary material/s referred to in this article, please visit https://doi.org/10.1017/S0007114517000125