The Coronaviridae family of single-stranded RNA viruses includes four genera: alpha-, beta-, gamma- and delta-coronavirus (Reference Fung and Liu1). Seven coronaviruses are known to infect humans. Two (229E and NL63) from the alpha genus and two (OC43 and HKU-1) from the beta genus cause mild upper respiratory tract infections and symptoms of the common cold. The remaining three human coronaviruses are also in the beta genus but cause severe lower respiratory tract infections, respiratory failure and death. These viruses are MERS-CoV, which causes the Middle East respiratory syndrome; SARS-CoV1, which causes severe acute respiratory syndrome, and SARS-CoV2, which is causing the current pandemic of coronavirus disease 2019 (COVID-19)(Reference Fung and Liu1,Reference Chen, Liu and Guo2) . SARS-CoV2 spreads readily from person-to-person due to the long incubation period of 2–14 d following infection(Reference Florindo, Kleiner and Vaskovich-Koubi3) and, according to data available to the WHO, has infected 75 million people and caused 1·7 million deaths worldwide between December 2019 and December 2020(4).
SARS-CoV2 infection causes both mild (in approximately 80 % of cases) and severe disease. Symptoms during mild disease include fever, dry cough, dyspnoea, sore throat, malaise, myalgia and, less frequently, gastrointestinal symptoms such as anorexia, nausea and diarrhoea. Mild cases are typically treated at home with supportive care, but no specific anti-viral therapy is currently recommended(Reference Gandhi, Lynch and Del Rio5). Some patients (approximately 20 % of all infections) progress from mild to more severe disease, the diagnosis of which involves a positive test for SARS-CoV2, tachypnoea and hypoxaemia and specific findings of decreased arterial oxygen concentration or a chest X-ray showing pneumonia, indicating extensive lung inflammation. Some patients with severe infection (approximately 5 % of all infections) develop renal failure and intravascular coagulation, require prolonged mechanical ventilation and may have involvement of multiple organ system failure. The survival rate in this most severe group is approximately 50 %(Reference Berlin, Gulick and Martinez6). Thus, SARS-CoV2 infection causes a multifaceted pathology, including heart attacks, coronary-related kidney damage and severe stroke in people with no previous history of CVD(Reference Florindo, Kleiner and Vaskovich-Koubi3). The most common complication of SARS-CoV2 is progressive consolidation of the lung leading to severe acute respiratory syndrome (SARS) due to extensive pulmonary fibrosis promoted by enhanced lipofibroblast–myofibroblast transition(Reference Yadav, Aggarwal and Singh7).
Many therapeutic interventions are being evaluated in severely affected patients including anti-viral, anti-malaria and anti-inflammatory interventions. The anti-viral drug remdesivir reduces time to hospital discharge in severe affected SARS-CoV2 patients, but fails to reduce respiratory tract viral load(Reference Johnson and Vinetz8). On the other hand, the anti-viral drug umifenovir reduces virus entry into target cells by inhibiting fusion of viral envelope(Reference Florindo, Kleiner and Vaskovich-Koubi3). The anti-malaria drug hydroxychloroquine sulphate could also block SARS-CoV2 infection by preventing the fusion of the virus with the cell membrane(Reference Florindo, Kleiner and Vaskovich-Koubi3), but has shown to be associated with heart arrythmias(Reference Hu, Lee and Asirvatham9) and induce retinopathy in some patients, potentially through inactivating lysosomal enzymes, blocking the digestion ability of phagocytes, binding to melanin and blocking organic anion transporter family member 1A2 OATP1A2(Reference Paniri, Hosseini and Rasoulinejad10). The anti-inflammatory corticosteroid dexamethasone was shown to reduce the death of SARS-CoV2-ventilated patients in the RECOVERY study by one-third(Reference Florindo, Kleiner and Vaskovich-Koubi3), but not in patients experiencing a milder disease(Reference Johnson and Vinetz8).
The purpose of this review is to examine how vitamin A (VA) status might affect host resistance to, or recovery from, COVID-19 disease. VA is of particular interest in this regard for at least three reasons. First, VA deficiency impairs host resistance to infection. VA was termed ‘the anti-infective’ vitamin approximately 90 years ago because of the increased risk of infections, particularly of lung infections, in VA-deficient experimental animals(Reference Green and Mellanby11), indicating that some aspect of host resistance to, or recovery from, infection was impaired. Research in the past 30 years has demonstrated that VA (retinol) is converted to its active metabolite retinoic acid by cells of the immune system and that retinoic acid then acts as an autocrine or paracrine factor to regulate many aspects of immune function, as has been reviewed recently(Reference Larange and Cheroutre12,Reference Guo, Brown and Ortiz13) . Second, the action of VA is crucial in normal lung development and in repair of lung tissue subsequent to injury(Reference Timoneda, Rodríguez-Fernández and Zaragozá14); thus, VA status may be particularly important during recovery from COVID-19. Third, severe infections are known to have a significant, negative impact on VA status by several mechanisms, including reduced absorption(Reference Aklamati, Mulenga and Dueker15), decreased hepatic mobilisation(Reference Gieng, Green and Green16) and urinary loss of VA due to glomerular and/or tubular dysfunction(Reference Stephensen17). Since severe COVID-19 cases often have prolonged hospitalisation with renal involvement, it is plausible that VA deficiency could develop during this period of severe disease. If this is the case, and VA deficiency impairs host resistance to infection and recovery of normal lung function, then recovery from severe COVID-19 disease might be improved by nutritional support involving VA supplementation, as well as other appropriate nutritional support. Fourth, medication used to reduce the impact of severe COVID-19 infection could alter the metabolism of VA in affected tissues and therefore induce tissue-specific VA deficiency(Reference Hind and Maden18), which could reduce the ability to repair affected tissues(Reference Timoneda, Rodríguez-Fernández and Zaragozá14).
SARS-CoV2 pathogenesis and effects of vitamin A on the immune system
SARS-CoV2 infection of the respiratory epithelium
Similarly to SARS-CoV1, the cellular infection mechanism of SARS-CoV2 is mainly mediated by the cell-surface receptor angiotensin-converting enzyme 2 (ACE2)(Reference Kuba, Imai and Rao19). SARS-CoV2 uses ACE2 for receptor-mediated cell entry and serine protease transmembrane serine protease 2 (TMPRSS2) for S protein priming(Reference Hoffmann, Begon and Lafon20). Cell entry of SARS-CoV2 can be partially blocked by the clinically proven serine protease inhibitor camostat mesylate, which is active against serine protease transmembrane serine protease 2 (TMPRSS2)(Reference Hoffmann, Begon and Lafon20). Although very low levels of ACE2 protein have been observed in the normal respiratory system(Reference Hikmet, Mear and Edvinsson21), the presence of ACE2 protein levels and viral RNA has been confirmed in ciliated epithelial cells, indicating that low-level protein expression in upper airway epithelial cells facilitates infection of ciliated cells, followed by a rapid interferon-induced increase of ACE2 expression in lower airway ciliated and type-2 alveolar epithelial cells, potentially allowing SARS-CoV2 to spread across the respiratory mucosa(Reference Nawijn and Timens22). The cell-surface ACE2 receptor internalises on binding to the SARS-CoV spike protein, leading to ACE2 receptor down-regulation(Reference Hamming, Cooper and Haagmans23,Reference Gagliardi, Tieri and Ortona24) . The SARS-CoV spike protein-mediated ACE2 down-regulation could contribute to the severity of lung pathologies such as pulmonary fibrosis and acute respiratory distress syndrome, since the counterbalance of ACE2 on angiotensin II production in the renin–angiotensin system is deregulated, affecting its ability to modulate the innate immune system and to regulate inflammation(Reference Kuba, Imai and Rao19,Reference Kuba, Imai and Penninger25,Reference Sodhi, Jenny and Yamaguchi26) . ACE2 knockout mice infected with SARS-CoV1 show reduced pathological alterations in the lung(Reference Kuba, Imai and Rao19). Increased incidence of intravascular coagulation with SARS-CoV2 infection could also be mediated via ACE2, since ACE2 deficiency is associated with up-regulation of putative mediators of atherogenesis, such as cytokines and adhesion molecules, and reduced ACE expression is observed in established atherosclerotic plaques(Reference Tikellis and Thomas27). Finally, the observed difference in SARS-CoV2 severity between children and adults could in part be attributed to the lower expression of the SARS-CoV2-targeted receptor ACE2 and serine protease serine protease transmembrane serine protease 2 (TMPRSS2) in nasal and bronchial airways in children compared with adults(Reference Saheb Sharif-Askari, Saheb Sharif-Askari and Alabed28).
Vitamin A and epithelial barriers
VA is needed to maintain healthy respiratory and intestinal epithelial barriers(Reference Stephensen17). VA deficiency may not produce obvious changes in healthy epithelial surfaces, but following viral infection in the intestine, for example, pathological changes to infected epithelial surfaces were found to be much greater in VA-deficient than in control mice(Reference Ahmed, Jones and Jackson29). In the respiratory tract, VA deficiency also increases epithelial damage and impairs recovery, sometimes leading to squamous metaplasia in alveoli and airways, following noxious exposures such as ozone treatment(Reference Paquette, Zhang and Ellis30) and infection with influenza virus(Reference Stephensen, Blount and Schoeb31). Sputum (mucus) also provides a solid physical barrier to pathogens(Reference Cone32) and contains many of the macromolecules of the innate immune system(Reference Martin and Frevert33). Sputum is a mixture of oligomeric mucins, MUC2, MUC5AC and MUC5B which are synthesised and secreted by the differentiated mucociliary epithelium(Reference Richardson34). Regulation of mucin production is induced by all-trans retinoic acid via retinoic acid receptor alpha as the major retinoid receptor subtype(Reference Koo, Jetten and Belloni35). Importantly, moderate VA supplementation, but not high-dose VA supplementation or VA deficiency, improves mucin secretion by regulating the gene expression of cytokines and epithelial growth factors(Reference Fan, Liu and Liu36).
The VA metabolite all-trans retinoic acid has also been shown to up-regulate the expression of ACE2(Reference Zhong, Huang and Yang37,Reference Zhou, Wu and Qin38) , resulting in the reduction of blood pressure and the attenuation of myocardial damage in spontaneously hypertensive rats(Reference Zhong, Huang and Yang37). Increased levels of ACE2 after VA supplementation could have two effects: 1) it could increase the risk of SARS-CoV2 infection at the time of virus exposure(Reference Fang, Karakiulakis and Roth39), particularly in individuals who have an adequate VA status; or 2) it could reduce the risk of sympathetic over-activation seen in severe SARS-CoV2 infection, obese and diabetic patients(Reference Aksoy, Karadag and Wollina40–Reference Porzionato, Emmi and Barbon42) through VA supplementation that activates the ACE2-Ang 1–7-Mas axis.
VA is also important during embryonic lung development(Reference Marquez and Chen43); thus, the role in tissue repair in postnatal life is not surprising. A recent review highlights the potential role of VA and retinoic acid as possible therapeutic interventions to prevent pulmonary fibrosis following lung injury(Reference Wang, Yu and Kane44), suggesting a role for VA in recovery from severe COVID-19 disease. Lipofibroblasts are the retinoid storage cells of the lung, similar to stellate cells of the liver(Reference Hind and Maden18), and contain many components of the retinoid signalling pathway including receptors and binding proteins(Reference Hind, Gilthorpe and Stinchcombe45). Importantly, the defining characteristic of hepatic fibrosis is the loss of VA from stellate cells(Reference Tsuchida and Friedman46). Similarly, chronic nutritional VA deficiency results in pulmonary fibrosis through decreased alveolar septation, squamous metaplasia of the respiratory epithelium and a thickening of the alveolar basement membrane followed by an increase in collagens and a deposition of ectopic collagen fibrils(Reference Timoneda, Rodríguez-Fernández and Zaragozá14). Pulmonary fibrosis is characterised by conversion of lipofibroblasts to FGF10-expressing myofibroblasts localised to αSMA expressing cells, down-regulation of PPARγ and activation of TGF-β signalling(Reference Habiel and Hogaboam47). PPARγ activation reverts fibrosis in both the lung and the liver through inhibition of αSMA, type I collagen and TGF-β expression(Reference Tsuchida and Friedman46,Reference Kruglikov and Scherer48) . The beneficial effects of retinoids on pulmonary fibrosis may be through activation of the retinoid X receptor via 9-cis-retinoic acid(Reference Belvisi, Hele and Birrell49).
Innate immune response to SARS-CoV2 infection
The immune response to respiratory viral infections, including coronaviruses, is initiated by barrier defences at the site of initial virus replication(Reference Sariol and Perlman50). SARS-CoV2 infects ciliated epithelial cells in the upper respiratory tract and alveolar type II pneumocytes in the lower respiratory tract via its cellular receptor ACE2(Reference Hou, Okuda and Edwards51). SARS-CoV2 thus first engages the innate immune system in respiratory epithelial cells and adjacent macrophages. Viral RNA in the cytoplasm or endosomes is recognised by pattern-recognition receptors including RIG-1, TLR3, TLR7 and TLR9. These receptors trigger transcription of type I and type III interferon (IFN) which act via cellular receptors to induce anti-viral programmes in infected and adjacent cells that render these cells refractory to viral infection. However, coronaviruses, including SARS-CoV2, can act on these pathways to block induction of anti-viral activity, which may be a factor in the development of more severe diseases. In addition to type I and III IFN, pattern-recognition receptor activation triggers production of other cytokines, including TNF-α, IL-1, IL-6 and IL-18 by innate immune cells, particularly macrophages. These cytokines help initiate inflammation, including recruitment of other immune cells to the site of initial infection. Innate lymphoid cells found at tissue sites are often involved in the initial response to viral (and other) infections, but their role in SARS-CoV2 infection is uncertain(Reference Vabret, Britton and Gruber52,Reference Subbarao and Mahanty53) .
Effects of vitamin A on innate immunity
Many cells of the innate immune system, including innate lymphoid cells, macrophages and granulocytes, are affected by VA. Type 1 innate lymphoid cells, including natural killer (NK) cells, play a role in responses to viral infection and their activity and number in peripheral blood is decreased by VA deficiency(Reference Ross and Stephensen54), while retinoic acid treatment can also dampen activity(Reference Oliveira, Teixeira and Sato55). Neutrophil and macrophage function is also directly affected by VA. Phagocytic and bacterial-killing activity of these cells is impaired by VA deficiency, leading to decreased resistance to some infections, though production of the CD4+ T-helper type 1 (Th1)-promoting cytokine IL-12 can be increased, causing increased type 1 inflammation(Reference Stephensen17). Ex vivo and animal studies show that treatment of macrophages with retinoic acid can have anti-inflammatory effects, dampening production of IL-12 and pro-inflammatory cytokines such as TNF-α, as well as increasing production of the regulatory cytokine IL-10(Reference Oliveira, Teixeira and Sato55). Other effects on macrophage phenotype are also caused by retinoic acid. For example, in Leishmania infection of mice, retinoic acid treatment diminishes the development of M1 macrophages, a component of protective type 1 immunity in this model system, and increases the development of M2 macrophages and type 2 immunity, which is not protective. Thus, retinoic acid intervention in this system decreases resistance to infection(Reference Vellozo, Pereira-Marques and Cabral-Piccin56). Conversely, in Schistosoma mansoni infection of mice, VA deficiency exacerbates disease due to excessive inflammation and tissue damage, while retinoic acid treatment increases conversion of macrophages into a tissue-resident phenotype that dampens inflammation and allows control of infection and enhancement of tissue repair(Reference Gundra, Girgis and Gonzalez57). While many of the effects of retinoic acid on innate immune cells can be seen as anti-inflammatory, a lesson from these and other studies is that intervention with retinoic acid during infections can have unpredictable effects on the course of a particular infection depending on the specifics of the immune response that are responsible for pathogen clearance. This topic has been discussed in a recent review(Reference Xavier-Elsas, Vieira and Masid-de-Brito58).
Adaptive immune response to SARS-CoV2 infection
Dendritic cells link the initial anti-viral response of the innate immune system to development of adaptive immunity, involving B and T lymphocytes, that will help clear infections that cannot be resolved by innate immunity alone. Adaptive immunity also has a memory component that, in the case of most acute viral infections, provides a rapid and robust response upon subsequent exposure to the same virus, typically providing protection against symptomatic infection. Such responses develop for other coronaviruses(Reference Sariol and Perlman50), and hopefully, this will also be true of SARS-CoV2.
To initiate adaptive immunity, dendritic cells at the site of infection are also activated by pattern-recognition receptor-mediated recognition of viruses, as well as by local cytokine production, and deliver viral antigen from the site of infection to a regional lymph node to initiate development of virus-specific effector and memory T cells, as well as memory B cells that results in development of antibody-producing plasma cells. Immunologists recognise three basic types of protective immunity against infectious diseases, types 1, 2 and 3(Reference Murphy and Weaver59). Type 1 immunity is typically induced in response to viruses and other intracellular pathogens and involves development of Th1 cells producing the signature cytokine IFN-γ and CD8+ cytotoxic T-lymphocyte which can directly kill virus-infected cells, and a robust serum IgG antibody response, often targeting the epitope of the viral glycoprotein involved in binding to its cellular receptor (the spike [S] protein in the case of SARS-CoV2), thus neutralising the ability of the virus to infect cells. Macrophages activated by Th1 cells at sites of infection are also a key component of type 1 immunity. Type 2 immunity provides protection against intestinal parasites, involves development of CD4+ Th2 cells producing IL-4, -5 and -13, induction of an antibody response involving IgE and activation of eosinophils and basophils at mucosal surfaces, near the sites of pathogen exposure. Type 3 immunity targets extracellular pathogens, involves development of CD4+ Th17 cells producing IL-17 and IL-22, development of appropriate antibody responses and engagement of mucosal epithelial cells and neutrophils to provide barrier protection. CD4+ follicular-helper T cells develop during all of these responses to provide help for development of antigen-specific memory B cells and a robust antibody response. CD4+ regulatory (Treg) cells also develop during most adaptive responses to provide a pathogen-specific cell type capable of dampening pro-inflammatory immunity by direct cell–cell interaction or production of the cytokines IL-10 and TGF-β.
Research on the adaptive immune response to SARS-CoV2 is in an early state, but two recent reviews indicate that patients recovering from COVID-19 have the Th1, CD8+ cytotoxic T-lymphocyte and neutralising antibody responses expected of a typical type 1 response against a viral infection(Reference Vabret, Britton and Gruber52,Reference Altmann and Boyton60) . Briefly, Th1 responses predominate over Th2 and Th17, as expected, and viral epitopes from all major structural proteins, including spike (S), matrix (M) and nucleocapsid (N), are seen in recovering patients. CD8 T-cell responses are also seen against the same proteins. The magnitude of the response is somewhat greater in patients with more severe disease, which could be due to greater antigenic stimulation. Similar patterns of response were seen against SARS-CoV1 in recovered SARS patients(Reference Yang, Peng and Zhu61), and protection against death by CD8 cells was demonstrated in a mouse model(Reference Channappanavar, Fett and Zhao62). Similar CD4 and CD8 responses were seen against MERS-CoV in MERS survivors 0·5–2 years after infection(Reference Zhao, Alshukairi and Baharoon63). These findings suggest that patients recovering from SARS-CoV2 infection may have protective T-cell memory responses. In contrast to the potential benefit of such Th1 responses, some evidence from SARS patients(Reference Li, Wu and Yan64) as well as preliminary work from COVID-19 patients cited in a recent review(Reference Altmann and Boyton60) suggests that Th2 responses may be associated with poor outcomes to infection. B-cell responses develop in response to SARS-CoV2, as demonstrated by the serum IgM and IgG antibody responses that are seen within 2 weeks of infection, as is also seen with MERS-CoV and SARS-CoV1(Reference Huang, Garcia-Carreras and Hitchings65). Antibodies are directed primarily against the S and N proteins, and the ACE2-binding site is quite immunogenic, resulting in robust neutralising antibody responses(Reference Vabret, Britton and Gruber52).
Effects of vitamin A on adaptive immunity
As discussed above, dendritic cells are a key bridge from innate to adaptive immunity during infection and immunisation. Dendritic cells provide the signals necessary to stimulate development of pathogen-appropriate adaptive immune responses. These signals include peptide antigen to provide pathogen-specificity (i.e. recognition of a specific strain of the flu virus by memory T cells), co-stimulation to insure continued proliferation of developing T cells and specific cytokines to steer CD4 T-cell development towards the pathogen-appropriate Th1, Th2, Th17, Treg and CD4+ follicular-helper T phenotypes. In addition, a fourth signal that is often provided by dendritic cells, particularly in the intestinal and other mucosal-associated immune tissues, is retinoic acid(Reference Iwata66), the production of which is diminished during VA deficiency. Retinoic acid affects several aspects of T-cell development, including modification of T-cell phenotype development and mucosal homing of lymphocytes. These effects have recently been reviewed(Reference Larange and Cheroutre12). They are pleiotropic and depend on modifying factors (e.g. the local cytokine environment), but several main points are clear.
Perhaps, the most consistent and biologically important effect of retinoic acid produced by dendritic cells, and a principal reason for retinoic acid production being constitutive in mucosal dendritic cells, is that retinoic acid induces transcription of two mucosal-homing molecules on lymphocytes, chemokine receptor 9 and α4β7 integrin. Thus, memory T cells, B cells and antibody-producing plasma cells which develop in the intestine or respiratory tract (both are initiating sites of the common mucosal immune response) will preferentially recirculate to these sites, whereas T cells developing from systemic lymph nodes are less likely to do so(Reference Iwata66). Mucosal infections often trigger secretory IgA responses, and the plasma cells responsible for secreting IgA are found at submucosal sites due to the imprinting of chemokine receptor 9 and α4β7 integrin expression. This mechanism may explain why VA deficiency sharply reduces the secretory IgA response to influenza A virus infection in the nasal mucosa of mice(Reference Stephensen, Moldoveanu and Gangopadhyay67). Furthermore, impaired IgA antibody and infrequent virus-specific CD8+ T response following respiratory virus infection induced a cytokine storm in the upper respiratory tract of VA-deficient mice(Reference Penkert, Surman and Jones68). Retinoic acid also enhances intestinal homing of other cell types, including Treg cells(Reference Bakdash, Vogelpoel and van Capel69).
Second, VA deficiency has contrasting effects on Th1/Th2 development, with deficiency not affecting or sometimes enhancing Th1 development (via increased IL-12 production, as discussed above) but dampening Th2 development, particularly during the early stages of phenotype commitment immediately after antigenic stimulation. In contrast, retinoic acid, or high-level dietary VA, can enhance type 2 immunity(Reference Schuster, Kenyon and Stephensen70). However, retinoic acid, at a somewhat later stage in development of Th1 memory cells, can help stabilise phenotype commitment to the Th1 lineage(Reference Brown, Esterhazy and Sarde71). Similarly, effects of VA on CD8+ cytotoxic T-lymphocyte development, a key component of type 1, anti-viral immunity are mixed(Reference Larange and Cheroutre12). Using model viral infections to explore the effects of VA deficiency has shown no impairment of clearance of acute influenza A virus infection in mice, a slight enhancement of some aspects of the type 1 response squamous metaplasia during recovery(Reference Stephensen, Blount and Schoeb31). Using chronic lymphocytic choriomeningitis virus infection in mice also showed an elevated type 1 response, more severe pathological changes, higher virus load and increased mortality, effects which were reversed with retinoic acid(Reference Liang, Yi and Wang72).
Third, VA regulates the balance between Th17 and Treg development. Both cell types often develop in the intestinal lymphoid tissue (as well as at other sites) with Treg cells being predominant when inflammation is minimal and constitutive TGF-β and retinoic acid production bias memory T-cell development towards a Treg phenotype which is appropriated for antigens derived from food and commensal bacteria. However, when inflammation develops, IL-6 is produced and this shifts the balance towards Th17. During VA deficiency, Treg development is diminished because retinoic acid production is reduced(Reference Oliveira, Teixeira and Sato55).
Fourth, retinoic acid affects B cell development, and VA deficiency decreases the antibody response to T-cell-dependent antigens, in particular(Reference Larange and Cheroutre12). While VA deficiency in mice slightly enhances type 1-related IgG2a antibody responses, the IgG1 response is diminished and, in particular, the IgA response is specifically diminished because retinoic acid can enhance class-switching to IgA(Reference Seo, Jang and Kim73,Reference Mora and von Andrian74) .
Contribution of the immune system to COVID-19 pathogenesis
Approximately 5 % of COVID-19 patients, many with underlying conditions such as CVD, diabetes mellitus and obesity, develop severe disease with a high risk of death. Male sex and age above 65 years are also risk factors(Reference Berlin, Gulick and Martinez6). The pathogenesis of severe disease involves excessive activation of the immune system that can result in immunopathology that increases disease severity, as has been reviewed recently(Reference Vabret, Britton and Gruber52,Reference Lo, Kemper and Woodruff75–Reference Mangalmurti and Hunter77) . During the initial innate immune response, a robust IFN type I/III response appears to predict a milder course of disease, presumably by controlling initial virus replication and allowing development of the adaptive response. However, patients with severe disease are more likely to have a lower initial IFN type I/II response (though it may be elevated later) which may result in a failure to control initial viral replication. Prolonged viral replication then appears to result in high-level production of pro-inflammatory cytokines (including TNF-α, IL-1, IL-6 and many others), likely produced by activated macrophages but perhaps by other innate cells such as neutrophils that may be attracted to the lungs. This local hyper-inflammation may dampen development of adaptive immunity and in patients with severe disease lymphopenia, including low levels of T cells, is pronounced. During this ‘cytokine storm’ with activation of innate immune cells, a system response develops that includes elevation of acute phase proteins, particularly CRP, and activation of the complement system which may result in vascular leakage as well as diffuse intravascular coagulation which can cause organ damage thus increasing disease severity. Developing therapeutic interventions to dampen this immune-mediated pathology is a high priority of current research. Potential drug targets include the NF-κB pathway to attenuate TNF-α and IL-6 expression, the JAK/STAT signalling pathway and the sphingosine-1-phosphate receptor 1 pathway(Reference Catanzaro, Fagiani and Racchi78). The JAK/STAT and sphingosine-1-phosphate receptor 1 signalling pathways are of particular interest since SARS-CoV2 potential down-regulation of ACE2 expression could result in over-production of angiotensin II via the related ACE enzyme, leading to enhanced IL-6 production. JAK/STAT inhibitors such as Baricitinib or sphingosine-1-phosphate receptor 1 receptor agonists such as Fingolimod could attenuate the cytokine storm(Reference Catanzaro, Fagiani and Racchi78).
Possible implications of vitamin A deficiency for immune response to SARS-CoV2
The effects of VA in the immune system discussed above have several possible implications for individuals with VA deficiency infected by SARS-CoV2. First, VA deficiency is not likely to impair a type 1 anti-viral response to SARS-CoV2, but could increase the severity of type 1 inflammation and tissue damage in the lungs following viral infection. Second, after clearance of SARS-CoV2 infection, VA deficiency could impair repair of damaged alveolar pneumocytes and airway epithelium. Third, protective immunity to SARS-CoV2 could be impaired by VA deficiency, particularly the mucosal IgA response, which could be important in resistance to reinfection, though development of memory Th1 and CD8+ cytotoxic T-lymphocyte response might also be affected.
Effect of infections on vitamin A metabolism and status
VA is absorbed in the intestine with high efficiency in healthy individuals (>80 %) and mainly stored in the liver (>90 %), with smaller amounts stored in intestine, kidney, adipose tissue and the lung(79). Release of serum VA bound to retinol-binding protein (RBP4) is homoeostatically controlled and does not change over a wide range of liver reserves(79). During infection, the acute protein response increases the synthesis of hepatic inflammatory cytokines, but at the same time reduces the release of negative acute phase proteins such as RBP4. The subsequent reduction of holo-RBP4 (retinol bound to apo-RBP4) causes a decline in circulating VA even before the acute-phase proteins CRP and AGP have reached peak concentrations(Reference Rubin, Ross and Stephensen80). Reduced serum retinol and RBP4 concentrations could potentially overestimate the prevalence of VA deficiency in populations with high levels of inflammation(Reference Larson, Guo and Williams81). Severe infections have also shown to reduce food intake(Reference Rubin, Ross and Stephensen80), impair absorption by 20–30 %(Reference Aklamati, Mulenga and Dueker15), increase metabolic requirements or catabolic losses(Reference Rubin, Ross and Stephensen80) and result in substantial urinary losses(Reference Stephensen17,Reference Stephensen, Alvarez and Kohatsu82) . Importantly, respiratory infections combined with low dietary VA intake could deplete liver VA stores in COVID-19 patients suffering from pneumonia to levels associated with VA deficiency, since urinary losses of >1000 μg retinol/d have been observed in 24 % of ICU patients with pneumonia and sepsis, representing a higher amount than the RDA for VA(Reference Stephensen, Alvarez and Kohatsu82). We estimate that this level of urinary loss, combined with a postulated decrease in intake of approximately 50 % of the RDA for women hospitalised with COVID-19, could lead to a loss of approximately 1350 μg/d from liver stores. Such losses would lead to deficient liver stores (<20 μg/g) in approximately 3 weeks for a 61 kg women with relatively low pre-existing liver stores (40 μg/g; estimated liver weight of 2·4 % of body weight). Using data on estimated liver stores among US women of 129 (sd 89) μg/g who consumed high levels of VA (a mean intake of 173 (sd 111) % of the RDA of 700 μg retinol)(Reference Valentine, Davis and Tanumihardjo83), we estimate that approximately 16 % of women (those at least one sd below the mean) in a population with similarly high intake would have reserves of 40 μg/g or less. In populations consuming lower levels of VA, the percentage of individuals at risk for systemic VA deficiency would be higher. It is important to note however that these systemic effects of VA are different to tissue-specific VA deficiency that could develop following COVID-19 infection (Fig. 1). First, pulmonary fibrosis induced by SARS-CoV2 is characterised by the conversion of lipofibroblasts to activated myofibroblasts that excessively deposit extracellular matrix proteins and lose their retinoid stores(Reference Hind, Gilthorpe and Stinchcombe45,Reference Kruglikov and Scherer48) . Second, inflammation induced reduction of retinoic acid uptake and metabolism to polar metabolites(Reference Rubin, Ross and Stephensen80) could affect the ability of the lung to regenerate from pulmonary fibrosis, since lipofibroblasts rely on retinoids to initiate, coordinate and regulate alveolar septal eruption and alveolo-genesis(Reference Hind, Gilthorpe and Stinchcombe45). Third, the ability to deliver retinoids to the lung is compromised during severe infection due to the decline in circulating VA(Reference Rubin, Ross and Stephensen80). Nutritionists have long recognised that acute infection exacerbates the risk of malnutrition, as there is potential to fall into a ‘vicious circle’ of acute illness, deficiency and susceptibility to secondary infection. However, the timing of VA supplementation during SARS-CoV2 infection and the recovery period may be critical, as early intervention during the infectious period may be contraindicative due to its impact on the ACE2 receptor(Reference Zhong, Huang and Yang37,Reference Zhou, Wu and Qin38) , but intervention during the recovery period may be critical to address the potentially developing tissue-specific VA deficiency and its effects on the respiratory immune response. Importantly, VA supplementation may not be beneficial depending on age and co-morbidity, since VA supplementation can increase the risk of acute respiratory tract infections in children with adequate VA status(Reference Grotto, Mimouni and Gdalevich84) and can delay recovery from community-acquired pneumonia in children(Reference Stephensen, Franchi and Hernandez85) and respiratory syncytial virus infection in infants(Reference Bresee, Fischer and Dowell86). However, VA supplementation was associated with improved recovery from Ebola virus infection in adults(Reference Aluisio, Perera and Yam87) and can have beneficial effects in obesity by correcting tissue VA deficiency, altering lung immune cell composition, improving vaccine responses and assisting in respiratory virus clearance(Reference Penkert, Cortez and Karlsson88). In particular, high-dose VA supplementation during recovery from measles has been shown to decrease mortality and improve recovery, including decreasing the severity of secondary bacterial pneumonia(Reference Hussey and Klein89). Delivering VA through inhalation may be more efficient to address the immunological lesions in VA-deficient individuals(Reference McGill, Kelly and Guerra-Maupome90,Reference Schaeffer, Roy and Mukherjee91) particularly since inflammation also reduces retinoic acid uptake and metabolism to polar metabolites(Reference Rubin, Ross and Stephensen80).
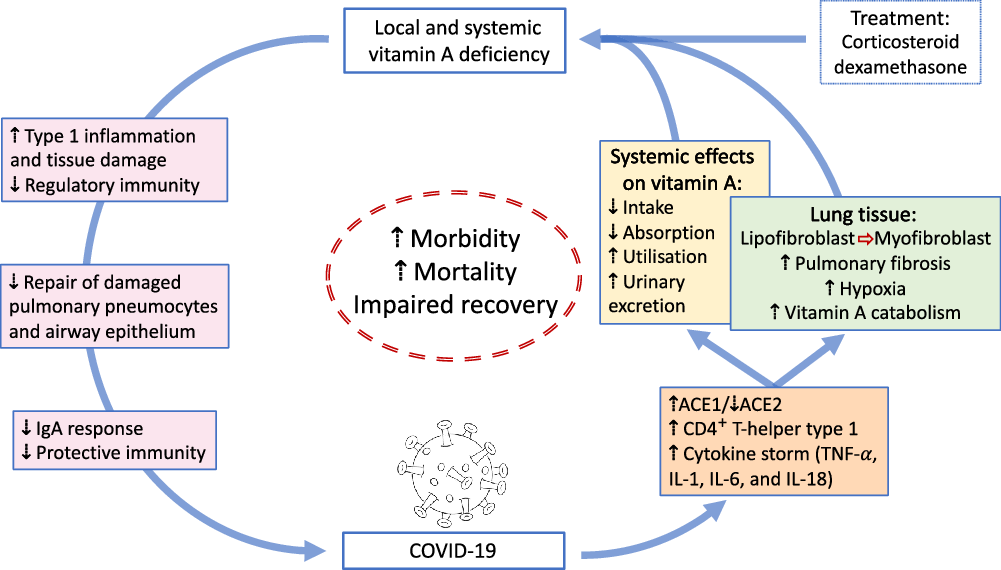
Fig. 1. Overview of potential interactions between vitamin A and COVID-19. SARS-CoV2 uses angiotensin-converting enzyme 2 (ACE2) for receptor-mediated cell entry, leading to ACE2 down-regulation and a deregulation of the renin–angiotensin system. Viral RNA triggers the production of CD4+ T-helper type 1 cells and cytokines, including TNF-α, IL-1, IL-6 and IL-18. In the lung, SARS-CoV2 can lead to severe acute respiratory syndrome due to extensive pulmonary fibrosis promoted by enhanced lipofibroblast–myofibroblast transition. Since lipofibroblasts rely on retinoids to initiate, coordinate and regulate alveolar septal eruption and alveolo-genesis, the loss of retinoids during the virus-induced lipofibroblast–myofibroblast transition could impair the ability of the lung to repair damaged epithelial surfaces, potentially leading to long-lasting scarring, lung fibrosis and reduced pulmonary capacity, which could manifest into a ‘long COVID’ effect. Treatment of COVID-19 patients with dexamethasone could further increase localised vitamin A deficiency through the reduction of retinoid-binding proteins and receptors. On the other hand, SARS-CoV2 could also lead to systemic vitamin A deficiency through a combination of increased urinary losses, reduced intake and absorption and increased utilisation. The effects of local and systemic vitamin A deficiency have been shown to reduce the ability of the immune system to maintain healthy respiratory and intestinal epithelial barriers, activity and numbers of type 1 innate lymphoid cells and secretory IgA responses to virus infection. This vicious cycle of increased vitamin A deficiency and decreased regulatory and protective immunity impairs recovery and is likely to increase morbidity and mortality.
Interaction of vitamin A with olfactory function
SARS-CoV2 causes olfactory and/or gustatory dysfunctions in COVID-19 patients(Reference Gori, Leone and Loffredo92–Reference Lechien, Chiesa-Estomba and De Siati95). A recent meta-analysis of eight studies confirmed that patients with COVID-19 had significantly higher risks of developing olfactory and/or gustatory dysfunctions compared with normal subjects (OR of 65·9) or patients with acute respiratory infection without detectable virus (OR of 11·3)(Reference Hoang, Kanjanaumporn and Aeumjaturapat94). On average, 50 % of COVID-19 patients suffered from olfactory and/or gustatory dysfunctions(Reference Hoang, Kanjanaumporn and Aeumjaturapat94), although olfactory impairment was reported to vary between 4·8 % in China, 68 % in the USA and 85·6 % in Europe(Reference Gorzkowski, Bevilacqua and Charmillon93). The mean time from olfactory loss to recovery onset was reported as 12 d, with complete olfactory recovery in only 51 % of patients(Reference Gorzkowski, Bevilacqua and Charmillon93). Viral infections are a common cause of olfactory dysfunction(Reference Murphy, Doty, Duncan and Doty96), although the pathogenesis of sensory loss after viral infections is not well characterised(Reference Gori, Leone and Loffredo92,Reference Lechien, Chiesa-Estomba and De Siati95) . It is assumed that the nasal epithelium and olfactory bulb could be critical in the pathological development of COVID-19, with mild outcomes of COVID-19 leading to localised effects with mild effects on olfactory function. Interestingly, the effects of SARS-CoV2 on chronic olfactory impairment increase with age, affecting up to 50 % of people aged ≥65 years and >80 % of people aged ≥80 years(Reference Gori, Leone and Loffredo92).
VA supplementation may play an important role in the regeneration of olfactory receptor neurons, particularly since retinoic acid signalling is involved in neuronal regeneration(Reference Rawson and LaMantia97). Indeed, a recent retrospective cohort analysis in patients with post-infectious olfactory dysfunction indicated that topically applied VA at a dose of 10 000 IU/d for 8 weeks improved clinical outcome(Reference Hummel, Whitcroft and Rueter98). This beneficial effect of VA to improve olfactory dysfunction is supposedly due to increased local concentration of VA at the olfactory neuroepithelium(Reference Hummel, Whitcroft and Rueter98). Thus, similarly for treating VA deficiency in the lung, topical administration of VA may increase bioavailability and reduce toxic side effects of high-dose systemic VA therapy needed to overcome localised VA deficiency(Reference Schaeffer, Roy and Mukherjee91,Reference Hummel, Whitcroft and Rueter98,Reference Brooks, Tong and Benedetti99) . However, since no studies have investigated the benefit of topical application of VA in COVID-19 patients suffering from olfactory dysfunction, prospective, double-blind and placebo-controlled trials are needed to confirm if VA therapy would be of benefit to those patients that struggle to recover from olfactory impairment.
Interaction of vitamin A with current SARS-CoV2 medication
The RECOVERY trial results demonstrated that low-dose dexamethasone reduced the death rate of patients that require respiratory support(Reference Florindo, Kleiner and Vaskovich-Koubi3). However, although corticosteroids have anti-inflammatory, antioxidant, pulmonary vasodilator and anti-edematous effects(Reference Mokra, Mikolka and Kosutova100), their impact on limiting immune responses might counteract viral clearance(Reference Lai101). Previous studies associate the use of corticosteroid in SARS, MERS and influenza with a higher risk of a delayed viral clearance and long-term complications in survivors(Reference Huang, Wu and Zheng102). Furthermore, dexamethasone decreases alveolar numbers, and this effect on alveolar architecture can be long term or permanent(Reference Hind and Maden18). The drug also reduces retinoid-binding proteins and receptors in mice(Reference Hind and Maden18), which potentially could affect the metabolism of VA in affected tissues. Since chronic nutritional VA deficiency can result in pulmonary fibrosis(Reference Timoneda, Rodríguez-Fernández and Zaragozá14), long-term complications in survivors after dexamethasone treatment could include lung tissue scaring due to medication-induced VA deficiency. On the other hand, supplementation with a nutrient-metabolite combination containing VA and retinoic acid in a 10:1 molar ratio (VARA) was effective in increasing the concentration of VA to a level that was not antagonised by dexamethasone, probably since the VARA-induced lung tissue VA accumulation compensated the VA loss caused by dexamethasone(Reference Ross and Ambalavanan103). Importantly, the delivery of retinoids to the lung tissue determines whether or not the negative side effects of dexamethasone on lung VA levels can be overcome. Treatment with retinoic acid was shown to reverse pulmonary emphysema(Reference Massaro and Massaro104), but caused significant toxic side effects(Reference Lippman, Lee and Karp105). To obtain biologically effective levels in the lung, inhalation of retinoids rather than oral administration may be a better approach(Reference Schaeffer, Roy and Mukherjee91), particularly since this approach induces cellular retinol-binding protein 1 protein expression which was shown to be reduced following dexamethasone treatment(Reference Hind and Maden18). However, effectiveness of either VARA or retinoic acid inhalation to optimise lung VA stores would need to be tested in COVID-19-affected individuals who have been treated with dexamethasone.
Summary
Traditionally, VA plays a very important role in the response of the immune system to infection, and this role may be particularly important in the development of a protective immune response after a SARS-CoV2 infection has occurred. This stresses the need for an adequate VA status prior to infection in order to support the immune system in its ability to deal with the inflammatory response due to SARS-CoV2 via a range of mechanisms that have been summarised in this review. However, in spite of an adequate a priori VA reserve, SARS-CoV2 infection itself may diminish VA stores. Such an infection-induced VA deficiency may impair the ability of the lung to repair damaged epithelial surfaces, potentially leading to long-lasting scarring, lung fibrosis and reduced pulmonary capacity. Thus, VA supplementation during recovery may be needed even though VA supplementation early in infection may not have been indicated due to a lack of VA deficiency prior to infection. In addition, earlier VA supplementation may have unpredictable effects for the following reasons: First, it is plausible that VA supplementation at the time of SARS-CoV2 exposure would increase the risk or severity of an infection since the cellular infection mechanism of SARS-CoV2 is mainly mediated by ACE2 which can be up-regulated by retinoic acid, as discussed above. Second, however, activation of ACE2 in infected patients could reduce the risk of sympathetic over-activation seen in severe SARS-CoV2 infection, and in particular in obese and diabetic COVID-19 patients. Topical application of VA may be beneficial to patients suffering from post-infectious olfactory dysfunction, since VA supplementation may play an important role in the regeneration of olfactory receptor neurons. Finally, there could be a beneficial use of VA as a co-medication in severe SARS-CoV2-affected patients who are treated with corticosteroids, particularly since dexamethasone was shown to negatively affect VA metabolism in lung tissues. It is plausible to assume that VA deficiency in at risk population groups would increase the risk of severe COVID-19 infection, but data to confirm this are currently missing. Importantly, COVID-19 has doubled the number of people in lower- and middle-income countries facing acute food insecurity and is predicted to reduce the coverage of VA supplementation programmes to VAD-affected populations between 15 and 50 %(Reference Headey, Heidkamp and Osendarp106). Since the risk of SARS-CoV2 infection is low in under 5-year-olds, and the benefit of VA supplementation outweighs the risk of developing a severe COVID-19 infection due to increased VA intake, VA supplementation programmes should continue for this age group.
Acknowledgements
We acknowledge the help of Kieran Finney and Chloe Elliott for the literature review and Catherine Meplan for her help in designing the figure.
Funding for C. B. S. was provided by USDA-ARS project number: 2032-51530-026-00-D.
The manuscript was written by C. B. S. and G. L.
Both authors have no conflicts of interest to declare.





